Key Points
Red blood cells from patients with sickle cell disease adhere to ICAM-1, which is associated with hemolysis and history of right-to-left shunts.
Red blood cell adhesion to ICAM-1 in SCD is mediated by membrane-bound fibrinogen, which acts as a bridging molecule.
Abstract
Sickle cell disease (SCD), which afflicts 100 000 Americans, as well as millions worldwide, is associated with anemia, lifelong morbidity, and early mortality. Abnormal adhesion of sickle red blood cells (RBCs) to activated vascular endothelium may contribute acutely to the initiation of painful vaso-occlusive crises and chronically to endothelial damage in SCD. Sickle RBCs adhere to activated endothelium through several adhesion mechanisms. In this study, using whole blood from 17 people with heterozygous SCD (HbS variant) and 55 people with homozygous SCD (HbSS) analyzed in an in vitro microfluidic assay, we present evidence for the adhesion of sickle RBCs to immobilized recombinant intercellular adhesion molecule 1 (ICAM-1). We show that sickle RBC adhesion to ICAM-1 in vitro is associated with evidence of hemolysis in vivo, marked by elevated lactate dehydrogenase levels, reticulocytosis, and lower fetal hemoglobin levels. Further, RBC adhesion to ICAM-1 correlates with a history of intracardiac or intrapulmonary right-to-left shunts. Studies of potential ICAM-1 ligands on RBC membranes revealed that RBC–ICAM-1 interactions were mediated by fibrinogen bound to the RBC membrane. We describe, for the first time, RBC rolling behavior on ICAM-1 under high shear rates. Our results suggest that firm adhesion of sickle RBCs to ICAM-1 most likely occurs in postcapillary venules at low physiological shear rates, which is facilitated by initial rolling in high shear regions (eg, capillaries). Inhibition of RBC and ICAM-1 interactions may constitute a novel therapeutic target in SCD.
Introduction
Sickle cell disease (SCD) leads to the production of abnormally adhesive and stiff red blood cells (RBCs) due to a mutation in the β-globin chain of hemoglobin.1 The clinical manifestations of SCD are mediated, in part, by abnormal cellular adhesion in the microvasculature. Impedance of microcirculatory flow is further disrupted in a locally hypoxic environment, in which sickle hemoglobin polymerizes into long and stiff chains under low oxygen tension, ultimately resulting in the formation of nondeformable RBCs.2-5 In SCD, abnormal cellular adhesion and the presence of highly rigid RBCs contribute to episodic and painful vaso-occlusive crises (VOCs), cumulative vasculopathy, and significant morbidity and early mortality.6
To date, a large group of endothelial- and subendothelial-associated adhesion molecules are recognized as mediators of sickle RBC adhesion to the vascular wall, including laminin,7 fibronectin,8 thrombospondin,9 VCAM-1,10 and P-selectin.11 However, it is still not clear which of, or to what degree, these molecules play a role in the progression of vaso-occlusion or endothelial activation. In an early study, there was a significant correlation between hypoxia-induced RBC adhesion and VCAM-1 expression levels on the endothelial cell surface but not under normoxic conditions.10 Notably, only the adhesion of less-dense RBCs (reticulocytes) was mediated via VCAM-1, suggesting an adhesion pathway involving α4β1, which has also been shown to mediate sickle reticulocyte adhesion to fibronectin.8 White et al recently demonstrated that white blood cell (WBC) and RBC adhesion to VCAM-1 correlated positively with the frequency of vaso-occlusive events in SCD.12 Sickle RBC adhesion to laminin has recently been reported to have clinical implications in normoxic and hypoxic conditions,3,13,14 consistent with the earlier studies that pointed to a link between RBC adhesion and clinical severity of the disease.15,16 In concert with these findings, it was recently shown that a P-selectin inhibitor significantly diminished the frequency of VOCs in SCD patients owing to the multifaceted role of P-selectin in SCD pathophysiology.17
Despite the recent efforts to better understand RBC adhesion mechanisms in SCD, there are adhesive ligands whose role in enhancing adhesion of sickle RBCs to the vascular wall, such as immobilized recombinant intercellular adhesion molecule 1 (ICAM-1; notwithstanding its extensively characterized contribution to WBC-endothelium interactions) have yet to be characterized. Firm adhesion of neutrophils to the endothelium requires the expression of ICAM-1, because neutrophils tend to have a rolling behavior on selectins rather than establishing a firm attachment.18 Neutrophil adhesion to ICAM-1 is mediated through β2 integrins (leukocyte function-associated antigen-1 [LFA-1] and macrophage antigen 1 [MAC-1]) and may be modulated by soluble fibrinogen.19,20 However, there has not been a systematic exploration of the adhesive interactions between sickle RBCs and ICAM-1, despite the abundant expression of ICAM-1 on the chronically activated and inflamed vasculature of SCD patients.21
In this study, we examined the adhesion of sickle RBCs from SCD subjects to immobilized ICAM-1 in microphysiological flow conditions utilizing an in vitro microfluidic adhesion assay. For the first time, we demonstrate that ICAM-1 supports the adhesion of sickle RBCs, in a subject-specific manner; adhesion of RBCs from homozygous subjects (HbSS) strongly correlated with high-grade hemolysis and a history of intracardiac or intrapulmonary right-to-left shunts. In particular, RBCs from subjects with HbSS and evidence for hemolysis and inflammation (elevated lactate dehydrogenase [LDH] levels, absolute reticulocyte counts [ARCs], and WBC count) have an increased propensity for adhesion. Notably, sickle RBC adhesion to ICAM-1 was mediated via fibrinogen, which forms intercellular bridges. Lastly, we observed that a fraction of adherent RBCs exhibited rolling motion at high shear rates and did not disassociate from the immobilized protein, suggesting a novel mechanism by which RBCs may contribute to the initiation of VOC events in SCD.
