Key Points
Hemolysis was caused by ABO blood-group–specific IgG2 antibodies leading to FcgRIIa-dependent clearance of opsonized erythrocytes.
IVIG-associated hemolysis was increased in the presence of soluble mediators of inflammation.
Abstract
Hemolytic anemia resulting from IV Immunoglobulin (IVIG) treatment can be a serious complication, especially for those with underlying conditions with a high level of inflammation and after administration of high IVIG dosages, such as Kawasaki disease (KD), a multisystem vasculitis affecting young children. This hemolysis is caused by antibodies against blood groups A and B, but the precise mechanism for hemolysis is not known. We performed a single center, partly retrospective, partly prospective study of a cohort of 581 patients who received IVIG for treatment of KD from 2006 to 2013. Factors associated with hemolysis were identified through univariable and multivariable logistic regression. Six IVIG preparations were assayed for their hemolytic effect with serological and cellular assays to clarify the mechanism of red cell destruction. During the study period, a sudden increase in the incidence of hemolysis was observed, which coincided with the introduction of new IVIG preparations in North America that contained relatively high titers of anti-A and anti-B. These blood-group–specific antibodies were of the immunoglobulin G2 (IgG2) subclass and resulted in phagocytosis by monocyte-derived macrophages in an FcγRIIa-dependent manner. Phagocytosis was increased in the presence of proinflammatory mediators that mimicked the inflammatory state of KD. An increased frequency of severe hemolysis following IVIG administration was caused by ABO blood-group–specific IgG2 antibodies leading to FcγRIIa-dependent clearance of erythrocytes. This increase in adverse events necessitates a reconsideration of the criteria for maximum titer (1:64) of anti-A and anti-B in IVIG preparations.
Introduction
IV immunoglobulin (IVIG) is a product of pooled immunoglobulin G (IgG) purified from the plasma of thousands of donors. It is used as replacement therapy in primary immune deficiencies, in which case it provides patients having hypogammaglobulinemia with protective antibodies against common pathogens. IVIG is also used as an immunomodulating agent in the treatment of multiple autoimmune and inflammatory diseases, such as immune thrombocytopenia (ITP), autoimmune hemolytic anemia (AIHA), Kawasaki disease (KD) and Guillain-Barré syndrome.1-6 As an immunomodulating agent, IVIG is administered at higher dosages (1-2 g/kg) compared with replacement therapy (0.4 g/kg).2,5,6
IVIG is generally considered to be a safe product. Most adverse effects are mild and transient, such as fatigue, headache, fever, and chills.6,7 However, an increasingly recognized adverse effect occurring in patients treated with IVIG is the development of hemolytic anemia.8,9 In most cases, there is only a mild decrease in hematocrit, but, rarely, the anemia can be severe, leading to cardiovascular compromise necessitating erythrocyte transfusions.6,10-12 It is generally believed that this hemolytic anemia is caused by passive transfer of anti-A and anti-B present in IVIG products9 and it occurs almost exclusively in non-O blood-group recipients,13,14 with an increased risk in recipients who have an heterozygous AB blood group.15 Several factors have been shown to be associated with an increased risk of developing hemolysis, including a high cumulative dose of IVIG in a short period14,16,17 and underlying inflammatory or immune-mediated disorders.18-20 Whether antibody titers of anti-A and anti-B relate to the risk of hemolysis remains a debate, as 1 study13 found an association between products with high anti-A and anti-B titers and severe cases of hemolysis spontaneously reported to a vigilance database, whereas another study16 did not find such an association. Likewise, it is not clear whether IVIG-associated hemolysis occurs more frequently with the use of specific IVIG preparations,9 although 1 study found a decreased risk of hemolysis with the use of lyophilized products when compared with liquid products.14 In a comparative analysis, KD and ITP were reported as the conditions with the highest risk of developing IVIG-associated hemolysis,14 with an incidence of 85 hemolytic events in 100 000 treatment episodes for KD. This study was based on spontaneous reporting of hemolysis cases and likely represents an underrepresentation of the actual incidence. Case series of severe hemolytic events post-IVIG in KD patients suggest a wide variation in the yearly proportion with hemolysis, ranging from 0.36%18 to as high as 16%.21 More recently, a retrospective cohort study of 123 KD patients who had been assessed for hemolysis estimated the incidence at 15%.17
The exact mechanism by which anti-A and anti-B antibodies in IVIG cause hemolysis is not well known. The anti-A and anti-B antibodies are predominantly of the IgG2 subclass22 and these antibodies are presumed to cause destruction of erythrocytes via Fcγ receptors (FcγRs) on splenic macrophages.23 However, no association with hemolysis was determined for the common polymorphism in FcγRIIA (rs1801274, FCGR2A-p.His166Arg), which greatly influences the binding of IgG2.15 IgG2 does not bind other FcγRs well.24
In this study, we report on the incidence of IVIG-associated hemolysis in KD patients in Canada, where a sudden increase in incidence over time was observed, starting in November 2007. This increase in serious adverse events led to heightened surveillance and detailed study of the at-risk population to understand the mechanisms involved and identify ways to improve patient safety. We assessed parameters associated with hemolysis pre- and post-IVIG treatment as well as differences between different preparations of IVIG and report the prevalence of hemolysis in 2 cohorts (1 retrospective and 1 prospective cohort).
