Key Points
HD-MTX CNS prophylaxis intercalated with R-CHOP caused increased toxicity and R-CHOP delay compared with delivery at EOT.
No differences in survival or CNS relapse were seen, and delays after i-HD-MTX were reduced by delivering R-CHOP before day 10.
Abstract
High-dose methotrexate (HD-MTX) is increasingly used as prophylaxis for patients with diffuse large B-cell lymphoma (DLBCL) at high risk of central nervous system (CNS) relapse. However, there is limited evidence to guide whether to intercalate HD-MTX (i-HD-MTX) between R-CHOP-21 (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone given at 21-day intervals) or to give it at the end of treatment (EOT) with R-CHOP-21. We conducted a retrospective, multicenter analysis of 334 patients with DLBCL who received CNS prophylaxis with i-HD-MTX (n = 204) or EOT HD-MTX (n = 130). Primary end points were R-CHOP delay rates and HD-MTX toxicity. Secondary end points were CNS relapse rate, progression-free survival, and overall survival. The EOT group had more patients with a high CNS international prognostic index (58% vs 39%; P < .001) and more concurrent intrathecal prophylaxis (56% vs 34%; P < .001). Of the 409 cycles of i-HD-MTX given, 82 (20%) were associated with a delay of next R-CHOP (median, 7 days). Delays were significantly increased when i-HD-MTX was given after day 9 post–R-CHOP (26% vs 16%; P = .01). On multivariable analysis, i-HD-MTX was independently associated with increased R-CHOP delays. Increased mucositis, febrile neutropenia, and longer median inpatient stay were recorded with i-HD-MTX delivery. Three-year cumulative CNS relapse incidence was 5.9%, with no differences between groups. There was no difference in survival between groups. We report increased toxicity and R-CHOP delay with i-HD-MTX compared with EOT delivery but no difference in CNS relapse or survival. Decisions on HD-MTX timing should be individualized and, where i-HD-MTX is favored, we recommend scheduling before day 10 of R-CHOP cycles.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma,1 comprising ∼40% of all cases of lymphoma in large population-based registries. Despite being an aggressive malignancy, the majority of cases can be cured with R-CHOP chemoimmunotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) given at 21-day intervals. Systemic progression or relapse remains the most common cause of treatment failure in DLBCL, but central nervous system (CNS) relapse may also occur either in isolation or in combination with systemic disease recurrence. The prognosis from CNS relapse is dismal, with most studies reporting a median survival of <6 months.2,3 Estimates of incidence of CNS relapse in DLBCL vary from 2% to 6%, with some discrepancy across published studies as to whether the introduction of rituximab has reduced this risk.4-7
Various patient and disease characteristics have been identified that confer a high risk of CNS relapse in DLBCL, including the total number of extranodal sites involved,8 involvement of specific high-risk sites (eg, testicular, breast), advanced stage disease, and increased lactate dehydrogenase (LDH) levels.9 Recently, the CNS international prognostic index (CNS-IPI) has increasingly been used to identify high-risk patients; this index was derived from a large population of patients in clinical trials for DLBCL and validated in a “real-world” registry.10 It incorporates all standard IPI features as well as an additional point for renal and/or adrenal involvement.
Although the evidence for identifying patients at increased risk of CNS relapse is relatively robust, data on the most effective way to reduce this risk are lacking, with many studies being retrospective and incorporating significant selection bias. Intrathecal (IT) chemotherapy (eg, methotrexate [MTX]), incorporated into R-CHOP therapy, was used for many years as a prophylactic regimen. However, with an increased recognition that the pattern of CNS relapse in DLBCL is predominantly parenchymal,11,12 an area inadequately penetrated by IT chemotherapy,13 there has been increased focus on the use of systemic prophylaxis such as intravenous high-dose MTX (HD-MTX). Indeed, several recent publications have cast further doubt on any benefit of IT prophylaxis14,15 as well as highlighting the potential for toxicity with this approach.16
Although several studies have suggested that HD-MTX is effective CNS prophylaxis in DLBCL,15,17,18 no prospective randomized trial has been performed to show the benefit of this strategy, and there remains a lack of consensus regarding how it should be delivered (ie, timing, number of cycles, dose). CNS relapses tend to occur early, with the median time from DLBCL diagnosis to CNS relapse reported in most studies at between 6 and 8 months.12,19 Therefore, there is rationale to deliver CNS prophylaxis as early as possible during treatment. “Intercalating” HD-MTX between cycles of R-CHOP has been adopted in many centers. However, the largest published study demonstrating this as a deliverable and effective strategy was retrospective in nature, single center, and included only 65 patients.18 Given that failure of systemic therapy in DLBCL poses a much greater risk than CNS relapse, concern exists that the toxicity of intercalated HD-MTX (i-HD-MTX) may compromise delivery of R-CHOP therapy. An alternative approach is to wait until completion of systemic therapy before delivering HD-MTX with the aim to retain R-CHOP dose intensity, albeit with concern that such a delay in delivery may not abrogate early CNS relapse in some patients.