Methods
Flow adhesion experiments
Fabrication and functionalization of the microfluidic channels were carried out as described in supplemental Materials. Functionalized microchannels were connected to blood-containing syringes through 40-cm inlet tubing. A total of 15 µL of whole blood sample was perfused into the microchannels at an approximate shear stress of 1 dyne/cm2, corresponding to the typical shear stress observed in postcapillary venules, via a constant displacement syringe pump (New Era NE-300; New Era Pump Systems, Farmingdale, NY). To remove the nonadherent cells, another syringe filled with wash buffer (1% bovine serum albumin and 0.09% sodium azide in 1× phosphate buffered saline [PBS]) was connected to the microchannels, and the buffer solution was injected at 10 µL/min, corresponding to a wall shear stress of 1 dyne/cm2, until all nonadherent cells had been cleared. Phase-contrast images of the microchannel surface were obtained using an inverted microscope at 20×. Quantification of ICAM-1–adherent RBCs was performed manually using Adobe Photoshop CS5 (Adobe, San Jose, CA) within a rectangular field of view (fov) with a surface area of 32 mm2.
Inhibition experiments
For the inhibition studies, whole HbSS blood samples were mixed with a monoclonal mouse antibody against human α4β1 or LFA-1 at a final antibody concentration of 50 μg/mL and incubated at 37°C for 1 hour prior to the adhesion experiments. As a control, samples were treated with the same concentration of isotype-control mouse immunoglobulin G1 for the same duration. Anti-human LFA-1 antibody and the isotype-control mouse immunoglobulin G were from BioLegend (San Diego, CA). Anti-human α4β1 antibody was purchased from Abcam (Cambridge, MA). To block the β2-associated adhesion pathways, a recombinant human β2 protein solution (Novus Biologicals, Centennial, CO) was perfused into ICAM-1–functionalized microchannels and incubated for 1 hour at 37°C. Before starting the adhesion experiments, the microchannels were rinsed 3 times with PBS. Similarly, to test the inhibitory effect of fibrinogen (Enzo Life Sciences, Farmingdale, NY) or low molecular weight heparin (LMWH; AMSBIO, Cambridge, MA), ICAM-1–functionalized microchannels were injected with fibrinogen (range, 1-20 mg/mL) or LMWH (range, 0.1-5 µg/mL). After an hour at 37°C, the microchannels were rinsed with PBS 3 times, and adhesion was assessed as above.
Statistical methods
Minitab 19 (Minitab, State College, PA) was used to analyze the results. A normality test was performed to determine whether the variables were normally distributed. Because of the largely nonnormally distributed variables, Mann-Whitney U tests were used to compare 2 groups, and Kruskal-Wallis tests with Dunn’s correction were used to compare multiple groups, unless stated otherwise. K-means clustering analysis was performed to identify distinct subject subpopulations based on hemolysis biomarkers. Throughout this article, the error bars represent ± standard error of the mean (SEM). P < .05 was considered statistically significant.
Results
Abnormal sickle RBC adhesion to ICAM-1 is heterogeneous in a clinically diverse population with SCD
We analyzed the ability of RBCs to adhere to ICAM-1 using whole blood samples from individuals with (HbSS, HbS variant) or without (HbAA) SCD in a physiologically relevant in vitro microfluidic adhesion platform. Our results showed that HbSS RBCs had significantly greater adhesion to immobilized ICAM-1 than did HbS variant RBCs or HbAA RBCs (Figure 1D; mean adhesion ± standard deviation [SD] = 1486 ± 3312 per fov for HbSS, 54 ± 59 per fov for HbS variant, and 3.5 ± 1.4 per fov for HbAA, P < .005). We also observed subject-specific and heterogeneous adhesion profiles for HbSS RBCs, ranging between 6 and 19 495 for the entire study population with homozygous SCD (n = 55; mean adhesion 1486 ± 3312 per fov).
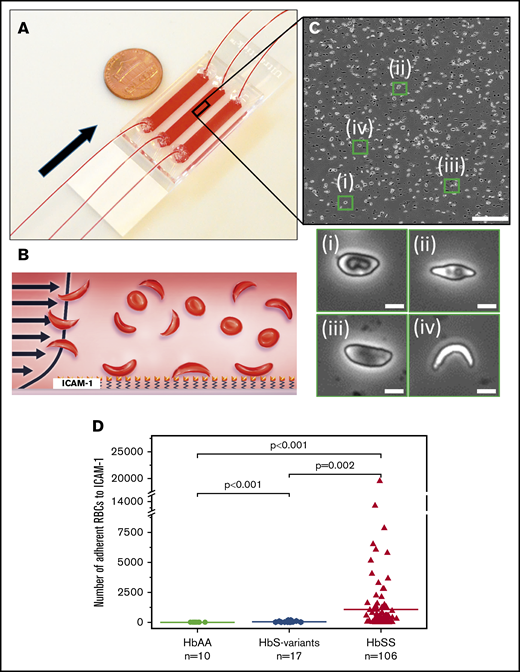
The microfluidic assay used to assess adhesion of sickle RBCs to immobilized ICAM-1 under physiological flow conditions. (A) Assembled microdevice containing 3 separate microchannels, each functionalized with ICAM-1. Arrow indicates the flow direction. (B) Recombinant human ICAM-1 protein was immobilized on the microchannel surface via a cross-linker agent, GMBS, to ensure consistent protein coverage throughout the microchannel, as well as to prevent protein dissociation from the surface at high shear rates. (C) Representative image obtained following an adhesion experiment (upper panel). Enlarged images of the green boxes are shown (lower panel). Adherent sickle RBC populations possessed distinct morphologies: (i) RBC with a characteristic biconcave morphology and elongated elliptic shape, (ii) RBC with a nearly absent biconcave morphology and further impaired elliptic shape, (iii) RBC with no biconcave morphology and elongated elliptic shape, (iv) highly sickled RBC with no biconcave morphology. (D) The number of adherent RBCs was significantly greater in samples from subjects with HbSS > HbS variant > HbAA. The horizontal lines represent the means; ‘‘n’’ represents the number of blood samples tested. A total of 106 blood samples were tested from 55 subjects with HbSS SCD. The scale bars are 5 μm.
