Key Points
Activity trackers may be a therapeutic modality to increase physical activity levels post-VTE but are limited by adherence.
Further studies are needed to improve the feasibility of exercise training in pediatric VTE for a successful randomized controlled trial.
Abstract
Increased physical activity is protective against worsening of postthrombotic syndrome (PTS) in adults. We assessed patient eligibility, consent, adherence, and retention rates in a pilot trial of prescribed physical activity following venous thromboembolism (VTE) in children. Secondary objectives were to describe the within-subject changes in PTS, quality of life, and coagulation biomarkers before and after the intervention in each group. We enrolled and randomized patients between 7 and 21 years of age to the physical activity group or the standard care (education-only) group in a 1:1 allocation ratio. The physical activity group wore a Fitbit for 4 weeks to determine habitual activity and then increased activity over an 8-week “active” period, followed by a 4-week “do-as-you-wish” period. Two hundred thirty-five children were diagnosed with VTE; 111 patients were screened, of whom 40 (36%) met study eligibility criteria. Of these, 23 (57%) consented to participate and were randomized (Fitbit,11; standard group, 12). The trial was of greater interest to overweight and obese children, as they comprised 83% of consented patients. Only 33% adhered to the activity prescription, and 65% (15/23) completed the trial. The PTS scores (P = .001) improved in the physical activity group compared with the education-only group. It is feasible to enroll and randomize pediatric VTE patients to a prescribed physical activity regimen 3 months following VTE. Metrics for adherence to enhanced physical activity and retention were not met. These results provide the rationale to explore low adherence and retention rates before moving forward with a larger trial of exercise training following VTE. This trial was registered at www.clinicaltrials.gov as #NCT03075761.
Introduction
The incidence of venous thromboembolism (VTE) is rising.1 Postthrombotic syndrome (PTS), a form of chronic venous insufficiency, develops in one-third of the children after extremity deep venous thrombosis (DVT).2-5 Post-pulmonary embolism (post-PE) syndrome, characterized by persistent dyspnea, impaired exercise capacity, and decreased quality of life (QoL), affects ≤50% of patients surviving PE.6,7 Both PTS and post-PE syndrome are associated with decreased physical activity levels.3,8
Exercise training after an acute VTE is safe but underutilized.9-11 Physical activity provides improvements in aerobic capacity, fatigue, and QoL and a decrease in PTS progression among VTE survivors.12-14 Obesity, a risk factor for VTE, may be worsened by sedentary behavior after an acute VTE.15 Only 50% of pediatric VTE survivors resume pre-VTE physical activity within 6 months.16 Extrapolating from above, we posited that a physical activity regimen for children after VTE is unlikely to be harmful, may be of benefit, and needs further study. In the past, investigator-initiated trials involving the prevention or treatment of pediatric VTE have been terminated prematurely for futility and feasibility concerns.17-19 Therefore, we designed a small-scale randomized controlled trial (RCT) to establish the feasibility of prescribing physical activity that would closely approximate physical activity prescription in the context of a large, fully powered RCT. A major feasibility issue that precedes a full RCT is the need to derive an effect-size estimate for the intervention, which precluded using a single-arm cohort design. We hypothesized that adherence to a physical activity regimen would be feasible and potentially effective post-VTE in children. Our primary objectives were to assess patient eligibility, consent, adherence, and retention in a pilot trial of prescribed physical activity following an initial VTE. Our secondary objectives were to obtain estimates of effect size associated with the prescribed activity by describing the within-subject changes in PTS, QoL, and coagulation biomarkers before and after intervention in each group.
Methods
Trial design
We conducted a parallel-group randomized controlled pilot trial of children with newly diagnosed, first, lower-extremity DVT. Patients were randomly allocated via a Web-based program to the physical activity/Fitbit group (physical activity prescription and education) or the standard care (education-only) group in a 1:1 allocation ratio. After assigning 6 patients (3 in each group), we recognized that accrual was slower than anticipated due to the exclusion of patients with PE and the timing of enrollment (4 weeks after diagnosis). Many patients with postoperative state– or infection-associated DVT had physical activity restrictions within the first month after diagnosis. We amended the protocol to delay enrollment up to 12 weeks after diagnosis, included patients with both DVT and PE, and randomly assigned participants to either intervention or standard care. At this time, we also changed the name of the trial from “Physical activity in children at risk of post-thrombotic syndrome (PACT): a pilot randomized controlled trial” to “Physical activity in children at risk of postthrombotic sequelae: a pilot randomized controlled trial.”
Participants
Twenty-three sequential eligible patients with VTE were recruited from the Bleeding Disorders and Thrombosis Program at the University of Texas Southwestern Medical Center (UTSW) between December 2016 and December 2018. The institutional review board approved this study at UTSW, and all participants gave informed consent. Participants were enrolled within 12 weeks of diagnosis and completed clinic visits corresponding to 3, 6, and 9 months after diagnosis. Inclusion criteria were (1) age 7 to 21 years; (2) a radiologically confirmed, acute, proximal (iliac, iliofemoral, and femoropopliteal), first lower-extremity DVT and/or acute PE; (3) 12 weeks (±2 weeks) after starting anticoagulation; and (4) outpatient ambulatory status. Participants were not eligible if they were unable to exercise or had a contraindication to increasing activity (eg, postoperative status, cast immobilization, or risk of pathological fracture). The research team screened inpatients and outpatients with VTE and identified potential participants that met the study inclusion criteria. Identification occurred through 3 sources: (1) new patient referrals to the Bleeding Disorders and Thrombosis outpatient clinic, (2) inpatient admissions to the hematology service, and (3) inpatient consultations for VTE that were not on primary hematology service. Information regarding the trial was also available on the UTSW clinical trials Web site and the www.clinicaltrials.gov Web site. We collected demographic and clinical information such as age, sex, body mass index (BMI), VTE location, and veno-occlusion at diagnosis. A BMI for age and sex between the 5th and 85th percentile was defined as normal, between the 85th and 95th percentile as overweight (OW), and >95th percentile as obese.20 We documented reasons for noneligibility, lack of consent, dropout, and lack of compliance.