In subsequent functional studies, we elucidated the mechanism by which anti-A and anti-B antibodies cause erythrocyte destruction. The extravascular hemolysis was mediated by FcγRIIA and only occurred after prior activation of phagocytes. We observed clear differences between different IVIG preparations in vitro, which correlated with their risk in vivo. Our findings clearly indicate the clinical impact of changes in IVIG preparations. Such changes, resulting from product availability or altered production procedures, can cause sudden and unexpected health issues regarding both clinical efficacy and adverse effects.
Methods
IVIG products
Six different IVIG products are described in this study, of which 5 were used in the Hospital for Sick Children (Toronto, ON, Canada). The 6 products were numbered in chronological order of use in the clinic. An overview of the use of the products in this study as well as some basic characteristics is given in Table 1.
IVIG products included in this study
| IVIG preparation . | Included in cohort description . | Included in laboratory studies . | Formulation . | Concentration, w/v . |
|---|---|---|---|---|
| Product I | Yes (2006-2007) | No (not available) | Lyophilized | 5% upon reconstitution |
| Product II | Yes (2007-2013) | Yes | Liquid | 9%-11% |
| Product III | Yes (2007-2013) | Yes | Liquid | 9%-11% |
| Product IV | Yes (2007-2013) | Yes | Liquid | 10% |
| Product V | No | Yes | Liquid | 5% |
| Product VI | No (rescue product) | Sparingly (limited supplies) | Lyophilized | 5% or 10% upon reconstitution |
| IVIG preparation . | Included in cohort description . | Included in laboratory studies . | Formulation . | Concentration, w/v . |
|---|---|---|---|---|
| Product I | Yes (2006-2007) | No (not available) | Lyophilized | 5% upon reconstitution |
| Product II | Yes (2007-2013) | Yes | Liquid | 9%-11% |
| Product III | Yes (2007-2013) | Yes | Liquid | 9%-11% |
| Product IV | Yes (2007-2013) | Yes | Liquid | 10% |
| Product V | No | Yes | Liquid | 5% |
| Product VI | No (rescue product) | Sparingly (limited supplies) | Lyophilized | 5% or 10% upon reconstitution |
w/v, weight-to-volume ratio (%).
Patient cohorts
This single center retrospective study identified 581 patients diagnosed with KD at The Hospital for Sick Children (Toronto, ON, Canada), between January 2006 and December 2013. Starting in 2012; additional monitoring for hemolysis was started in the hospital as a standard safety procedure, which yielded additional information incorporated in the study; hence, we describe 2 different cohorts: cohort 1 from January 2006 to December 2011 (440 patients) and cohort 2 from January 2012 to December 2013 (141 patients).