To address this clinically important and unanswered question, we conducted a retrospective, multicenter national analysis of patients with DLBCL who had received R-CHOP therapy as well as CNS prophylaxis with HD-MTX. Within this large data set, our primary aim was to analyze the toxicity of HD-MTX and its effect on R-CHOP relative dose intensity, comparing an i-HD-MTX approach to delivery at end of treatment (EOT). Secondary aims were to determine whether there were differences in survival and relapse outcome (including rates of CNS recurrence).
Methods
Data on 334 consecutive patients with DLBCL who received R-CHOP given at 21-day intervals in addition to HD-MTX CNS prophylaxis between 2011 and 2018 were collected from 11 centers in the United Kingdom who used either the i-HD-MTX or the EOT approach according to center preference. Patients with transformed indolent non-Hodgkin lymphoma were included, but patients with HIV-associated DLBCL, posttransplant or immunosuppression-related lymphoproliferative disorders, and any patients with known CNS involvement at diagnosis were excluded. Baseline CNS evaluation was not mandated but was performed according to local treating clinician discretion for patients with clinical suspicion of CNS disease at diagnosis. Patients receiving additional IT prophylaxis were not excluded.
Patients were selected for CNS prophylaxis per local policies on the basis of published risk models, including involvement of ≥2 extranodal sites plus increased LDH levels9 or high CNS-IPI score,10 or due to involvement of specific high-risk sites (testicular, renal/adrenal, breast, paranasal sinus, paraspinal, or ovarian involvement).
Baseline characteristics were collected, including several risk factors known to influence CNS relapse rates. Continuous variables are expressed as median and range; intergroup comparisons were performed by using the Mann-Whitney U test. Categorical variables are presented as proportions and were compared by using the χ2 test.
R-CHOP was scheduled in 21-day cycles for all patients. R-CHOP delays were analyzed in 2 ways. First, all cycles of i-HD-MTX administered were reviewed and any delays to subsequent R-CHOP cycles recorded, with univariable and multivariable analyses (MVA) of risk factors for delay performed using logistic regression. Second, to determine if i-HD-MTX was an independent risk factor for delay, an analysis of all R-CHOP delays throughout therapy for both groups was performed, including MVA with timing of HD-MTX included as a risk factor.
Progression-free survival (PFS), overall survival (OS), and time to CNS relapse were determined by using Kaplan-Meier survival analysis20 and Cox regression with comparison between treatment groups made using the log-rank test. Time-to-event analyses were measured from the date of initial DLBCL diagnosis. An “event” for PFS was defined by CNS or systemic relapse, or death from any cause. Patients were censored at the date last seen if alive and event free. Time-to-CNS relapse and the cumulative incidence of CNS relapse at 2 and 3 years were calculated. Landmark survival analyses of PFS, OS, and CNS relapse were performed for patients who were alive and event free at 6 months from diagnosis to address potential immortality bias. Statistical analyses were performed by using IBM SPSS Statistics for Windows, version 26 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) with 95% confidence intervals presented and P < .05 considered significant.
Results
Baseline characteristics
Baseline characteristics of all 334 patients are summarized in Table 1, with further stratification by timing of HD-MTX (i-HD-MTX [n = 204] vs EOT [n = 130]). Across both cohorts, the median age was 61 years (range, 20-82 years) with a male predominance (59%). Sixty-two percent had involvement of ≥2 extranodal sites, and 46% had a high CNS IPI (score, 4-6). Only 3% had “double-hit” lymphoma (presence of MYC with BCL2 and/or BCL6 translocations), reflecting the preference in most centers to treat such patients with more intensive regimens than R-CHOP. Ninety-six percent of patients received 6 cycles of R-CHOP, and the median number of cycles of HD-MTX delivered was 2 (range, 1-4 cycles).