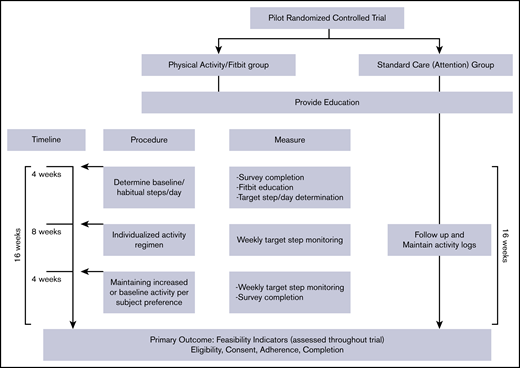
Schedule and procedures for all participants.
We used a prospective randomized, open, blinded end-point (PROBE) design due to the nature of our study and inability to completely blind the patients, principal investigator (PI), and study coordinator to the treatment group. At the first study visit at 3 months after diagnosis, the PI (A.Z.), blinded to treatment allocation, provided a standardized 15- to 20-minute education session to both arms on the potential benefits of physical activity after VTE, including recommended physical activity guidelines from the American College of Sports Medicine. The primary study coordinator (K.M.) then revealed the allocated arm to the participants and the PI and explained the study procedures for each group. A trained physical therapist (E.T.), blinded to the laterality of DVT and treatment allocation, performed PTS assessment at the 6- and 9-month postdiagnosis visits.
Experimental intervention
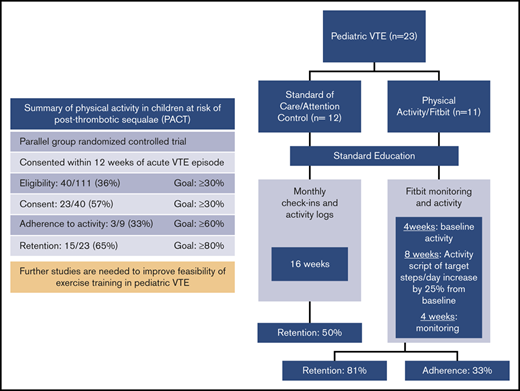
The intervention was started at 3 months post-DVT and occurred over a 16-week period that included 8 weeks of prescribed physical activity, preceded by 4 weeks to determine “habitual or baseline” activity, and followed by 4 weeks of “do-as-you-wish” physical activity (Figure 1).
General principles and rationale.
The prescribed physical activity consisted of aerobic exercise. We chose step counts per day as the metric to quantify and prescribe physical activity using a validated activity tracker, the Fitbit Charge 2, an accelerometer-based device that tracks steps, distance, active minutes, and hourly activity. Steps at the light-, moderate-, and vigorous-intensity levels provide a range of exertion choices in the context of physical activity monitoring and prescription. As the principal muscles affected by PTS are the lower limb muscles, we opted to use a walking-jogging program to target all potentially affected muscles (quadriceps, hamstrings, and gastrocnemius/soleus). Walking-jogging is an acceptable and accessible exercise activity, especially among subgroups with a low prevalence of leisure-time physical activity such as adolescents.21,22 If preferred or already involved, muscle-strengthening or bone-strengthening exercises could replace the walk/jog (eg, lifting weights, jumping rope, dancing, or team sports such as basketball or volleyball) as long as the target goal for physical activity was met.
Schedule and procedures.
At 3-months after diagnosis, the research coordinator (K.M.) provided hands-on training to promote successful use of the Fitbit and made a study account for each participant to sync their Fitbits with the online data platform, the Fitabase, to display the physical activity metrics. A valid day was defined as having ≥10 hours of Fitbit wear. A valid week was defined when a participant had ≥4 valid days of Fitbit wear.23 Participants wore the Fitbit for 4 weeks to establish a baseline or “habitual'” activity. Using steps/day data and the type of physical activities received at the end of 4 weeks, we provided an individualized activity prescription of “target” steps/day, corresponding to a 25% increase above the participant’s baseline (see supplemental Appendix, page 7, for an example activity prescription). We included occupational, and transportation activity in this target but also provided examples of aerobic exercises within their target heart rate zone (60% to 85% of maximal heart rate). Participants were asked to maintain the goal for 8 weeks. At the end of this period, participants were given the preference to either maintain the increased activity or resume habitual activity for another 4 weeks. A phone call was made weekly for encouragement and to ensure adherence throughout the 16 weeks. For participants who had difficulty with the activity prescription or use of the Fitbit, a phone call or face-to-face session was arranged with the research coordinator and physical therapist. The effort required for participating in the trial was more for the physical activity group than the standard care group, related to wearing the Fitbit, syncing with the Fitabase, and receiving weekly phone calls.
Control intervention
The controls were chosen to be attention controls that received intervention over the same 16-week period consisting of (1) education on benefits of physical activity at the outset, (2) monthly phone contacts to check in, reinforce education, and provide encouragement to exercise, and (3) complete monthly physical activity logs. Physical activity was not explicitly addressed and was up to the preference of the participant. The goal of the “attention” intervention is to provide intervention in the form of education and to simulate more or less the same contact that participants in the physical activity groups received.
Outcomes
Feasibility outcomes.