The study was approved by the Hospital’s Research Ethics Board, and the requisite for individual consent was waived for the retrospective study design. Children with suspected KD underwent standardized KD assessments which included complete clinical examination together with laboratory testing, electrocardiogram and echocardiogram examinations as per protocol. All data were captured on standardized data capture forms in a prospective manner. Patients were diagnosed with KD in accordance with the most recent guidelines published by the American Heart Association,25 and admitted to hospital and commenced on an identical treatment protocol. The KD treatment protocol used at The Hospital for Sick Children included IVIG (2 g/kg; maximum, 70 g) plus high-dose acetylsalicylic acid (ASA; 80-100 mg/kg per day) until afebrile for 24 hours. Low-dose ASA, 3 to 5 mg/kg per day, was continued until follow-up echocardiogram at 6 weeks. Sequential echocardiogram studies were performed as described before.25,26 For the purposes of this analysis, hemolysis was defined a drop in hemoglobin of >2 g/dL post-IVIG infusion together with a positive direct antiglobulin test (DAT), and reticulocytosis. Blood smears were performed to look for morphologic evidence of hemolysis such as polychromasia or schistocytes.
From 2006 to 2007, 116 patients received product I. Once product I was totally discontinued in 2007, all IVIG preparations available in the institution were given in sequential order for KD patients. First product III alternating with product II, and subsequently with the introduction of product IV, the 3 products were given in the same sequential order with 173, 172, and 85 patients receiving product III, product II, and product IV, respectively, between 2008 and 2013.
Determination of antibody titers of IVIG preparations
Antibody titers were determined with the direct hemagglutination assay according to the European Pharmacopeia (version 9.0, July 2011, guideline 2.6.20; method B). Erythrocytes obtained from peripheral blood of healthy volunteers, positive for blood groups A, B, and O, RhD−, were washed 4 times in phosphate-buffered saline (PBS) and incubated with 5% papain (Biotest), for 15 minutes at 37°C. The cells were subsequently washed 4 times in PBS and diluted to a final concentration of 3% in PBS containing 0.2% bovine serum albumin (BSA).
A 2× dilution range of the IVIG preparations was made, starting with 25 g/L, in PBS plus 0.2% BSA, until a final dilution of 1/256. The diluted IVIG preparations were incubated with the papain-treated erythrocytes in a 1:1 ratio, in a 96-well plate. After mixing for 10 seconds, the cells were spun down for 1 minute at 650 rpm. The plate was placed at an angle of 60° for 10 minutes, after which the plate was rotated and placed at 60° for another 3 minutes. The lowest dilution where agglutination was observed, indicated the antibody titer of the IVIG preparation.
Erythrocyte isolation and opsonization
Peripheral blood from healthy volunteers with blood group O, A, B, or AB was obtained in heparinized tubes. Erythrocytes were isolated and opsonized with different IVIG products (product II, product III, product IV, product V, or product VI), all in a final concentration of 10 mg/mL, for 30 minutes at 37°C or left unopsonized. After washing twice, the deposition of antibodies was determined by staining with goat-anti-human immunoglobulin (Life Technologies) or with anti-subclass–specific antibodies and analyzed by flow cytometry using FACSCANTO II (BD Biosciences). The median fluorescence intensity was used as a measure of the amount of antibody binding.
Monocyte isolation and culture
Monocytes from healthy individuals were isolated from peripheral blood mononuclear cells by positive selection using the CD14 magnetic-activated cell separation (MACS) isolation kit (Miltenyi Biotec), according to manufacturer’s description. The isolated monocytes were cultured in 24-well (0.2 × 106 cells per well) plates in Iscove modified Dulbecco medium (IMDM; Gibco) supplemented with 10% fetal calf serum (FCS; Bodinco, Alkmaar), l-glutamine and penicillin and streptomycin, with either 50 ng/mL macrophage colony-stimulating factor (M-CSF; eBioscience) or 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech), for 9 days used for genotyping, immunophenotyping, and functional testing, as has been described before (see supplemental Methods).27
Phagocytosis assay
Phagocytosis assay was performed as described previously.27,28 In short, erythrocytes of blood group O or A were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Life Technologies) and opsonized with 1 of the IVIG batches as described in the previous section. Erythrocytes were added to the macrophages in a ratio of 10 erythrocytes to 1 macrophage. After 20-minute (M-CSF macrophages) or 120-minute (GM-CSF macrophages) incubation at 37°C, the reaction was stopped by putting the cells on ice and lysing the unphagocytosed erythrocytes. The percentage of macrophages that had taken up erythrocytes was determined by flow cytometry using FACSCANTO II (BD Biosciences). Inflammation was mimicked by prestimulating the macrophages with 2 ng/mL lipopolysaccharide (LPS) or 10 ng/mL Tumor necrosis factor-α (TNFα), 60 minutes prior to the start of the phagocytosis assay. In some of the experiments, FcγRs were blocked by adding 10 µg/mL of F(ab′)2 fragments of anti-CD32 clone 7.3 (Ancell) or anti-CD16 clone 3G8 (Ancell),27 5 minutes prior to the addition of erythrocytes. Isotype F(ab′)2 fragments (Ancell) were used as control.