Baseline characteristics
| Characteristic . | All patients (N = 334) . | Intercalated (n = 204) . | EOT (n = 130) . | P . |
|---|---|---|---|---|
| Age, median (range), y | 61 (20-82) | 60 (20-81) | 62 (20-82) | .78 |
| Male sex | 197 (59) | 116 (57) | 81 (62) | .32 |
| Creatinine clearance, median (range), mL/min | 111 (44-299) | 115 (45-299) | 107 (44-236) | .03* |
| Advanced stage | 266 (82) | 168 (82) | 107 (82) | .99 |
| Elevated LDH | 242 (72) | 143 (70) | 99 (76) | .33 |
| ECOG PS ≥2 | 88 (27) | 45 (22) | 44 (34) | .02* |
| 1 EN site | 123 (37) | 81 (40) | 42 (32) | .38 |
| 2 EN sites | 116 (35) | 66 (32) | 50 (38) | |
| ≥3 EN sites | 90 (27) | 55 (27) | 35 (27) | |
| Renal/adrenal involvement | 55 (16) | 26 (13) | 29 (22) | .02* |
| “Double hit”† | 10 (3) | 5 (3) | 5 (4) | .65 |
| CNS-IPI | ||||
| Low (0-1) | 51 (16) | 32 (16) | 19 (15) | |
| Intermediate (2-3) | 123 (35) | 88 (45) | 35 (27) | |
| High (4-6) | 151 (46) | 77 (39) | 74 (58) | <.001* |
| IT prophylaxis | 142 (42) | 69 (34) | 73 (56) | <.001* |
| Received 6 cycles of R-CHOP | 319 (96) | 194 (95) | 125 (96) | .65 |
| No. HD-MTX received, median (range) | 2 (1-4) | 2 (1-4) | 2 (1-3) | .62 |
| Received ≥3 g/m2 HD-MTX | 309 (93) | 191 (94) | 118 (91) | .33 |
| Characteristic . | All patients (N = 334) . | Intercalated (n = 204) . | EOT (n = 130) . | P . |
|---|---|---|---|---|
| Age, median (range), y | 61 (20-82) | 60 (20-81) | 62 (20-82) | .78 |
| Male sex | 197 (59) | 116 (57) | 81 (62) | .32 |
| Creatinine clearance, median (range), mL/min | 111 (44-299) | 115 (45-299) | 107 (44-236) | .03* |
| Advanced stage | 266 (82) | 168 (82) | 107 (82) | .99 |
| Elevated LDH | 242 (72) | 143 (70) | 99 (76) | .33 |
| ECOG PS ≥2 | 88 (27) | 45 (22) | 44 (34) | .02* |
| 1 EN site | 123 (37) | 81 (40) | 42 (32) | .38 |
| 2 EN sites | 116 (35) | 66 (32) | 50 (38) | |
| ≥3 EN sites | 90 (27) | 55 (27) | 35 (27) | |
| Renal/adrenal involvement | 55 (16) | 26 (13) | 29 (22) | .02* |
| “Double hit”† | 10 (3) | 5 (3) | 5 (4) | .65 |
| CNS-IPI | ||||
| Low (0-1) | 51 (16) | 32 (16) | 19 (15) | |
| Intermediate (2-3) | 123 (35) | 88 (45) | 35 (27) | |
| High (4-6) | 151 (46) | 77 (39) | 74 (58) | <.001* |
| IT prophylaxis | 142 (42) | 69 (34) | 73 (56) | <.001* |
| Received 6 cycles of R-CHOP | 319 (96) | 194 (95) | 125 (96) | .65 |
| No. HD-MTX received, median (range) | 2 (1-4) | 2 (1-4) | 2 (1-3) | .62 |
| Received ≥3 g/m2 HD-MTX | 309 (93) | 191 (94) | 118 (91) | .33 |
Data are n (%) unless otherwise noted. Missing data: LDH, n = 8; PS, n = 3; Renal/adrenal involvement, n = 2; Double hit, n = 38; CNS-IPI, n = 9. ECOG PS, Eastern Cooperative Oncology Group performance status; EN, extranodal; IT, intrathecal.
Statistically significant.
Presence of MYC with BCL2 and/or BCL6 translocations.
Baseline characteristics were broadly similar between the 2 treatment groups. Of note, the EOT group had a higher proportion of patients with poor performance status and with renal/adrenal involvement; as a result, more patients were in the high CNS-IPI category in this group. A higher proportion of patients in the EOT group (73 of 130 [56%]) received IT prophylaxis in addition to HD-MTX compared with the intercalated group (69 of 204 [34%]). The most frequently used IT chemotherapy was MTX, with a median number of treatments of 2 (range, 1-6).
Delays with i-HD-MTX
A total of 409 cycles of HD-MTX were given intercalated between cycles of R-CHOP from 204 patients. Eighty-two (20%) of these were associated with a delay in the subsequent R-CHOP cycle, with a median delay of 7 days (range, 2-150 days). Clinicians were asked to determine whether they felt the R-CHOP delay was directly attributable to HD-MTX. Fifty-six (14%) of 409 cycles had an R-CHOP delay attributed to MTX, with reasons for delay as follows: infection (n = 19), mucositis (n = 11), cytopenias (n = 10), renal toxicity (n = 7), delayed MTX clearance (n = 2), hepatotoxicity (n = 2), and other/unknown (n = 4). Delays were significantly increased when i-HD-MTX was given after day 9 following R-CHOP (48 of 185 [26%] vs 32 of 207 [16%]; P = .01). Univariable and multivariable analysis of factors associated with R-CHOP delay after intercalated MTX identified that delivering MTX later in the R-CHOP cycle (on or after day 10) was the most significant factor contributing to R-CHOP delay (Table 2). Full details of timing of delivery of i-HD-MTX are displayed in supplemental Figure 1.