The primary outcomes were the following feasibility indicators: (1) rate of eligibility (proportion of subjects screened who fully meet eligibility criteria to participate in the trial); (2) rate of consent (the proportion of eligible subjects who consented to participate); (3) compliance in the physical activity group, calculated as the proportion meeting target weekly steps/day goal on ≥4 valid days during the “active” 8-week period and compliance in the standard-of-care arm, calculated as the proportion who completed and submitted physical activity questionnaires; and (4) trial completion (the proportion who complete the trial after randomization). We established a priori that we will consider the trial successful if the following criteria are met: rate of eligibility ≥30%, rate of consent ≥30%, level of compliance ≥60%, and rate of trial completion ≥80% within 24 months of trial initiation. Our conservative estimate for the rates of consent was based on the evidence that prevention trials, especially exercise prevention trials, recruit a smaller proportion of those screened than treatment trials and on previous exercise trials in venous insufficiency where the rate of consent achieved or consent decided a priori was similar to our trial.10,24,25
Secondary outcomes.
We assessed secondary outcomes in all participants at VTE diagnosis and at the 3-, 6- and 9-month visits. QoL was measured using the Pediatric Quality of Life Inventory, a validated tool for measurement of health-related QoL for healthy children and adolescents as well as those with acute or chronic medical conditions.1 The Godin-Shephard Leisure-Time Physical Activity Questionnaire was used to quantify physical activity levels. Individuals reporting moderate-to-strenuous activity levels ≥24 were classified as active, whereas individuals reporting moderate-to-strenuous activity levels ≤23 were classified were insufficiently active.2 For assessment of PTS, the Manco-Johnson Instrument was used to assess children at the 6- and 9-month visits. A score of ≥1 with findings in both physical examination and functional assessment yields a diagnosis of functionally significant PTS.3 Coagulation biomarkers, including D-dimer (Stago; STA-LIATEST immuno-turbidimetric assay), factor VII (FVIII):C (one-stage clotting assay), C-reactive protein (CRP; using a latex-enhanced immunoturbidimetric assay), thrombin generation assay using calibrated thrombogram, and fibrinolysis using modified thromboelastography, were measured at all study visits (supplemental Appendix, pages 8-9).4-6 We provided prescriptions to obtain custom-fitted elastic compression stockings (ECS) (knee length, 20-30 mm Hg) with a contracted garment boutique to all participants. The use of ECS was not mandated but was recorded as dedicated use (6 or more days/ week), moderate use (4-5 days/week), poor use (<4 days/week), or no use.26
Sample size calculation
We estimated a feasible sample size based on institutional VTE numbers and published flat rules of thumbs for an overall pilot trial sample size of a 2-armed trial as cited previously.27-29 We identified 268 children with VTE in the preceding 2 years (n = 130 in 2014 and n = 138 in 2015). Of these, half involved lower-extremity thrombosis (n = 134 of 268 [50%]), and of these, 52% fell within the age range of our trial (n = 67). Considering contraindications to exercise, we estimated 60 eligible children over a 2-year period. We decided on a conservative estimate of 44 (20 in each arm). We reasoned that it would be a large enough sample size to inform about the practicalities of eligibility, consent, adherence to exercise, and retention that might arise for a single site in a definitive trial of exercise in pediatric VTE. Post-hoc power analyses show that with 111 patients screened, we were powered to estimate an eligibility rate of 30% to within a 95% confidence interval of ±8.5%. At an eligibility rate of 30%, 33 patients were expected be eligible and an estimated consent rate of 30% to within a 95% confidence interval of ±15.6%.
Statistical analyses
We calculated the proportion of screened patients who fully met trial eligibility criteria, the proportion of eligible patients who consented to participate, the proportion in the physical activity/Fitbit group who adhered to prescribed activity, and the proportion of randomized patients who completed the trial (in total, and by allocated intervention).
The feasibility outcomes are reported descriptively using mean (standard deviations [SD]) for continuous outcomes, and raw counts (%) for categorical outcomes are reported. For the clinical end points, using an intent-to-treat analysis that includes all participants with data at baseline and 6 months (end of the intervention period), we compared within-patient change from baseline (diagnosis or pre-VTE levels) to 6 months (mean, SD) in the physical activity vs standard care in the following measures: D-dimer, FVIII, CRP, thrombin generation assay parameters of endogenous thrombin potential and peak thrombin, fibrinolysis on thromboelastography, QoL (total and physical), self-reported physical activity levels, and PTS (change from 6 to 9 months). This was averaged for each group, and within-group differences were compared between the standard care and physical activity groups using a t test. ECS use was descriptively described but not included in a formal analysis.
Safety monitoring
A safety officer was assigned for the trial and charged with assessing the safety of study participants. The safety officer (1) reviewed the protocol when funded and made suggestions for safety-related changes, (2) assessed the agreed-upon interim data reports, (3) reviewed end points for safety, and (4) could add to or modify this list of objectives. The safety officer did not have any other involvement with the study.