The microfluidic assay used to assess adhesion of sickle RBCs to immobilized ICAM-1 under physiological flow conditions. (A) Assembled microdevice containing 3 separate microchannels, each functionalized with ICAM-1. Arrow indicates the flow direction. (B) Recombinant human ICAM-1 protein was immobilized on the microchannel surface via a cross-linker agent, GMBS, to ensure consistent protein coverage throughout the microchannel, as well as to prevent protein dissociation from the surface at high shear rates. (C) Representative image obtained following an adhesion experiment (upper panel). Enlarged images of the green boxes are shown (lower panel). Adherent sickle RBC populations possessed distinct morphologies: (i) RBC with a characteristic biconcave morphology and elongated elliptic shape, (ii) RBC with a nearly absent biconcave morphology and further impaired elliptic shape, (iii) RBC with no biconcave morphology and elongated elliptic shape, (iv) highly sickled RBC with no biconcave morphology. (D) The number of adherent RBCs was significantly greater in samples from subjects with HbSS > HbS variant > HbAA. The horizontal lines represent the means; ‘‘n’’ represents the number of blood samples tested. A total of 106 blood samples were tested from 55 subjects with HbSS SCD. The scale bars are 5 μm.
Sickle RBC adhesion to ICAM-1 is associated with clinical biomarkers of hemolysis
We found an association between HbSS RBC adhesion to ICAM-1 and clinical variables, in samples obtained at the clinical steady-state. We performed a k-means clustering analysis based on subject LDH levels and ARCs, which resulted in 2 distinct HbSS subgroups (Figure 2A). We found that RBCs from samples in group 1 (n = 34; lower LDH and lower ARC laboratory profiles) had significantly less adhesion to ICAM-1 compared with RBCs from samples in group 2 (n = 21; higher LDH levels and higher ARC laboratory profiles) consistent with hemolysis (Figure 2B; 453 ± 199 per fov vs 3159 ± 1038 per fov; mean adhesion ± SEM, respectively; P = .002). These findings suggest an association between RBC adhesion to the vascular bed, hemolysis, and shortened RBC half-life. In addition, we found an association between HbSS RBC adhesion to ICAM-1 and WBC count (Figure 2C-D). Subjects with an elevated WBC count (>11 × 109 per liter) exhibited significantly higher RBC adhesion compared with those with a normal WBC count (Figure 2D; 2532 ± 1017 per fov vs 789 ± 265 per fov; mean adhesion ± SEM, respectively; P = .02). The clinical variables of subjects in group 1 and group 2 are summarized in Table 1.
Adhesion of HbSS RBCs to immobilized ICAM-1 in vitro is associated with subject hematological parameters. (A) Subjects were categorized into 2 groups based on their LDH levels and ARCs; group 1 (●) had higher LDH and ARCs. The categorization was performed based on the k-means clustering method. The shaded green and blue regions indicate reference ranges (normal) for LDH and ARC levels, respectively. (B) Subjects in group 2 had significantly higher RBC adhesion levels compared with the patients in group 1 (mean, 3159 ± 4758 vs 453 ± 1159, respectively). (C) There is a positive correlation between adherent RBC numbers and WBC counts. The shaded blue region represents the normal WBC range in healthy adults. (D) Subjects with clinically high WBC counts (>11 × 109/L) have significantly greater RBC adhesion to ICAM-1 in vitro. The horizontal lines in panels B and D represent the mean; ‘‘n’’ represents the number of subjects tested. The P values were calculated using the Mann-Whitney nonparametric U test. PCC, Pearson’s correlation coefficient.
Adhesion of HbSS RBCs to immobilized ICAM-1 in vitro is associated with subject hematological parameters. (A) Subjects were categorized into 2 groups based on their LDH levels and ARCs; group 1 (●) had higher LDH and ARCs. The categorization was performed based on the k-means clustering method. The shaded green and blue regions indicate reference ranges (normal) for LDH and ARC levels, respectively. (B) Subjects in group 2 had significantly higher RBC adhesion levels compared with the patients in group 1 (mean, 3159 ± 4758 vs 453 ± 1159, respectively). (C) There is a positive correlation between adherent RBC numbers and WBC counts. The shaded blue region represents the normal WBC range in healthy adults. (D) Subjects with clinically high WBC counts (>11 × 109/L) have significantly greater RBC adhesion to ICAM-1 in vitro. The horizontal lines in panels B and D represent the mean; ‘‘n’’ represents the number of subjects tested. The P values were calculated using the Mann-Whitney nonparametric U test. PCC, Pearson’s correlation coefficient.