Univariable and multivariable analysis of factors influencing delay of subsequent R-CHOP when i-HD-MTX given
| Parameter . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | |
| i-HD-MTX given after day 9 post R-CHOP | 1.92 (1.16-3.16) | .01* | 1.74 (1.03-2.93) | .04* |
| Age | 1.02 (1.00-1.04) | .07 | 1.02 (0.99-1.05) | .16 |
| Male sex | 1.41 (0.86-2.32) | .17 | 1.50 (0.88-2.56) | .13 |
| Advanced stage | 0.61 (0.34-1.11) | .11 | 0.56 (0.26-1.21) | .14 |
| ECOG PS ≥2 | 0.84 (0.52-1.71) | .84 | 0.98 (0.52-1.84) | .94 |
| No. extranodal sites | 0.95 (0.74-1.20) | .65 | 1.00 (0.76-1.33) | .98 |
| Elevated LDH | 1.12 (0.65-1.93) | .70 | 1.72 (0.87-3.40) | .12 |
| Baseline creatinine clearance | 1.00 (0.99-1.00) | .16 | 1.00 (0.99-1.01) | .80 |
| Parameter . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | |
| i-HD-MTX given after day 9 post R-CHOP | 1.92 (1.16-3.16) | .01* | 1.74 (1.03-2.93) | .04* |
| Age | 1.02 (1.00-1.04) | .07 | 1.02 (0.99-1.05) | .16 |
| Male sex | 1.41 (0.86-2.32) | .17 | 1.50 (0.88-2.56) | .13 |
| Advanced stage | 0.61 (0.34-1.11) | .11 | 0.56 (0.26-1.21) | .14 |
| ECOG PS ≥2 | 0.84 (0.52-1.71) | .84 | 0.98 (0.52-1.84) | .94 |
| No. extranodal sites | 0.95 (0.74-1.20) | .65 | 1.00 (0.76-1.33) | .98 |
| Elevated LDH | 1.12 (0.65-1.93) | .70 | 1.72 (0.87-3.40) | .12 |
| Baseline creatinine clearance | 1.00 (0.99-1.00) | .16 | 1.00 (0.99-1.01) | .80 |
Missing data: day of i-HD-MTX, n = 17.
*Statistically significant.
Comparison of R-CHOP delays between treatment groups
Sixty-five (32%) of 203 patients in the i-HD-MTX group had at least one R-CHOP delay during therapy of ≥7 days compared with 18 of 119 (15%) in the EOT group (P = .001). Ninety (44%) of 203 had at least 1 delay of ≥3 days in the i-HD-MTX group compared with 27 of 119 (23%) in the EOT group (P < .001). Further breakdown of number of cycles delayed for each patient is outlined in supplemental Table 1. On multivariable analysis of the whole cohort, including several baseline and prognostic factors, intercalation of HD-MTX and male sex were the only parameters independently associated with increased R-CHOP delays (Table 3).
Univariable and multivariable analysis of factors associated with R-CHOP delays in whole study population
| Parameter . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | |
| i-HD-MTX | 2.64 (1.48-4.72) | .001* | 3.06 (1.62-5.77) | .001* |
| Age | 1.01 (0.99-1.03) | .58 | 1.00 (0.97-1.03) | .88 |
| Baseline creatinine clearance | 1.00 (0.99-1.01) | .78 | 1.00 (0.99-1.01) | .39 |
| Male sex | 1.60 (0.95-2.70) | .08* | 1.84 (1.04-3.26) | .04* |
| Advanced stage | 0.82 (0.43-1.56) | .54 | 0.69 (0.29-1.63) | .40 |
| ECOG PS ≥2 | 0.81 (0.45-1.43) | .46 | 0.86 (0.46-1.60) | .63 |
| ≥2 extranodal sites | 1.23 (0.73-2.07) | .44 | 1.76 (0.89-3.45) | .10 |
| Elevated LDH | 0.91 (0.51-1.60) | .73 | 0.94 (0.50-1.91) | .94 |
| IT therapy given | 0.63 (0.38-1.06) | .08 | 0.74 (0.42-1.30) | .29 |
| HD-MTX dose | 0.76 (0.45-1.30) | .31 | 0.65 (0.37-1.16) | .14 |
| Parameter . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | |
| i-HD-MTX | 2.64 (1.48-4.72) | .001* | 3.06 (1.62-5.77) | .001* |
| Age | 1.01 (0.99-1.03) | .58 | 1.00 (0.97-1.03) | .88 |
| Baseline creatinine clearance | 1.00 (0.99-1.01) | .78 | 1.00 (0.99-1.01) | .39 |
| Male sex | 1.60 (0.95-2.70) | .08* | 1.84 (1.04-3.26) | .04* |
| Advanced stage | 0.82 (0.43-1.56) | .54 | 0.69 (0.29-1.63) | .40 |
| ECOG PS ≥2 | 0.81 (0.45-1.43) | .46 | 0.86 (0.46-1.60) | .63 |
| ≥2 extranodal sites | 1.23 (0.73-2.07) | .44 | 1.76 (0.89-3.45) | .10 |
| Elevated LDH | 0.91 (0.51-1.60) | .73 | 0.94 (0.50-1.91) | .94 |
| IT therapy given | 0.63 (0.38-1.06) | .08 | 0.74 (0.42-1.30) | .29 |
| HD-MTX dose | 0.76 (0.45-1.30) | .31 | 0.65 (0.37-1.16) | .14 |
*Statistically significant.