Results
Patient flow, baseline characteristics, and feasibility outcomes
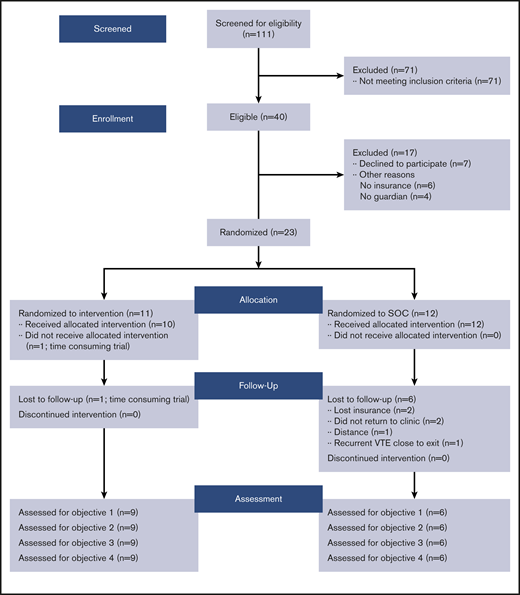
From 2016 to 2018, 235 children at our center were diagnosed with VTE and 111 patients were screened, of whom 40 (36%) met study eligibility criteria (Table 1). Of these, 23 (57.5%) consented to participate (Table 2) (11 randomized to physical activity arm and 12 to education or standard care arm). Among consenting patients, 23 completed the baseline and 3-month assessment, 18 completed the 6-month visit, and 15 completed the end-of-study visit (Figure 2).
Sample characteristics of VTE patients by eligibility criteria
| . | Met eligibility criteria (n = 40) . | Did not meet eligibility criteria (n = 71) . |
|---|---|---|
| Age, mean (SD), y | 15 (2.6) | 13.8 (3.8) |
| Sex, n (%) | ||
| Female | 28 (70) | 33 (46) |
| Male | 12 (30) | 38 (54) |
| Race, n (%) | ||
| White | 30 (75) | 40 (56) |
| Nonwhite | 10 (25) | 31 (44) |
| BMI, n (%), kg/m2 | ||
| Normal weight | 12 (30) | 36 (51) |
| OW/obese | 28 (70) | 35 (49) |
| VTE location, n (%) | ||
| DVT | 22 (55) | 58 (82)* |
| PE | 12 (30) | 8 (11) |
| DVT + PE | 6 (15) | 5 (7) |
| Concomitant medical disorders, n (%) | ||
| Yes | 22(55) | 55 (77) |
| No | 18 (45) | 16 (22) |
| . | Met eligibility criteria (n = 40) . | Did not meet eligibility criteria (n = 71) . |
|---|---|---|
| Age, mean (SD), y | 15 (2.6) | 13.8 (3.8) |
| Sex, n (%) | ||
| Female | 28 (70) | 33 (46) |
| Male | 12 (30) | 38 (54) |
| Race, n (%) | ||
| White | 30 (75) | 40 (56) |
| Nonwhite | 10 (25) | 31 (44) |
| BMI, n (%), kg/m2 | ||
| Normal weight | 12 (30) | 36 (51) |
| OW/obese | 28 (70) | 35 (49) |
| VTE location, n (%) | ||
| DVT | 22 (55) | 58 (82)* |
| PE | 12 (30) | 8 (11) |
| DVT + PE | 6 (15) | 5 (7) |
| Concomitant medical disorders, n (%) | ||
| Yes | 22(55) | 55 (77) |
| No | 18 (45) | 16 (22) |
Of 58 patients, 48 had upper-extremity DVT.
Sample characteristics of VTE patients by consent
| . | Provided consent (n = 23) . | Did not provide consent (n = 17) . |
|---|---|---|
| Age, mean (SD), y | 14.7 (2.9) | 15.5 (2.2) |
| Sex, n (%) | ||
| Female | 15 (65) | 13 (76) |
| Male | 8 (35) | 4 (24) |
| Race, n (%) | ||
| White | 19 (83) | 9 (53) |
| Nonwhite | 4 (17) | 8 (47) |
| BMI, n (%), kg/m2 | ||
| Normal weight | 4 (17) | 8 (47) |
| OW/obese | 19 (83) | 9 (53) |
| VTE location, n (%) | ||
| DVT | 15 (65) | 12 (70) |
| PE | 2 (8) | 3 (17) |
| DVT + PE | 6 (27) | 2 (13) |
| Concomitant medical disorders, n (%) | ||
| Yes | 4 (17) | 6 (35) |
| No | 19 (83) | 11 (65) |
| Distance to UTSW (miles) | 38.8 | 51.6 |
| . | Provided consent (n = 23) . | Did not provide consent (n = 17) . |
|---|---|---|
| Age, mean (SD), y | 14.7 (2.9) | 15.5 (2.2) |
| Sex, n (%) | ||
| Female | 15 (65) | 13 (76) |
| Male | 8 (35) | 4 (24) |
| Race, n (%) | ||
| White | 19 (83) | 9 (53) |
| Nonwhite | 4 (17) | 8 (47) |
| BMI, n (%), kg/m2 | ||
| Normal weight | 4 (17) | 8 (47) |
| OW/obese | 19 (83) | 9 (53) |
| VTE location, n (%) | ||
| DVT | 15 (65) | 12 (70) |
| PE | 2 (8) | 3 (17) |
| DVT + PE | 6 (27) | 2 (13) |
| Concomitant medical disorders, n (%) | ||
| Yes | 4 (17) | 6 (35) |
| No | 19 (83) | 11 (65) |
| Distance to UTSW (miles) | 38.8 | 51.6 |
Consort diagram for pilot study of physical activity in children at risk of postthrombotic sequelae. SOC, standard of care.
Consort diagram for pilot study of physical activity in children at risk of postthrombotic sequelae. SOC, standard of care.
One physical activity/Fitbit participant withdrew between the baseline and 6-month visit (for time constraint issues), and 1 participant was lost to follow-up after the 6-month visit. Three participants were lost to follow-up in the standard care group due to the inability to return to the clinic and 3 due to reasons unrelated to the trial (2 lost insurance and 1 developed recurrent DVT before the exit visit). There were no protocol violations in regard to eligibility, consent, or randomization. The most common reasons for the inability to meet feasibility criteria are described in Table 3.