Clinical variables of the tested subjects with HbSS SCD
| . | Group 1 (n = 34) . | Group 2 (n = 21) . | P . |
|---|---|---|---|
| Age, y | 37.8 ± 13.6 | 35.9 ± 13.0 | .628** |
| Hemoglobin, g/dL | 8.6 ± 1.4 | 8.6 ± 1.3 | .965** |
| Mean corpuscular volume, fL | 96.7 ± 14.2 | 92.6 ± 5.4 | .222* |
| Platelets, ×109/L | 313.7 ± 140.9 | 364.3 ± 89.4 | .147* |
| WBC count, ×109/L | 9.6 ± 3.2 | 12.7 ± 3.8 | .002* |
| Absolute neutrophil count, ×106/L | 5514 ± 2303 | 7359 ± 2841 | .014* |
| ARC, ×109/L | 214.7 ± 89.1 | 482.2 ± 174.9 | <.001* |
| LDH, U/L | 271.9 ± 64.0 | 510.0 ± 200.2 | <.001* |
| Ferritin, μg/L | 2680.2 ± 2626.3 | 1920.4 ± 2179.3 | .472* |
| Hemoglobin S, % | 58.5 ± 23.7 | 56.5 ± 20.2 | .523* |
| Hemoglobin A, % | 25.8 ± 24.6 | 30.0 ± 21.8 | .601* |
| Hemoglobin F, % | 8.2 ± 7.6 | 4.1 ± 4.3 | .143* |
| . | Group 1 (n = 34) . | Group 2 (n = 21) . | P . |
|---|---|---|---|
| Age, y | 37.8 ± 13.6 | 35.9 ± 13.0 | .628** |
| Hemoglobin, g/dL | 8.6 ± 1.4 | 8.6 ± 1.3 | .965** |
| Mean corpuscular volume, fL | 96.7 ± 14.2 | 92.6 ± 5.4 | .222* |
| Platelets, ×109/L | 313.7 ± 140.9 | 364.3 ± 89.4 | .147* |
| WBC count, ×109/L | 9.6 ± 3.2 | 12.7 ± 3.8 | .002* |
| Absolute neutrophil count, ×106/L | 5514 ± 2303 | 7359 ± 2841 | .014* |
| ARC, ×109/L | 214.7 ± 89.1 | 482.2 ± 174.9 | <.001* |
| LDH, U/L | 271.9 ± 64.0 | 510.0 ± 200.2 | <.001* |
| Ferritin, μg/L | 2680.2 ± 2626.3 | 1920.4 ± 2179.3 | .472* |
| Hemoglobin S, % | 58.5 ± 23.7 | 56.5 ± 20.2 | .523* |
| Hemoglobin A, % | 25.8 ± 24.6 | 30.0 ± 21.8 | .601* |
| Hemoglobin F, % | 8.2 ± 7.6 | 4.1 ± 4.3 | .143* |
A total of 106 blood samples were obtained from 55 patients with homozygous HbSS (29 males and 26 females); the mean value was used for a subject tested more than once. Data are mean ± standard deviation. P values that represent a statistically significant difference between group 1 and 2 are denoted with boldface.
Nonparametric Mann-Whitney U test.
Parametric 1-way analysis of variance (ANOVA).
Next, we examined associations between fetal hemoglobin (HbF) and RBC adhesion to ICAM-1 (Figure 3A-B). We assessed RBC adhesion with respect to subjects’ HbF levels, with a cutoff value of 8.6%, which was associated with improved life expectancy and a decrease in VOCs in natural history studies.22 We found that HbSS RBCs from subjects with higher HbF levels (n = 18) had significantly lower adhesion to ICAM-1 compared with those with lower HbF levels (n = 37) (Figure 3C; 283 ± 107 per fov vs 2127 ± 649 per fov; mean adhesion ± SEM, respectively; P = .007). Moreover, 14 of 37 subjects with lower HbF levels (<8.6%) had RBC adhesion that was higher than the mean adhesion level, whereas only 1 of 19 subjects had HbF levels > 8.6%. Hydroxyurea (HU) treatment had a significant impact on HbF levels in our cohort (P < .001, χ2 test). Fourteen of 18 (77.8%) subjects with an HbF level > 8.6% were on HU compared with only 5 of 37 subjects (13.5%) with an HbF level < 8.6%. Of note, RBC adhesion did not correlate with subjects’ hemoglobin A levels (ie, prior transfusions; data not shown).
Adhesion of HbSS RBCs to immobilized ICAM-1 in vitro correlates clinically with HbF levels. (A) There is an inverse relationship between RBC adhesion to ICAM-1 and HbF level. The P value was based on 1-way ANOVA. (B) Subjects with higher HbF levels (using a previously defined ameliorative cutoff of 8.6%22 ) had significantly lower numbers of adherent RBCs compared with those with lower HbF levels. The horizontal lines represent the means; ‘‘n’’ represents the number of subjects. The Mann-Whitney nonparametric U test was used to calculate the P value in panel B. PCC, Pearson’s correlation coefficient.
Adhesion of HbSS RBCs to immobilized ICAM-1 in vitro correlates clinically with HbF levels. (A) There is an inverse relationship between RBC adhesion to ICAM-1 and HbF level. The P value was based on 1-way ANOVA. (B) Subjects with higher HbF levels (using a previously defined ameliorative cutoff of 8.6%22 ) had significantly lower numbers of adherent RBCs compared with those with lower HbF levels. The horizontal lines represent the means; ‘‘n’’ represents the number of subjects. The Mann-Whitney nonparametric U test was used to calculate the P value in panel B. PCC, Pearson’s correlation coefficient.
History of intracardiac/intrapulmonary right-to-left shunts is associated with higher RBC adhesion to ICAM-1
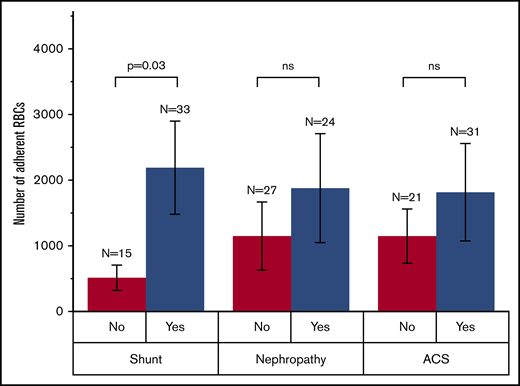
We next analyzed the adhesion data for select clinical comorbidities, including intracardiac or intrapulmonary shunts, concurrent nephropathy, and history of acute chest syndrome (ACS). Our results showed that people with HbSS and a history of right-to-left shunts had significantly higher RBC adhesion to ICAM-1 compared with people with HbSS and no history of right-to-left shunts (Figure 4; 2184 ± 717 per fov vs 515 ± 738 per fov; mean adhesion ± SEM, respectively; P = .03). Although RBC adhesion was typically higher for subjects with a history of nephropathy or ACS compared with those without, we did not detect a statistically significant difference. These results suggest that higher RBC adhesion to ICAM-1 may be a surrogate biomarker of right-to-left intrapulmonary or intracardiac shunts in people with HbSS.