MTX toxicity
Toxicity data were collected for a total of 729 cycles of HD-MTX (Table 4). The overall rate of renal toxicity was 5% and was similar across groups. Focusing on the period post–HD-MTX administration, i-HD-MTX was associated with significantly increased mucositis (10% vs 4%; P = .001), neutropenic fever (10% vs 2%; P < .001), and longer median inpatient stay (5 vs 4 days; P < .001), likely reflecting the delivery of MTX during the neutrophil nadir after R-CHOP.
Summary of HD-MTX toxicity
| Parameter . | All (N = 729) . | Intercalated (n = 409) . | EOT (n = 320) . | P . |
|---|---|---|---|---|
| No. of inpatient days, median (range) | 5 (2-60) | 5 (2-60) | 4 (3-80) | <.001* |
| Toxicity | ||||
| Renal (any) | 38 (5) | 21 (5) | 17 (5) | .92 |
| Grade 1 (creatinine 1.5-1.9 × baseline) | 22 (3) | 12 (3) | 10 (3) | |
| Grade 2 (creatinine 2-2.9 × baseline) | 6 (1) | 3 (1) | 3 (1) | |
| Grade 3 (creatinine >3 × baseline) | 10 (1) | 6 (1) | 4 (1) | |
| Liver (grade 2 or worse) | 17 (2) | 7 (2) | 10 (3) | .21 |
| Mucositis | 54 (7) | 42 (10) | 12 (4) | .001* |
| Neutropenic fever | 49 (7) | 42 (10) | 7 (2) | <.001* |
| Parameter . | All (N = 729) . | Intercalated (n = 409) . | EOT (n = 320) . | P . |
|---|---|---|---|---|
| No. of inpatient days, median (range) | 5 (2-60) | 5 (2-60) | 4 (3-80) | <.001* |
| Toxicity | ||||
| Renal (any) | 38 (5) | 21 (5) | 17 (5) | .92 |
| Grade 1 (creatinine 1.5-1.9 × baseline) | 22 (3) | 12 (3) | 10 (3) | |
| Grade 2 (creatinine 2-2.9 × baseline) | 6 (1) | 3 (1) | 3 (1) | |
| Grade 3 (creatinine >3 × baseline) | 10 (1) | 6 (1) | 4 (1) | |
| Liver (grade 2 or worse) | 17 (2) | 7 (2) | 10 (3) | .21 |
| Mucositis | 54 (7) | 42 (10) | 12 (4) | .001* |
| Neutropenic fever | 49 (7) | 42 (10) | 7 (2) | <.001* |
Data are n (%) unless otherwise noted.
*Statistically significant.
Survival outcomes and CNS relapse
There were 19 CNS relapses in the whole study cohort (5.7%), with a median time from diagnosis to relapse of 8.1 months (range, 5-46 months). Fourteen were parenchymal (74%), 2 (11%) involved both the parenchyma and leptomeninges, and 3 (16%) were isolated to the leptomeninges. Four of the five patients with leptomeningeal involvement at relapse had received concurrent IT prophylaxis. Two of the patients who experienced a CNS relapse had only received 1 cycle of HD-MTX (both in the i-HD-MTX group), with the remainder receiving ≥2 cycles.