Top 3 reasons for an inability to meet feasibility criteria
| Noneligibility |
| Location of DVT |
| Concurrent medical problems |
| Malignancy |
| Lack of consent |
| Time constraints |
| Funding/insurance |
| No guardian |
| Lack of adherence |
| Time constraints |
| Motivation |
| Pain/discomfort and fatigue |
| Dropout by allocated intervention |
| Activity/Fitbit group (n = 1) |
| Time constraints |
| Standard care group (n = 6) |
| Time constraints (n = 3) |
| Insurance reasons (n = 2) |
| Recurrence (n = 1) |
| Noneligibility |
| Location of DVT |
| Concurrent medical problems |
| Malignancy |
| Lack of consent |
| Time constraints |
| Funding/insurance |
| No guardian |
| Lack of adherence |
| Time constraints |
| Motivation |
| Pain/discomfort and fatigue |
| Dropout by allocated intervention |
| Activity/Fitbit group (n = 1) |
| Time constraints |
| Standard care group (n = 6) |
| Time constraints (n = 3) |
| Insurance reasons (n = 2) |
| Recurrence (n = 1) |
The mean age of the study participants was 14.7 (SD 2.9) years (Table 4). The sex distribution was 63% (7/11 patients) female in the physical activity group and 42% (5/12 patients) female in the standard care group. OW or obese patients made up 83% (19 of 23 patients) of those who provided consent and 65% (15 of 23 patients) of those who were successfully enrolled. Approximately 54% (6 of 11 patients) in the physical activity group were OW or obese compared with 75% (9 of 12) in the standard care group. Functionally significant PTS was detected in 3 participants at the 9-month postdiagnosis visit.
Baseline characteristics of study patients
| Characteristic . | Entire cohort (N = 23) . | Physical activity group (n = 11) . | Standard care group (n = 12) . |
|---|---|---|---|
| Age, mean (SD), y | 14.70 (2.32) | 15.18 (2.04) | 14.25 (2.56) |
| Sex (female), n (%) | 12 (52) | 7 (63) | 5 (42) |
| BMI, mean (SD), kg/m2 | 28.28 (9.69) | 29.83 (12.34) | 26.86 (5.58) |
| OW/obese, n (%) | 15 (65.22) | 6 (54.54) | 9 (75) |
| Clot burden at diagnosis occlusive, n, (%) | 14 (60.87) | 7 (63.64) | 7 (58.33) |
| VTE location, n (%) | |||
| DVT | 17 (73.91) | 8 (72.73) | 9 (75) |
| PE | 3 (13.04) | 2 (18.18) | 1 (8.335) |
| Concurrent DVT and PE | 3 (13.04) | 1 (9.09) | 2 (16.67) |
| D-dimer, ng/mL | |||
| At diagnosis, mean (SD) | 5792.24 (7222.35) | 5416.67 (6997.21) | 8077.6 (8783.46) |
| At exit visit, mean (SD) | 352.86 (96.32) | 406.67 (118.65) | 300 (103.57) |
| Pre-VTE PA score, mean (95% CI) | 60 (40, 79.7) | 66 (33.7, 98.9) | 57 (34, 80.2) |
| QoL score at diagnosis, mean (95% CI) | 67 (57.5, 76.5) | 71 (56, 87) | 64 (52, 76.5) |
| PTS at exit visit, n (%) | 3 (20) | 0 | 3 |
| Characteristic . | Entire cohort (N = 23) . | Physical activity group (n = 11) . | Standard care group (n = 12) . |
|---|---|---|---|
| Age, mean (SD), y | 14.70 (2.32) | 15.18 (2.04) | 14.25 (2.56) |
| Sex (female), n (%) | 12 (52) | 7 (63) | 5 (42) |
| BMI, mean (SD), kg/m2 | 28.28 (9.69) | 29.83 (12.34) | 26.86 (5.58) |
| OW/obese, n (%) | 15 (65.22) | 6 (54.54) | 9 (75) |
| Clot burden at diagnosis occlusive, n, (%) | 14 (60.87) | 7 (63.64) | 7 (58.33) |
| VTE location, n (%) | |||
| DVT | 17 (73.91) | 8 (72.73) | 9 (75) |
| PE | 3 (13.04) | 2 (18.18) | 1 (8.335) |
| Concurrent DVT and PE | 3 (13.04) | 1 (9.09) | 2 (16.67) |
| D-dimer, ng/mL | |||
| At diagnosis, mean (SD) | 5792.24 (7222.35) | 5416.67 (6997.21) | 8077.6 (8783.46) |
| At exit visit, mean (SD) | 352.86 (96.32) | 406.67 (118.65) | 300 (103.57) |
| Pre-VTE PA score, mean (95% CI) | 60 (40, 79.7) | 66 (33.7, 98.9) | 57 (34, 80.2) |
| QoL score at diagnosis, mean (95% CI) | 67 (57.5, 76.5) | 71 (56, 87) | 64 (52, 76.5) |
| PTS at exit visit, n (%) | 3 (20) | 0 | 3 |
CI, confidence interval; PA, physical activity.
Adherence to the allocated intervention
The mean number of participants that adhered to the prescribed activity during the 8-week active phase was only 3, and the predefined adherence threshold of ≥60% participants meeting target activity was not met during any week of the active phase (Table 5). There were no differences in the self-reported physical activity scores in the physical activity/Fitbit group or the standard care group at any time during the study trial (Table 5). In the postintervention phase, this declined further; only 20% of patients maintaining target activity, and the self-reported habitual physical activity level was low in one-third of participants in both groups by the exit visit.