Association of RBC adhesion to ICAM-1 with select clinical phenotype in HbSS SCD. Subjects with a history of intracardiac or intrapulmonary shunt have significantly higher RBC adhesion compared with those with no history of shunt. A history of nephropathy or ACS does not have a significant association with RBC adhesion levels. The P value was calculated using a 2-sample Student t test. ns, not significant.
Association of RBC adhesion to ICAM-1 with select clinical phenotype in HbSS SCD. Subjects with a history of intracardiac or intrapulmonary shunt have significantly higher RBC adhesion compared with those with no history of shunt. A history of nephropathy or ACS does not have a significant association with RBC adhesion levels. The P value was calculated using a 2-sample Student t test. ns, not significant.
Longitudinal monitoring of RBC adhesion to ICAM-1
We obtained multiple data points from 12 individuals with HbSS SCD at steady-state to monitor the variation in RBC adhesion and its association with clinical biomarkers (LDH, ARC, and WBC count) over time. Four subjects had very low adhesion levels, which did not change over time (<50 RBCs). Nineteen data points were obtained from 8 people: 6 subjects had RBC adhesion data from 2 time points, and the other 2 subjects had data from 3 and 4 time points (supplemental Figure 1). Between each time point, or interval, we determined whether subjects’ RBC adhesion and clinical biomarkers changed or remained the same, with a total of 11 evaluable intervals (ie, 1 interval for subjects with 2 time points and 2 and 3 intervals for subjects with 3 and 4 time points, respectively). We found that an increase or decrease in LDH levels and ARC was typically accompanied by an increase or decrease in RBC adhesion, whereas WBC counts did not seem to correlate with RBC adhesion. A change in LDH levels and ARC was reflected by a change in RBC adhesion for 8 of 11 (73%) intervals and 7 of 11 (64%) intervals, respectively. On the other hand, WBC counts reflected a change in RBC adhesion in only 3 of 11 intervals (27%). These results suggest that RBC adhesion may correlate with LDH and ARC over time. Nevertheless, it is still possible to detect changes in RBC adhesion, despite stable LDH levels and ARC, indicating that different factors could be at play with regard to RBC adhesion at different time points.
HbSS RBC adhesion to ICAM-1 is mediated by fibrinogen, which acts as a bridging molecule between RBCs and immobilized ICAM-1
To determine possible mediators of HbSS RBC adhesion to immobilized ICAM-1, we performed adhesion-inhibition experiments. First, we inhibited α4β1 integrin on the surface of RBCs to assess the adhesion of reticulocytes that were previously shown to adhere to fibronectin and VCAM-1 through α4β1. However, inhibition of α4β1 integrin did not result in reduced RBC adhesion (Figure 5A). Next, we tested whether HbSS RBC adhesion to ICAM-1 is mediated by integrin-type receptors on the red cell membrane, considering the extensive characterization of the role of β2-associated integrin in WBC–ICAM-1 interactions. Again, we found that neither the treatment of the blood samples with anti–LFA-1 antibodies nor incubation of the ICAM-1–immobilized microchannels with recombinant human β2 protein prior to sample application had an inhibitory effect on the adhesion of HbSS RBCs to ICAM-1 (Figure 5A). Therefore, we used fibrinogen to block the fibrinogen-binding domain of the immobilized ICAM-1 on the channel surface to prevent sample plasma fibrinogen from enhancing adhesive interactions during blood flow. Notably, treatment of microchannels with fibrinogen significantly decreased RBC adhesion in a concentration-dependent manner (Figure 5B). It has been reported that plasma fibrinogen levels were in the range of 2 to 5.5 mg/mL for healthy subjects and 3 to 11.5 mg/mL for subjects with HbSS SCD.23 Therefore, we used 4 fibrinogen concentrations to simulate physiologic and pathophysiologic conditions: 1, 5, 10, and 20 mg/mL. Incubation of ICAM-1–immobilized microchannels with 1 mg/mL of fibrinogen did not inhibit RBC adhesion, whereas using a fibrinogen concentration of 5 mg/mL blocked adhesion by ∼50% (Figure 5B), suggesting that supranormal fibrinogen levels in HbSS blood samples may decrease RBC adhesion to ICAM-1. Because LMWH can bind to fibrinogen, we next treated whole blood samples with LMWH (1:1 volume to volume ratio) at concentrations of 0.1, 1, and 5 µg/mL, all of which significantly blocked RBC adhesion to ICAM-1 (Figure 5C); on the other hand, preincubation of microfluidic channels with LMWH had no effect (data not shown). These findings suggest a mediating role for plasma fibrinogen in HbSS RBC adhesion to ICAM-1.
The adhesion of HbSS RBCs to immobilized ICAM-1 is mediated by fibrinogen and is inhibited by LMWH. (A) Mean percentages of RBCs adherent to immobilized ICAM-1 following the treatment of blood samples with anti-α4β1 or anti–LFA-1 antibodies or the treatment of microchannels with recombinant human β2 protein in 5 experiments. With vehicle treatment, a mean of 100% of RBCs adhered to immobilized ICAM-1. No significant reduction in HbSS RBC adhesion to immobilized ICAM-1 was observed (P > .05). (B) Treatment of microchannels with fibrinogen decreased HbSS RBC adhesion to ICAM-1 in a concentration-dependent manner. Shown are mean percentages of adherent RBCs after treatment with 1, 5, 10, or 20 mg/mL fibrinogen (n = 5). (C) Treatment of blood samples with LMWH significantly inhibited HbSS RBC adhesion to ICAM-1. Shown are mean percentages of adherent RBCs following pretreatment with 0.1, 1, or 5 μg/mL LMWH in 5 experiments. The P values were calculated using 1-way ANOVA with the Dunnett post hoc test. Error bars represent ± SEM.