IgG receptor genotyping and phenotyping
The copy number variations and single nucleotide polymorphisms of the low-affinity FCGRs of all donors were determined by multiplex ligation-dependent probe amplification (MLPA) assay, as described previously.29 Expression levels of IgG receptors were determined by flow cytometry as described previously for all activating receptors of this family (FcγRI [CD64], FcγRIIa [CD32a], FcγRIIIa [CD16], and the single inhibitory receptor (FcγRIIb [CD32b]).28 We excluded individuals carrying an FCGR2C open reading frame in the biological assays to avoid the contribution of the activating FcγRIIc in a minority of control individuals (15% to 20%) as confounding factor in our phagocytosis assay.
Statistical analysis
Data are described as means with standard deviations, medians and interquartile range, and frequencies as appropriate. Variables and categories with low frequency are reported in the descriptive statistics but were collapsed (when possible) or excluded from further analyses. Basic comparisons between hemolyzed and nonhemolyzed groups and between first (2006-2011) and second cohort (2012-2013) were performed using the Fisher exact test for all categorical variables and the Student t test assuming unequal variance between samples (Satterthwaite method). After excluding patients with unknown/combined IVIG preparations, comparisons between IVIG preparation groups were performed and the 4-way Wald χ2P value obtained from linear regression models for continuous characteristics and logistic regression models for dichotomized categorical variables is reported.
For all patients and patients in the second cohort (2012-2013), factors associated with hemolysis were identified and modeled in logistic regression models (maximum-likelihood method for parameter estimation). All statistical analyses were performed using SAS v9.4 (SAS statistical software).
Results
IVIG-associated hemolysis varied according to IVIG preparations
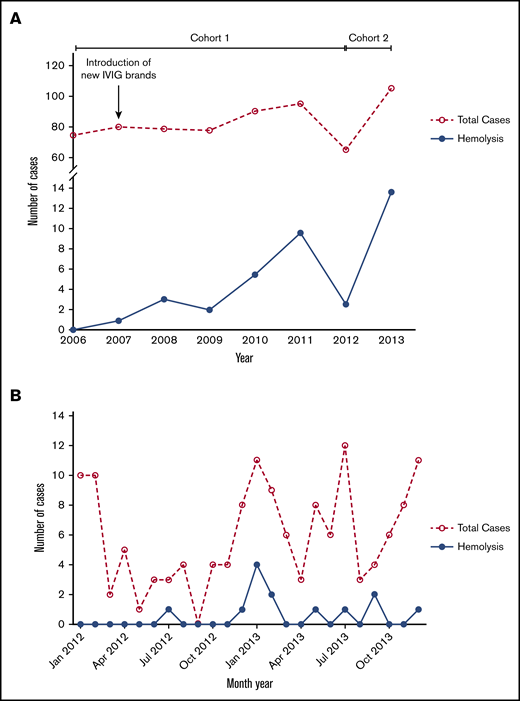
From 1999 to 2007, no cases of IVIG-associated hemolysis were observed in the study hospital (data not shown). Starting in November 2007, coincident with the introduction of new preparations of IVIG, an increasing incidence of IVIG-associated hemolysis was observed in children with KD (cohort 1; Figure 1A). Twenty cases of IVIG-associated hemolysis were observed between 2008 and 2011, 8 of which required erythrocyte transfusion, increasing over time as more new preparations of IVIG were used for treatment of KD. All cases of IVIG-associated hemolysis identified in this first cohort of patients came to attention when they required clinical intervention, thus, the true incidence of hemolysis was likely underreported given the lack of standardized testing for hemolytic markers for this previously rare adverse event. Starting in 2012, we instituted additional monitoring (ABO typing and hemolytic markers) for patient-safety reasons to further characterize this serious adverse event (SAE) and improve patient safety, identifying 13 cases in 2012 to 2013 (cohort 2; Figure 1B). The incidence of hemolysis corresponded directly with the incidence of KD as indicated by the analysis for the years 2012 to 2013 (Figure 1B). The only variable that differed between pre- and post-2007 were the commercial preparations of IVIG used to treat children with KD. Detailed analysis of the clinical features of the patient cohorts pre- and post-2007 did not show any difference for the patient characteristics (data not shown). The clinical features of the children treated with the various different preparations of IVIG, including product I, product II, product III, and product IV, did not differ (supplemental Tables 1-3), but the prevalence of hemolysis did vary per brand (Table 2), showing an increased incidence of hemolysis associated with product II, product III, and product IV when compared with product I (which was discontinued in 2007). This increased incidence could be found in the period of 2006 to 2011 as well as in the period of 2012 to 2013.