Indicators of adherence to physical activity in the Fitbit and standard care group
| Intervention period . | Physical activity/Fitbit group . | Standard care group . | |||
|---|---|---|---|---|---|
| Adherence to target activity, n (%) . | Strenuous exercise, n (%) . | Average daily steps . | Range of daily steps . | ||
| Preintervention | Not applicable | ||||
| Week 1 (n = 9) | — | 4 (44.4) | 7936 | 1990-13 418 | |
| Week 2 (n = 9) | — | 3 (33.3) | 5832 | 1920-9172 | |
| Week 3 (n = 8) | — | 2 (25) | 6274 | 2602-10 279 | |
| Week 4 (n = 8) | — | 3 (37.5) | 5924 | 2745-7897 | |
| Active phase | |||||
| Week 1 (n = 8) | 4 (50) | 3 (37.5) | 6402 | 1761-9929 | |
| Week 2 (n = 7) | 2 (28) | 6 (85.7) | 6662 | 2494-8544 | |
| Week 3 (n = 9) | 4 (43) | 4 (44.4) | 6524 | 1595-11 557 | |
| Week 4 (n = 7) | 3 (42) | 2 (28.6) | 6170 | 1284-8909 | |
| Week 5 (n = 6) | 1 (16) | 1 (16.7) | 4376 | 1882-6930 | |
| Week 6 (n = 8) | 4 (50) | 2 (25) | 6238 | 2157-9967 | |
| Week 7 (n = 8) | 4 (50) | 3 (37.5) | 6452 | 1719-12 218 | |
| Week 8 (n = 6) | 2 (33) | 1 (16.7) | 5388 | 1670-8290 | |
| Postintervention | |||||
| Week 1 (n = 3) | 0 (0) | 1 (33) | 6589 | 1944-10 164 | |
| Week 2 (n = 5) | 1 (20) | 2 (40) | 4023 | 1369-6516 | |
| Week 3 (n = 5) | 1 (20) | 0 (0) | 4246 | 1877-8596 | |
| Week 4 (n = 4) | 0 (0) | 0 (0) | 3276 | 2186-4222 | |
| Intervention period . | Physical activity/Fitbit group . | Standard care group . | |||
|---|---|---|---|---|---|
| Adherence to target activity, n (%) . | Strenuous exercise, n (%) . | Average daily steps . | Range of daily steps . | ||
| Preintervention | Not applicable | ||||
| Week 1 (n = 9) | — | 4 (44.4) | 7936 | 1990-13 418 | |
| Week 2 (n = 9) | — | 3 (33.3) | 5832 | 1920-9172 | |
| Week 3 (n = 8) | — | 2 (25) | 6274 | 2602-10 279 | |
| Week 4 (n = 8) | — | 3 (37.5) | 5924 | 2745-7897 | |
| Active phase | |||||
| Week 1 (n = 8) | 4 (50) | 3 (37.5) | 6402 | 1761-9929 | |
| Week 2 (n = 7) | 2 (28) | 6 (85.7) | 6662 | 2494-8544 | |
| Week 3 (n = 9) | 4 (43) | 4 (44.4) | 6524 | 1595-11 557 | |
| Week 4 (n = 7) | 3 (42) | 2 (28.6) | 6170 | 1284-8909 | |
| Week 5 (n = 6) | 1 (16) | 1 (16.7) | 4376 | 1882-6930 | |
| Week 6 (n = 8) | 4 (50) | 2 (25) | 6238 | 2157-9967 | |
| Week 7 (n = 8) | 4 (50) | 3 (37.5) | 6452 | 1719-12 218 | |
| Week 8 (n = 6) | 2 (33) | 1 (16.7) | 5388 | 1670-8290 | |
| Postintervention | |||||
| Week 1 (n = 3) | 0 (0) | 1 (33) | 6589 | 1944-10 164 | |
| Week 2 (n = 5) | 1 (20) | 2 (40) | 4023 | 1369-6516 | |
| Week 3 (n = 5) | 1 (20) | 0 (0) | 4246 | 1877-8596 | |
| Week 4 (n = 4) | 0 (0) | 0 (0) | 3276 | 2186-4222 | |
| . | 3 mo (n = 11) . | 6 mo (n = 10) . | 9 mo (n = 9) . | 3 mo (n = 11) . | 6 mo (n = 8) . | 9 mo (n = 6) . |
|---|---|---|---|---|---|---|
| Physical activity score, mean (SD)* | 56 (29) | 52 (32) | 38 (36) | 60 (41) | 65 (38) | 67 (47) |
| Active, n | 8 | 8 | 6 | 8 | 7 | 4 |
| Insufficiently active, n | 2 | 2 | 3 | 3 | 1 | 2 |
| . | 3 mo (n = 11) . | 6 mo (n = 10) . | 9 mo (n = 9) . | 3 mo (n = 11) . | 6 mo (n = 8) . | 9 mo (n = 6) . |
|---|---|---|---|---|---|---|
| Physical activity score, mean (SD)* | 56 (29) | 52 (32) | 38 (36) | 60 (41) | 65 (38) | 67 (47) |
| Active, n | 8 | 8 | 6 | 8 | 7 | 4 |
| Insufficiently active, n | 2 | 2 | 3 | 3 | 1 | 2 |
Measured by self-reported Godin leisure-time physical activity questionnaire. Eleven participants in the standard care group completed physical activity questionnaires.