The adhesion of HbSS RBCs to immobilized ICAM-1 is mediated by fibrinogen and is inhibited by LMWH. (A) Mean percentages of RBCs adherent to immobilized ICAM-1 following the treatment of blood samples with anti-α4β1 or anti–LFA-1 antibodies or the treatment of microchannels with recombinant human β2 protein in 5 experiments. With vehicle treatment, a mean of 100% of RBCs adhered to immobilized ICAM-1. No significant reduction in HbSS RBC adhesion to immobilized ICAM-1 was observed (P > .05). (B) Treatment of microchannels with fibrinogen decreased HbSS RBC adhesion to ICAM-1 in a concentration-dependent manner. Shown are mean percentages of adherent RBCs after treatment with 1, 5, 10, or 20 mg/mL fibrinogen (n = 5). (C) Treatment of blood samples with LMWH significantly inhibited HbSS RBC adhesion to ICAM-1. Shown are mean percentages of adherent RBCs following pretreatment with 0.1, 1, or 5 μg/mL LMWH in 5 experiments. The P values were calculated using 1-way ANOVA with the Dunnett post hoc test. Error bars represent ± SEM.
HbSS RBCs roll on ICAM-1 at higher shear rates and establish a firm attachment at lower shear rates
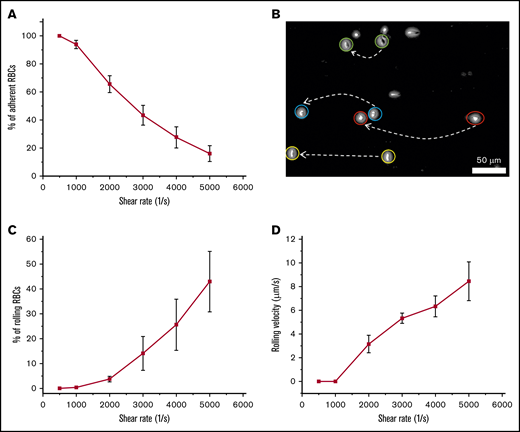
We observed that the total number of adherent RBCs decreased with increasing shear rate (Figure 6A), whereas adherent RBCs persisted even after exposure to extreme shear rates (up to 5000 s−1). We also observed a distinct motion of adherent RBCs, depending on flow shear rate. Some of the adherent RBCs exhibited rolling adhesion on ICAM-1, particularly at higher shear rates (Figure 6B), and the percentages of rolling RBCs increased at shear rates of 3000, 4000, and 5000 s−1 compared with those at 500, 1000, and 2000 s−1 (Figure 6C). Similarly, RBC rolling velocities increased at higher flow shear rates, with the maximum occurring at 5000 s−1 (Figure 6D), suggesting that the characteristic adhesion behavior shown herein may prevent physiological flow rates from removing persistently attached RBCs in the microvasculature.
Rolling adhesion of HbSS RBCs onto immobilized ICAM-1 under flow conditions. The data shown are the mean percentages of adherent or rolling RBCs onto immobilized ICAM-1 and the mean rolling velocities under shear rates of 500, 1000, 2000, 3000, 4000, and 5000 s−1. A mean of 100% of HbSS RBCs adhered to immobilized ICAM-1 (including rolling adhesion and firm adhesion). (A) The number of adherent RBCs to immobilized ICAM-1 decreased with increasing shear rates. Shown are mean percentages of adherent RBCs (n = 5). (B) Two overlapped frames, taken 1 second apart, extracted from a video depicting cell rolling behavior. (C) The number of rolling RBCs increased with increasing shear rates (n = 5). (D) The velocities of rolling RBCs on immobilized ICAM-1 increased with increasing shear rates (n = 5). Error bars represent ± SEM.
Rolling adhesion of HbSS RBCs onto immobilized ICAM-1 under flow conditions. The data shown are the mean percentages of adherent or rolling RBCs onto immobilized ICAM-1 and the mean rolling velocities under shear rates of 500, 1000, 2000, 3000, 4000, and 5000 s−1. A mean of 100% of HbSS RBCs adhered to immobilized ICAM-1 (including rolling adhesion and firm adhesion). (A) The number of adherent RBCs to immobilized ICAM-1 decreased with increasing shear rates. Shown are mean percentages of adherent RBCs (n = 5). (B) Two overlapped frames, taken 1 second apart, extracted from a video depicting cell rolling behavior. (C) The number of rolling RBCs increased with increasing shear rates (n = 5). (D) The velocities of rolling RBCs on immobilized ICAM-1 increased with increasing shear rates (n = 5). Error bars represent ± SEM.