Increasing incidence of hemolysis. (A) Percentage of patients with IVIG-associated hemolysis among KD patients in the period 2006 to 2013. (B) Number of total KD cases and hemolysis cases per month in the period 2012 to 2013 (cohort 2).
Increasing incidence of hemolysis. (A) Percentage of patients with IVIG-associated hemolysis among KD patients in the period 2006 to 2013. (B) Number of total KD cases and hemolysis cases per month in the period 2012 to 2013 (cohort 2).
Incidence of hemolysis
| IVIG preparation . | Hemolysis, patients (%) . | |
|---|---|---|
| 2006-2011 . | 2012-2013 . | |
| Product I | 0/116 (0) | NA |
| Product II | 7/121 (6) | 5/51 (10) |
| Product III | 3/127 (2) | 2/46 (4) |
| Product IV | 6/46 (13) | 6/39 (15) |
| Unknown | 4/30 (13) | 0/5 (0) |
| IVIG preparation . | Hemolysis, patients (%) . | |
|---|---|---|
| 2006-2011 . | 2012-2013 . | |
| Product I | 0/116 (0) | NA |
| Product II | 7/121 (6) | 5/51 (10) |
| Product III | 3/127 (2) | 2/46 (4) |
| Product IV | 6/46 (13) | 6/39 (15) |
| Unknown | 4/30 (13) | 0/5 (0) |
NA, not applicable.
Risk factors for hemolysis include IVIG product, amount of IVIG infused, and blood group
For all patients in the second cohort (2012-2013) who received additional hemolytic marker testing, risk factors associated with hemolysis were identified and modeled in logistic regression models. The following risk factors for hemolysis were identified (Table 3), with infusion of >1 IVIG preparation as the highest risk factor with an odds ratio (OR) of 5.54, followed by the recipient having blood group AB (OR, 5.31; 95% confidence interval, 2.06-13.69 [P < .001]), then blood group A (OR, 3.98), receiving product IV as the IVIG preparation (OR, 2.86), then the total dose of IVIG received per kg body weight (OR, 2.2). Children with blood group AB had the highest risk of hemolysis with 33% to 42% of those with blood group AB developing hemolysis when receiving IVIG (Table 4). No children with blood group O developed IVIG-associated hemolysis.
Risk factors for hemolysis
| Risk factor . | OR . | 95% CI . | P . |
|---|---|---|---|
| >1 IVIG | 5.54 | 2.37-12.91 | <.001 |
| Blood group: AB vs other | 5.31 | 2.06-13.69 | .001 |
| Blood group: A vs other | 3.98 | 1.75-9.08 | .001 |
| Product IV | 2.86 | 1.26-6.49 | .01 |
| Total IVIG, g/kg | 2.20 | 1.44-3.35 | <.001 |
| Risk factor . | OR . | 95% CI . | P . |
|---|---|---|---|
| >1 IVIG | 5.54 | 2.37-12.91 | <.001 |
| Blood group: AB vs other | 5.31 | 2.06-13.69 | .001 |
| Blood group: A vs other | 3.98 | 1.75-9.08 | .001 |
| Product IV | 2.86 | 1.26-6.49 | .01 |
| Total IVIG, g/kg | 2.20 | 1.44-3.35 | <.001 |
CI, confidence interval; OR, odds ratio.