Secondary outcomes
PTS scores were lower in the Fitbit group compared with the standard group (Table 6). Three patients in the standard care group developed PTS as compared with 0 patients in the Fitbit group. The mean difference in total PTS score from baseline to 6 months was 0 points (SD, 0.5) among the physical activity/Fitbit group versus 1.3 points (SD 0.8) among the standard care group, with a between-group difference of −1.3 points (SD 0.6; P = .0017). There was a significant between-group difference of −19 points (SD 14.3; P = .023) in total QoL score with the larger within-group difference (24.5 points; SD 14.2) found in the standard care group compared with Fitbit (5.4 points; SD 14.3). No significant intergroup differences were found in the coagulation biomarkers (Table 6). Some degree of ECS use was reported by 44% at 6 months in the physical activity group and 85% in the standard care group.
Secondary outcomes
| Variable . | Fitbit group (n = 11), mean (SD) . | Standard care group (n = 12), mean (SD) . | Fitbit vs standard care . | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline . | 6 mo . | Within-patient change . | Baseline . | 6 mo . | Within-patient change . | Difference, within-patient change, mean (SD) . | P* . | |
| Coagulation biomarkers | ||||||||
| CRP, mg/dL | 3.74 (1.9) | 3.34 (0.9) | −0.4 (1.6) | 3.43 (1.3) | 3.7 (1.2) | 0.2 (1.5) | −0.6 (1.6) | .462 |
| D-dimer, µg/mL | 4992 (5598) | 317 (52) | −4675 (5633) | 6531 (6455) | 290 (65) | −6241 (6780) | 3087 (6118) | .322 |
| ETP, nM/min | 1704 (335) | 1841 (346) | 41.0 (223) | 2062 (403) | 1962 (440) | −60.1 (148) | 101.1 (183.9) | .439 |
| FVIII, % | 176 (56) | 149 (55) | −39.6 (92) | 238 (77) | 181 (67) | −50.4 (82.5) | 10.8 (88.6) | .808 |
| Lysis 30, % | 3.5 (4.0) | 2.7 (2.3) | −0.05 (3.3) | 4.9 (4.0) | 5.7 (2.8) | 2.1 (3.1) | −2.2 (3.2) | .221 |
| Lysis 60, % | 15.5 (13) | 14 (8.0) | −0.4 (11.6) | 18 (12.4) | 20 (8.7) | 6.2 (6.5) | −6.6 (9.4) | .209 |
| Thrombin peak, nmol/L | 290 (85) | 375 (85) | 60.5 (164) | 381 (80) | 338 (88) | 0.2 (28.4) | 60.3 (118) | .52 |
| Physical activity | ||||||||
| PTS score | 1.4 (0.5) | 1.4 (0.5) | 0.0 (0.50) | 1.2 (0.7) | 2.2 (0.8) | 1.3 (0.8) | −1.3 (0.6) | .0017 |
| Physical activity | 53 (37.6) | 49 (33) | 3.0 (20.6) | 60.7 (40.5) | 71.6 (40) | 8.1 (34.4) | −5.0 (29.5) | .716 |
| QoL, physical | 65 (27.7) | 87 (22) | 11.2 (20.6) | 49.5 (34) | 94 (10) | 38.6 (29.0) | −27.4 (25.5) | .058 |
| QoL, total | 71 (26) | 83 (24) | 5.4 (14.3) | 64 (22) | 87 (12) | 24.5 (14.2) | −19.0 (14.3) | .023 |
| Variable . | Fitbit group (n = 11), mean (SD) . | Standard care group (n = 12), mean (SD) . | Fitbit vs standard care . | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline . | 6 mo . | Within-patient change . | Baseline . | 6 mo . | Within-patient change . | Difference, within-patient change, mean (SD) . | P* . | |
| Coagulation biomarkers | ||||||||
| CRP, mg/dL | 3.74 (1.9) | 3.34 (0.9) | −0.4 (1.6) | 3.43 (1.3) | 3.7 (1.2) | 0.2 (1.5) | −0.6 (1.6) | .462 |
| D-dimer, µg/mL | 4992 (5598) | 317 (52) | −4675 (5633) | 6531 (6455) | 290 (65) | −6241 (6780) | 3087 (6118) | .322 |
| ETP, nM/min | 1704 (335) | 1841 (346) | 41.0 (223) | 2062 (403) | 1962 (440) | −60.1 (148) | 101.1 (183.9) | .439 |
| FVIII, % | 176 (56) | 149 (55) | −39.6 (92) | 238 (77) | 181 (67) | −50.4 (82.5) | 10.8 (88.6) | .808 |
| Lysis 30, % | 3.5 (4.0) | 2.7 (2.3) | −0.05 (3.3) | 4.9 (4.0) | 5.7 (2.8) | 2.1 (3.1) | −2.2 (3.2) | .221 |
| Lysis 60, % | 15.5 (13) | 14 (8.0) | −0.4 (11.6) | 18 (12.4) | 20 (8.7) | 6.2 (6.5) | −6.6 (9.4) | .209 |
| Thrombin peak, nmol/L | 290 (85) | 375 (85) | 60.5 (164) | 381 (80) | 338 (88) | 0.2 (28.4) | 60.3 (118) | .52 |
| Physical activity | ||||||||
| PTS score | 1.4 (0.5) | 1.4 (0.5) | 0.0 (0.50) | 1.2 (0.7) | 2.2 (0.8) | 1.3 (0.8) | −1.3 (0.6) | .0017 |
| Physical activity | 53 (37.6) | 49 (33) | 3.0 (20.6) | 60.7 (40.5) | 71.6 (40) | 8.1 (34.4) | −5.0 (29.5) | .716 |
| QoL, physical | 65 (27.7) | 87 (22) | 11.2 (20.6) | 49.5 (34) | 94 (10) | 38.6 (29.0) | −27.4 (25.5) | .058 |
| QoL, total | 71 (26) | 83 (24) | 5.4 (14.3) | 64 (22) | 87 (12) | 24.5 (14.2) | −19.0 (14.3) | .023 |
Lysis 30% and Lysis 60% are measurements of percent amplitude reduction 30 and 60 minutes after maximum amplitude, respectively, on thromboelastography.