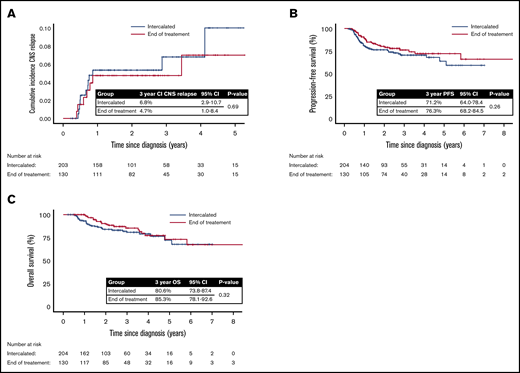
The overall estimated 2- and 3-year cumulative incidence of CNS relapse was 5.1% (95% CI, 2.7-7.5) and 5.9% (95% CI, 3.0-8.8), respectively. According to HD-MTX timing, the 3-year cumulative incidence of CNS relapse was: i-HD-MTX, 6.8% (95% CI, 2.9-10.7); and EOT, 4.7% (95% CI, 1.0-8.4). There was no statistically significant difference between the groups (unadjusted hazard ratio, 1.21; 95% CI, 0.48-3.07; P = .691) (Figure 1A).
CNS relapse rates and survival outcomes according to timing of HD-MTX CNS prophylaxis. (A) Cumulative incidence of CNS relapse according to HD-MTX timing. (B) PFS according to HD-MTX timing. (C) OS according to HD-MTX timing.
CNS relapse rates and survival outcomes according to timing of HD-MTX CNS prophylaxis. (A) Cumulative incidence of CNS relapse according to HD-MTX timing. (B) PFS according to HD-MTX timing. (C) OS according to HD-MTX timing.
On univariable analysis, the only significant risk factor for CNS relapse identified was involvement of ≥2 extranodal sites (P = .04). Timing of HD-MTX and use of IT prophylaxis were not associated with CNS relapse risk on MVA (supplemental Table 2). There was no reduction in CNS relapse rate in the 72 patients in the EOT group who had IT prophylaxis compared with those who did not (5.8% vs 5.5%; P = .96).
An analysis focusing on patients who developed early CNS relapse (defined as earlier than 8 months from original DLBCL diagnosis) identified 9 patients in this category, with clinical and prognostic features described in supplemental Table 3. Of note, these patients were enriched for high-risk features such as advanced stage and raised LDH levels (all patients), number of extranodal sites (5 of 9 with ≥3 extranodal sites), and renal or adrenal involvement (4 of 9 patients). However, 4 of 9 patients did not fall into the high-risk CNS-IPI category (due to age ≤60 years and Eastern Cooperative Oncology Group performance status <2). The outcomes were poor, with all but 1 patient dying of lymphoma. Of note, 6 of 9 patients had concurrent systemic progression at the time of CNS relapse.
With a median follow up of 2.4 years (range, 0.3-8.7 years), the 3-year PFS and OS of the i-HD-MTX group were 71.2% (95% CI, 64.0-78.4) and 80.6% (95% CI, 73.8-87.4), respectively, and in the EOT group, 3-year PFS and OS were 76.3% (95% CI, 68.2-84.5) and 85.3% (95% CI, 78.1-92.6). There was no statistically significant difference in either PFS (P = .26) or OS (P = .32) between the groups (Figure 1B-C). On landmark analysis including only those who were alive and event-free at 6 months, there remained no difference in PFS, OS, or CNS relapse rate between the 2 groups (supplemental Figure 2). No significant difference in CNS relapse, PFS, or OS was seen when analysis was restricted to patients with high CNS-IPI, but an increased risk of treatment delays remained with i-HD-MTX (data not shown). There was no significant difference in 3-year PFS between patients who did or did not have ≥1 R-CHOP delay of ≥7 days (66.8% vs 75.1%; P = .12).
Discussion
CNS prophylaxis in DLBCL is a contentious issue, with wide variation in practice throughout the United Kingdom and worldwide. This disparity is largely due to the paucity of robust, prospective evidence to guide how patients are selected for prophylaxis and the optimum method of delivery. Cumulatively, there appears to be sufficient data to suggest that intravenous HD-MTX is an effective method for delivering CNS prophylaxis. Although the median time from diagnosis to CNS relapse reported in most studies is 6 to 8 months,12,19 early CNS relapses during primary R-CHOP therapy do occur. Therefore, although not supported by prospective data, there is theoretical rationale for administering HD-MTX as early as possible. However, HD-MTX can result in significant toxicity, and careful patient selection is crucial. Patients at highest risk of CNS relapse are also those at greater risk of systemic treatment failure, and there are concerns that delivering HD-MTX in an intercalated fashion with R-CHOP may compromise the timing and relative dose intensity of systemic therapy. Some clinicians fear that this risk outweighs the relatively low likelihood of early CNS relapse, and they choose to wait until after R-CHOP completion before administering HD-MTX.
To the authors’ knowledge, this multicenter retrospective analysis of 334 patients is the largest of its type, specifically assessing the deliverability and toxicity of HD-MTX as CNS prophylaxis with R-CHOP chemoimmunotherapy, either intercalated or delivered at the end of systemic treatment. We have shown that intercalating a cycle of HD-MTX resulted in a delay of the subsequent R-CHOP cycle in 20% of instances, with a median delay of 7 days. Although clinicians reported that the HD-MTX itself caused a delay in 14% of cycles, delays due to the inherent toxicity of R-CHOP are inevitable for some patients, and it is difficult to ascertain the true contribution of HD-MTX in these delays.