Discussion
SCD limits the lifespan of millions of individuals worldwide through a single hemoglobin mutation.24 Disease severity has been linked to abnormal RBC adhesion to the vascular endothelium, thereby contributing acutely to VOC and chronically to endothelial activation.15,25 Although the pathways leading to aberrant RBC adhesion to vascular endothelium and associated proteins are complex and have not been fully explicated yet, RBC adhesion plays a pivotal role in the initiation and propagation of unpredictable VOC episodes, thereby contributing to the pathophysiology of SCD.15,26,27
Endothelial activation has been thought to have an important role in SCD pathology and VOC.28 There are several adhesion molecules that have been associated with an activated phenotype in SCD, such as ICAM-1, VCAM-1, E-selectin, and P-selectin.29,30 These adhesion molecules mediate the attachment and movement of blood cells to the endothelium and into peripheral tissue while increasing microvascular permeability. ICAM-1 expression has been shown to be induced by various inflammatory signaling pathways.31 Although the interactions between WBCs and ICAM-1, abundantly found on the activated endothelial lining, have been well characterized, the role of ICAM-1 in supporting HbSS RBC adhesion to ECs has never been identified. Here, our results demonstrated significant HbSS RBC adhesion to immobilized ICAM-1 presenting unique dynamics of motion, which were heterogeneous and subject-specific. In our previous work, we correlated HbSS RBC adhesion to laminin with increased hemolysis and inflammation.13,25,32 High LDH levels and ARCs have been suggested as markers of hemolysis-associated endothelial dysfunction and disease severity.33 On the other hand, ICAM-1 is located directly on the endothelium, which may result in a more physiologically meaningful representation of cellular adhesion to vascular endothelium. Our results show that increased RBC adhesion to ICAM-1 correlates with hemolysis and a history of right-to-left shunts. Moreover, variations in RBC adhesion to ICAM-1 over time appear to correlate with LDH and ARC: an increasing or decreasing level of LDH or ARC corresponds to an increase or decrease in RBC adhesion. These observations are consistent with the “hyperhemolytic paradigm” that establishes that hemolysis in SCD increases endothelial dysfunction along with increased free plasma hemoglobin and NO biodeficiency and consumption.
HbF is one of the most studied genetic modulators of SCD because of its protective role in the disease. An increase in HbF levels correlates with improved survival and a milder disease phenotype, as evidenced by the lower frequency of VOCs and decreased mortality.22,34 Here, we found that HbF was inversely related to the adhesion of RBCs to ICAM-1. Interestingly, HbF levels seem to have a stronger effect than transfusions on decreasing RBC adhesion. Our data indicated that, even subjects with relatively lower HbS levels may exhibit enhanced RBC adhesion to ICAM-1 (data not shown). Because the majority of subjects with higher HbF levels were on HU therapy, we cannot rule out the potential direct impacts of HU on RBC adhesion, independent of an HbF-related mechanism. For instance, Bartolucci et al showed that HU led to diminished phosphorylation of Lu/BCAM, regardless of the HbF expression levels, thereby decreasing HbSS RBC adhesion to laminin.35 It is also possible that downregulation of several adhesion molecules, such as CD36 and α4β1,36 could result in attenuated overall RBC adhesion. Nevertheless, a direct inhibitory effect of HbF on RBC adhesion was also noted in a cohort consisting of infants and young children with no HU therapy. These findings suggest that increased HbF can inhibit, at least in part, RBC adhesion in an HU-independent mechanism.
Although adhesion of Plasmodium falciparum–infected RBCs (iRBCs) to ICAM-1 has been well established,37 HbSS RBC adhesion to ICAM-1 has not yet been documented. It has been shown that P falciparum erythrocyte membrane protein 1 is implicated in iRBC adhesion to ICAM-1 as the result of a binding site on the first domain of ICAM-1, which overlaps with the fibrinogen binding domain.38 iRBCs were shown to bind to ICAM-1 at a site that was distinct from LFA-1, MAC-1, and human rhinovirus.39 Further, blocking the fibrinogen binding site of ICAM-1 diminished iRBC adhesion to ICAM-1 in static and flow adhesion assays. Fibrinogen plays a vital role in the establishment of intercellular bridging between WBCs and ICAM-1, independently from LFA-1–and MAC-1–related pathways. We hypothesized that adhesion of HbSS RBCs to immobilized ICAM-1 in our microfluidic model was driven through a pathway involving plasma fibrinogen, because the HbSS RBC membrane lacks β2 integrins. Indeed, mixing the blood samples with an antibody against LFA-1 or incubating the ICAM-1–immobilized microchannels with β2 integrin did not have any effect on RBC adhesion. Similarly, blocking the α4β1-dependent pathway did not have any effect on RBC adhesion, suggesting that little or no contribution was made by reticulocytes.
Our results reveal that HbSS RBC adhesion was significantly attenuated upon fibrinogen pretreatment of the microchannels immobilized with ICAM-1 in a concentration-dependent manner. These results indicate that HbSS RBCs do not directly interact with ICAM-1; rather, membrane-bound fibrinogen, which was characterized previously,40 triggers the adhesive connection by forming an intercellular bridge. To further support this, we incubated whole blood samples with varying concentrations of LMWH, which has been shown to bind to fibrinogen, prior to the flow adhesion experiments. Notably, LMWH pretreatment of blood samples, but not of the microchannels themselves, significantly blocked RBC adhesion to ICAM-1, even at very low doses (ie, >80% inhibition at 0.1 µg/mL).
Although elevated plasma fibrinogen levels are linked to an increased risk for thrombosis,41 they may also interfere with ICAM-1–mediated RBC adhesion to decrease the frequency of VOC episodes. Upregulation of ICAM-1 through the vascular endothelium is triggered by various plasma cytokines and takes place at different levels, depending on the origin of the tissue. Therefore, a high fibrinogen level may alleviate cellular adhesion at sites where ICAM-1 is predominantly expressed while increasing the likelihood of thrombotic complications. Our results suggest that novel therapeutic approaches targeting the fibrinogen binding domain of ICAM-1 may decrease ICAM-1–mediated HbSS RBC adhesion.