Incidence of hemolysis among different blood groups
| Blood group . | 2006-2011,* patients (%) . | 2012-2013,* patients (%) . |
|---|---|---|
| A | 12/46 (26) | 7/35 (20) |
| B | 0/17 (0) | 1/21 (5) |
| AB | 5/12 (42) | 4/12 (33) |
| O | 0/33 (0) | 0/45 (0) |
| Blood group . | 2006-2011,* patients (%) . | 2012-2013,* patients (%) . |
|---|---|---|
| A | 12/46 (26) | 7/35 (20) |
| B | 0/17 (0) | 1/21 (5) |
| AB | 5/12 (42) | 4/12 (33) |
| O | 0/33 (0) | 0/45 (0) |
In cohort I (2006-2011), 108 of 440 patients had ABO typing performed. In cohort II (2012-2013), 113 of 141 patients had ABO typing performed.
Hemolysis-associated IVIG preparations are also associated with reduced efficacy
In addition to an increase in the incidence of SAEs in the form of severe hemolysis associated with the new IVIG preparations, treatment efficacy was also adversely affected. The clinical features of the children treated with the various preparations of IVIG did not differ (supplemental Tables 1 and 2), but the clinical outcomes did vary per brand. Table 5 highlights several clinically relevant outcomes posttreatment, including significant differences between IVIG preparations with regard to days of fever post-IVIG infusion and the treatment failure rate. There was also an important trend toward need for acute readmission posttreatment of all the new preparations of IVIG (Table 5).
Outcome metrics
| . | Product I, n = 116 . | Product II, n = 164 . | Product III, n = 168 . | Product IV, n = 82 . | P . |
|---|---|---|---|---|---|
| Clinical outcome | |||||
| Days of fever post-IVIG, median (IQR) | 0 (0-1) | 1 (0-2) | 0 (0-2) | 1 (0-2) | .006 |
| Retreatment rate/IVIG failure, n (%) | 17 (15) | 54 (31) | 42 (24) | 20 (24) | .01 |
| Acute readmission, n (%) | 6 (5) | 19 (11) | 16 (9) | 9 (11) | .32 |
| Coronary outcome, n (%) | n = 115 | n = 164 | n = 168 | n = 82 | |
| No CAA z <2.5 | 103 (90) | 144 (88) | 144 (86) | 69 (84) | |
| CAA z >2.5 | 12 (10) | 20 (12) | 24 (14) | 13 (16) | .66 |
| . | Product I, n = 116 . | Product II, n = 164 . | Product III, n = 168 . | Product IV, n = 82 . | P . |
|---|---|---|---|---|---|
| Clinical outcome | |||||
| Days of fever post-IVIG, median (IQR) | 0 (0-1) | 1 (0-2) | 0 (0-2) | 1 (0-2) | .006 |
| Retreatment rate/IVIG failure, n (%) | 17 (15) | 54 (31) | 42 (24) | 20 (24) | .01 |
| Acute readmission, n (%) | 6 (5) | 19 (11) | 16 (9) | 9 (11) | .32 |
| Coronary outcome, n (%) | n = 115 | n = 164 | n = 168 | n = 82 | |
| No CAA z <2.5 | 103 (90) | 144 (88) | 144 (86) | 69 (84) | |
| CAA z >2.5 | 12 (10) | 20 (12) | 24 (14) | 13 (16) | .66 |
CAA, coronary artery aneurysm; IQR, interquartile range.