We addressed this issue by comparing patients receiving i-HD-MTX vs those who received it after R-CHOP; the latter group acted as a “control” to show how many delays are seen with R-CHOP alone in this high-risk patient group. We acknowledge that those who received EOT HD-MTX were potentially more likely to have completed R-CHOP therapy without significant complication, and there may be a degree of selection bias in using this group as a control for delays. However, we found that 32% of patients in the intercalated group had at least one R-CHOP delay of ≥7 days compared with only 15% in the EOT group. Importantly, on multivariable analysis, timing of MTX (intercalated vs EOT) was the only independent risk factor influencing number of R-CHOP delays. Although a delay of ≥3 days may not be considered clinically relevant in isolation, it should be noted that 15% of patients in the intercalated group had ≥2 delays of ≥3 days during treatment compared with only 1% in the EOT group.
Although we have shown that i-HD-MTX increased the risk of R-CHOP delay, the clinical significance of this finding is a matter for debate. Given the need to maintain dose intensity in a high-grade, proliferative malignancy such as DLBCL, delays of ≥7 days might be considered as potentially clinically relevant. This is particularly concerning in this patient cohort who are inherently at high risk of systemic relapse, as shown by a median IPI of 3 and 123 (37%) of 334 with an IPI of 4 to 5. On analysis of all patients who experienced a delay of ≥7 days, there was a trend toward improved PFS in the no delay group, but this did not reach statistical significance. We should acknowledge there may be other confounding variables associated with delay that we have not identified in this retrospective analysis. Furthermore, it should be noted that for 56 of the 65 patients in the i-HD-MTX group who had a delay of ≥7 days, the rest of the cycles were delivered with no further delays of a similar length.
When considering patients for i-HD-MTX CNS prophylaxis, clinicians must assess the patients’ fitness for such an approach. However, other than ensuring adequate renal function, this is done in a mainly subjective manner, and often it can be difficult to predict the tolerability of this approach in individual patients. From this data set, we attempted to identify factors that may help identify patients more likely to experience R-CHOP delays after i-HD-MTX. Timing of i-HD-MTX following R-CHOP was the most significant factor identified on both univariable and multivariable analyses, with a higher rate of delay seen when i-HD-MTX was given on day 10 or later. Therefore, based on these data, it may be more suitable to bring forward i-HD-MTX to earlier within the R-CHOP 21-day cycle to minimize the risk of delay to the next treatment. It is recognized that such an approach cannot be substantiated with high-quality evidence and may lead to as-yet unidentified toxicities.
The rate of CNS relapse in the entire cohort was low (5.7%). Although the study was not designed or powered to address the efficacy of HD-MTX CNS prophylaxis, given the high-risk nature of the patient group, this does seem to be a relatively low rate of CNS recurrence. For example, patients with a high CNS IPI (score, 4-6) have a predicted 2-year CNS relapse rate of 10.2%10 ; 151 patients in our study fell into this category but had a 2-year CNS relapse risk of 6.4%. We feel that we have provided some indirect evidence of efficacy of HD-MTX CNS prophylaxis but acknowledge that this is an area requiring further investigation, ideally within the setting of a prospective randomized trial. Furthermore, with such a low event rate, it is difficult to draw definitive conclusions on any potential difference in CNS relapse risk when considering the different approaches for delivering HD-MTX. However, there did not seem to be any signal toward a difference in CNS recurrence between the 2 strategies.
Toxicity is the main concern when selecting patients for HD-MTX, and it was therefore important to assess and quantify the frequency of various toxicities in this real-world cohort. Accepting that this is a patient group deemed by clinicians to be “fit” for HD-MTX, we showed that renal toxicity occurred in 5% of HD-MTX cycles, with the majority being relatively mild and only 2% of cycles causing grade 2 toxicity or worse. There was a significant increase in mucositis and infection after i-HD-MTX, which is likely to be the main explanation for the longer median inpatient stay with this approach.
The main limitations of the current study are those inherent to retrospective, nonrandomized observation analyses, with some imbalances in baseline characteristics between groups. We acknowledge that selection criteria for CNS prophylaxis varied between centers, reflecting the limited evidence to guide such decisions, particularly before the introduction of the CNS-IPI. Survival outcomes were a secondary end point of the study with no preplanned power calculation, and thus there is a risk that the study is underpowered to detect a difference in PFS or OS between the 2 groups. There is also potential for survivorship bias in retrospectively identifying patients who had HD-MTX after R-CHOP completion, as data from those who progressed early or died before R-CHOP completion may not have been captured. However, data from recent large prospective trials suggest that the number of patients with disease progression or treatment-related mortality during R-CHOP induction therapy is very small (approximately <5%).21-23 Furthermore, on landmark analysis including only those who were alive and event free at 6 months, there remained no difference in PFS, OS, or CNS relapse rate between the groups.