ETP, endogenous thrombin potential.
P value by Student t test.
Discussion
Results of our single-center pilot trial suggest that it is feasible to enroll and randomize pediatric VTE patients to a prescribed physical activity regimen 3 months following VTE diagnosis, but metrics for adherence to enhanced physical activity and retention were not met. These results provide the rationale to explore the reasons for lack of adherence, such as exercise intolerance and motivation to exercise, and low retention before moving forward with a larger definitive trial of exercise training in children following VTE.
Thirty-three percent of participants met the criteria for adherence to prescribed physical activity. Lack of adherence was related not to wearing compliance but to an inability to adhere to physical activity recommendations due to leg symptoms, motivation, or time constraints. The activity prescription of a simple walking/jogging program at 25% above baseline was chosen for several reasons. The most dramatic decreases in physical activity occur among adolescents, and many studies underscore the importance of walking/jogging among the many available options for leisure-time physical activity among inactive groups, such as adolescents.21 Moreover, light- to moderate-intensity activities such as walking provide some of the same health benefits as do more vigorous types of physical activity, along with a lower risk of injury and sudden death, safety factors that were unknown at the time the trial was designed. Similar to our study, Fitbit and Facebook interventions to motivate physical activity in childhood cancer survivors to decrease risk of chronic diseases such as type 2 diabetes and obesity were unable to promote moderate to vigorous physical activity or decrease sedentary time despite a 72% wearing compliance.30 In an exercise training trial of adults with PTS of varying severities, exercise training occurred over several training sessions (either in person or over the phone) with a reported adherence rate of ∼62%.10 Patients in this trial were eligible if they had established PTS and might have been more invested to exercise; therefore, it is difficult to compare those results with ours. Weekly phone calls may have been a limited intervention compared with face-to-face sessions for assessing the patient’s pain or discomfort with the prescribed physical activity. Furthermore, objective exercise impairment is prevalent in 70% of patients for ≤6 months and 40% at ≤1 post-PE.6,7 While these limitations are thought to be from general deconditioning rather than cardiopulmonary causes, our exercise prescriptions in the PE population likely needed to be more individualized to improve aerobic fitness and adherence. A more gradual activity increment may increase compliance in future pilot RCTs.
Relying on the self-reported Godin questionnaire alone, the majority of the participants in both groups fell into the “sufficiently active” group. Indeed, there is sometimes a discrepancy between subjective and objective measures of active, as self-reported questionnaires will show improvements in activity, while objective measures of steps per day show no significant differences in activity levels with the Fitbit.30,31 Despite attempts to calibrate the responses by providing example activities and physiologic cues in the Godin questionnaire, respondents may have overestimated their physical activity or the activity duration. Alternatively, the accelerometer-based activity recorded can be underestimated, and this difference is attributable to certain types of activity, such as bicycling, swimming, and upper body movements, which is not captured by the Fitbit. In future studies, it would be worthwhile investigating supervised home-based exercise training or innovative approaches such as telerehabilitation to promote and sustain physical activity levels.
In exploring potential effectiveness of enhanced physical activity to decrease PTS incidence and severity, we found a significant difference in PTS and total QoL but did not find associations among Godin physical activity scores, physical QoL, or coagulation biomarkers between the 2 groups. However, our small sample size and inadequate adherence to prescribed physical activity makes it impossible to make definitive conclusions. Other ongoing studies by the authors and the thrombosis community will shed light on the natural trajectory of coagulation biomarkers and QoL following VTE in children.
The strengths of our trial include several strategies to protect against bias. Randomization with allocation concealment was used to assign treatment group. We enrolled consecutive patients with VTE. The use of attention controls reduced the likelihood that any observed changes in the Fitbit group were from a Hawthorne effect. We reduced the risk of treatment contamination in the control group by asking participants not to change the usual levels of physical activity. Outcomes assessors (for PTS and coagulation biomarkers) were blinded to the treatment allocation. Some limitations of our study merit discussion. We estimated our sample size based on the flat rules of thumb and did not stratify the treatment groups based on BMI or baseline physical activity, which may be have been effect modifiers of adherence to exercise. On the contrary, our trial was of greater interest to OW and obese children, as they comprised 83% of consented patients, and thus our results are most applicable to OW and obese children ≥7 years of age with VTE. We realize that having step counts on controls would have yielded a better comparison, but we wanted to simulate a true standard care group. Our trial, despite its inability to meet some of its primary objectives, provides a new and sobering picture of objectively obtained physical activity levels in pediatric VTE survivors. The secondary findings of our small trial should be interpreted with caution. Future studies should focus on understanding the trajectory of physical activity after VTE, mechanisms of exercise intolerance, and the direction and strength of the relationship of physical activity levels with VTE outcomes.
Requests for data sharing should be e-mailed to the corresponding author, Ayesha Zia (e-mail: ayesha.zia@utsouthwestern.edu).
Acknowledgments
A.Z. is supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (1K23HL132054-01).
The funding source was not involved in the study design, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Authorship
Contribution: A.Z. conceptualized and designed research; R.S. and J.J. provided critical input in the research design; K.M. and A.Z. enrolled participants; S.Z., A.Z., and K.M. analyzed data; A.Z. and R.H. wrote the manuscript; and all other authors critically edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayesha Zia, Division of Hematology-Oncology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390; e-mail: ayesha.zia@utsouthwestern.edu.
References
Author notes
The full-text version of this article contains a data supplement.