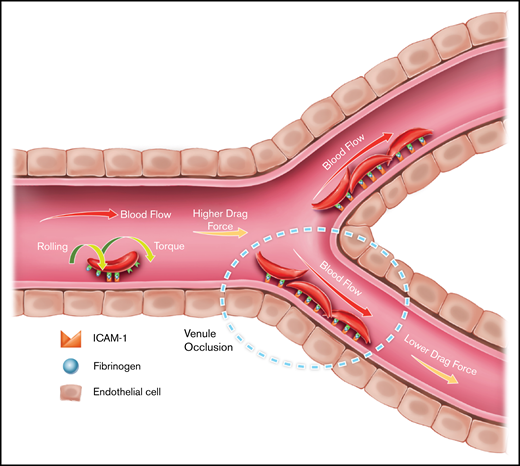
Furthermore, our results revealed that interactions analogous to leukocyte rolling on endothelium occur when HbSS RBCs bind to ICAM-1 at high shear rates. Similar rolling behavior of iRBCs interacting with ICAM-1 has also been documented.42,43 In the case of leukocytes, initial capture and rolling are established through receptors from the selectin family (eg, E-selectin), after which firm attachment is accomplished via integrins LFA-1 and MAC-1.44 Meanwhile, in the case of P falciparum infection, adhesion of iRBCs mediated by ICAM-1 causes rolling and static adhesion over a wide range of shear rates.45 However, it was reported that ICAM-1 was not able to successfully immobilize iRBCs in the absence of CD36,46 which leads to the following question: Why does ICAM-1 act as a rolling receptor and a firm adhesion receptor? In accordance with previous discovery, we reported the rolling behavior of HbSS RBCs on immobilized ICAM-1, as well as that the ratio of rolling cells/firm adherent cells increased with increasing shear rate. Because we have shown that such HbSS RBC–ICAM-1 interaction is mediated by an intermediate molecule, fibrinogen, we speculate that the bridging of fibrinogen to ICAM-1 might have a cutoff stress threshold, such that the bond might be distorted under high shear and, thus, rupture under a critical stress load. It is also likely that rolling RBCs may have fewer adhesion receptors for fibrinogen on their membrane, preventing them from establishing a firm attachment to ICAM-1 under high shear stress. Because reticulocytes have more binding sites for fibrinogen,47,48 it is tempting to speculate that the majority of firmly attached RBCs are younger relative to the rolling RBCs. On the other hand, adhesion characteristics of HbSS RBCs onto ICAM-1, whereby firm adhesion dominates under low shear rates but rolling with increasing velocities occurs under high shear rates, may provide unique insights into the mechanism of the initiation of VOC-involved HbSS RBC–ICAM-1 interactions (Figure 7). We postulate that, under inflammatory conditions in which endothelial ICAM-1 levels are upregulated, HbSS RBCs roll in capillaries, where the physiological shear rates are relatively higher, and firmly attach to the vasculature in postcapillary venules where shear rates are lower, thus contributing to increased resistance to blood flow and vaso-occlusion.
Proposed ICAM-1–mediated HbSS RBC adhesion mechanism in SCD. Results are consistent with a model of firm adhesion of HbSS RBCs to the vasculature in the postcapillary venules under low physiological shear, which may be mediated by initial rolling adhesion of RBCs in the capillary under high physiological shear, facilitated by ICAM-1. RBCs may form firm attachment with ICAM-1 near the low shear sites throughout the microvasculature, contributing to impaired local flow conditions, as illustrated by the dashed oval.
Proposed ICAM-1–mediated HbSS RBC adhesion mechanism in SCD. Results are consistent with a model of firm adhesion of HbSS RBCs to the vasculature in the postcapillary venules under low physiological shear, which may be mediated by initial rolling adhesion of RBCs in the capillary under high physiological shear, facilitated by ICAM-1. RBCs may form firm attachment with ICAM-1 near the low shear sites throughout the microvasculature, contributing to impaired local flow conditions, as illustrated by the dashed oval.
In conclusion, we used a physiologically relevant microfluidic platform to quantitatively evaluate adhesion of HbSS RBCs to immobilized ICAM-1. Our results pointed to a link between adhesion levels and high-grade hemolysis, as well as a history of right-to-left shunt development for subjects with HbSS SCD. Subjects with higher HbF levels had a milder adhesion profile. We further uncovered the role of plasma fibrinogen in mediating such HbSS RBC–ICAM-1 interactions and characterized the motion of adherent RBCs on ICAM-1, where they adhered firmly under low shear rates but rolled with increasing velocities under high shear rates. Future studies will aim to establish a physical model of HbSS RBCs rolling on ICAM-1, including the role of fibrinogen. In addition to HbSS SCD, the contribution that RBC adhesion to ICAM-1 might make to the pathophysiology of other diseases that include abnormal RBC-endothelium interactions, such as diabetes and retinal vein occlusion, warrants further investigation.
Data sharing requests should be sent to Umut A. Gurkan (e-mail: umut@case.edu).
Acknowledgments
The authors thank Grace Gongaware (Cleveland Institute of Art) for a scientific illustration used in this work. The authors also acknowledge with gratitude the contributions of patients and clinicians at Seidman Cancer Center (University Hospitals, Cleveland).
This work was supported by the Doris Duke Charitable Foundation (2013126 and 2015191); National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL133574, OT2HL152643, U01HL117659, and T32HL134622); the Clinical and Translational Science Collaborative of Cleveland; National Institutes of Health, National Center for Advancing Translational Sciences (UL1TR002548); and National Institutes of Health Roadmap for Medical Research, Case-Coulter Translational Research Partnership Program. U.A.G. acknowledges National Science Foundation CAREER Award 1552782, which supported this study in part, as well as the University Center for Innovation in Teaching and Education, Case Western Reserve University, for the Glennan Fellowship, which supports the scientific art program and the art student internship in Case Biomanufacturing and Microfabrication Laboratory.
Authorship
Contribution: E.K. and U.A.G designed the study, analyzed data, and interpreted data; E.K., Y.M., E.Q., N.T., and R.A. performed experiments; A.I., J.A.L., and N.S.K. interpreted data; E.K. wrote the manuscript; and A.I., J.A.L., N.S.K., and U.A.G. edited the manuscript; and J.A.L. analyzed the associations between adhesion data and clinical variables of the subjects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Umut A. Gurkan, Department of Mechanical and Aerospace Engineering, Department of Biomedical Engineering, Case School of Engineering, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106; e-mail: umut@case.edu.
References
Author notes
The full-text version of this article contains a data supplement.