Despite a higher proportion of patients with high CNS-IPI in the EOT group, there appeared to be no increased CNS relapse with this approach. However, the number of patients receiving IT therapy in this group (56%) may be considered a confounding factor. Accepting the caveat of low event rates in a retrospective analysis, in the EOT group there was no increase in CNS relapse rate in the 54 patients who had no IT therapy, and in the whole study population use of IT therapy was not found to be a significant predictor for CNS relapse on multivariable analysis. Furthermore, there is growing evidence to suggest that IT therapy is ineffective in reducing CNS relapses in DLBCL,14-16 although no prospective trial has definitively answered this question.
The current study addressed 2 methods for HD-MTX delivery (intercalated or at end of R-CHOP therapy), but a potential third option is to attempt delivery at the beginning of treatment. This approach was investigated in a recent phase 2 trial in which HD-MTX was given with the first 2 cycles of 14-day R-CHOP therapy, followed by an additional 4 cycles of 14-day R-CHOP and etoposide with IT cytarabine given as further CNS prophylaxis.24 Although the rates of systemic and CNS relapse were low, whether this intensive approach is deliverable in a routine clinical setting remains to be seen. Other potential methods for reducing CNS relapse in DLBCL under investigation mainly involve incorporation of novel agents capable of crossing the blood–brain barrier. For example, ibrutinib, a Bruton tyrosine kinase inhibitor, has shown activity in CNS involvement of mantle cell lymphoma,25 lymphoplasmacytic lymphoma,26 and DLBCL.27 Similarly, the immunomodulatory agents lenalidomide and pomalidomide have shown activity in primary and secondary CNS involvement with B-cell malignancies.28,29 Both ibrutinib and lenalidomide have failed to show overall benefit for patients with DLBCL when incorporated into R-CHOP therapy in large phase 3 trials30,31 ; whether these drugs could specifically benefit the small subset of patients at high risk of CNS relapse remains an unanswered question.
In conclusion, although our data suggest that HD-MTX may be deferred until EOT with less risk of causing R-CHOP delay, the clinical significance of such delays is unclear, and the additional value of IT therapy during R-CHOP in this setting remains uncertain. There continues to be theoretical rationale for intercalating HD-MTX with R-CHOP to reduce the risk of very early CNS relapse and, where this approach is favored, we recommend that HD-MTX is scheduled before day 10 of the R-CHOP cycle to minimize risk of delay to the next treatment. Delivery at EOT seems to be a valid alternative strategy, particularly where there is concern about fitness and ability to maintain R-CHOP dose intensity, accepting a risk that early CNS relapse may not be prevented. In the absence of a prospective, randomized trial to inform decision-making in this area, our data may help make a careful analysis of competing risks on an individual patient basis.
Requests for data sharing may be submitted to the corresponding author (Matthew R. Wilson; e-mail: matthewwilson1@nhs.net).
Authorship
Contribution: M.R.W., P.M., C.P.F., F.M., and K.C. conceived the study; M.R.W. coordinated the collection of national data; M.R.W., T.A.E., N.M.-C., M.A., K.E.P., G.P., J.K., J. Schofield, J.E., K.L., A.M.K., N.S., C.-K.C., M.A.T., T.C., and J. Smith collected data; and M.R.W. performed statistical analysis and wrote the manuscript, which all authors critically reviewed.
Conflict-of-interest disclosure: G.P. received travel expenses from Takeda and AbbVie. J. Smith received travel expenses from AbbVie and Janssen. K.C. served a consulting/advisory role and received travel expenses from Roche and Janssen; and served a consulting/advisory role for Celgene. M.A. received honoraria from Roche; served a consultancy role for Takeda and Gilead; received travel expenses from AbbVie; and received research funding from Pfizer. N.M.-C. received travel support and honoraria from AbbVie. N.S. served consultancy roles for AbbVie and Roche. P.M. served a consultancy role and received travel expenses from Roche. T.A.E. received honoraria from Roche, Janssen, and Celgene. K.C. received consultancy/speaker fees from AbbVie, AZ, Celgene, Gilead, Janssen, Roche, and Takeda; and received research funding from Adienne, AbbVie, Roche, Gilead, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Matthew R. Wilson, Beatson West of Scotland Cancer Centre, 1053 Great Western Rd, Glasgow G12 0YN, United Kingdom; e-mail: matthewwilson1@nhs.net.
References
Author notes
The full-text version of this article contains a data supplement.