Hemolysis by IVIG is associated with anti-A and anti-B titers in the product
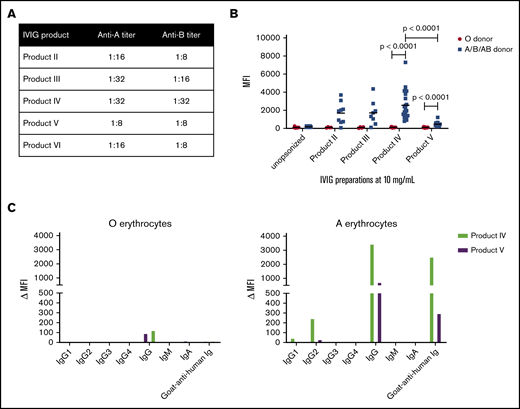
Upon determining the IgG titers against A and B blood group antigens in different IVIG products, we found that the titers of anti-A and anti-B IgG antibodies were highest in product IV (Figure 2A). Unfortunately, product I was no longer available for these tests. For product VI, a product that was similar to product I that was used as rescue IVIG product at the Hospital for Sick Children for patients needing retreatment, titers were relatively low. The lowest titers were found for product V. Product V was not used at the Hospital for Sick Children, but has been used for the treatment of KD and was never registered the SAE of severe hemolysis in a pharmacovigilance database.
Blood-group–specific antibodies present in IVIG products are of the IgG2subclass and cause opsonization of erythrocytes of the A, B, and AB blood group. (A) Antibody titers against A and B blood group antigens in product II, product III, product IV, product V, and product VI. (B) Erythrocytes of blood group O and blood groups A, B, or AB were opsonized with either of the 4 IVIG products mentioned (10 mg/mL) and subsequently stained with fluorescently labeled goat-anti-human immunoglobulin. The median fluorescence intensity (MFI) is shown (n = 14-23 erythrocyte donors). (C) Erythrocytes of the O and A blood group were opsonized with product IV or product V and stained with subclass- and isotype-specific antibodies: anti-IgG1, anti-IgG2, anti-IgG3, anti-IgG4, anti-IgG, anti-IgM, and anti-IgA. The MFI is shown. Graphs are representative graphs of 2 independent experiments.
Blood-group–specific antibodies present in IVIG products are of the IgG2subclass and cause opsonization of erythrocytes of the A, B, and AB blood group. (A) Antibody titers against A and B blood group antigens in product II, product III, product IV, product V, and product VI. (B) Erythrocytes of blood group O and blood groups A, B, or AB were opsonized with either of the 4 IVIG products mentioned (10 mg/mL) and subsequently stained with fluorescently labeled goat-anti-human immunoglobulin. The median fluorescence intensity (MFI) is shown (n = 14-23 erythrocyte donors). (C) Erythrocytes of the O and A blood group were opsonized with product IV or product V and stained with subclass- and isotype-specific antibodies: anti-IgG1, anti-IgG2, anti-IgG3, anti-IgG4, anti-IgG, anti-IgM, and anti-IgA. The MFI is shown. Graphs are representative graphs of 2 independent experiments.
Erythrocytes of the non-O blood group become opsonized by IVIG products
We opsonized erythrocytes of healthy donors with blood group O or non-O (ie, blood group A, B, or AB) with products II, III, IV, and V and determined the amount of immunoglobulin deposition on these erythrocytes by flow cytometry (Figure 2B). Products I and VI could not be tested because of very limited supplies. We found no antibody deposition on group O erythrocytes with any of the IVIG products tested. We found clear antibody binding to non-O–typed erythrocytes and this was significantly greater for product IV as compared with product V, which had the lowest opsonization.
Antibodies against blood groups A and B in IVIG preparations are of the IgG2 subclass and induce hemolysis via FcγR-mediated phagocytosis by macrophages
By staining with anti-IgG subclass-specific antibodies, anti-A and anti-B that caused opsonization of the erythrocytes were identified to be of the IgG2 subclass (Figure 2C), as confirmed in an IgG subclass-specific enzyme-linked immunosorbent assay (data not shown). We did not detect binding of IgM nor of IgA.
All patients who developed IVIG-associated hemolysis showed a positive DAT with anti-IgG, meaning there were antibodies present on erythrocytes. The eluent from DAT+ patients only showed the presence of anti-A or anti-B antibodies, and not other erythrocyte-specific antibodies. The DAT of patients with IVIG-associated hemolysis was negative with anti-complement. When complement deposition on erythrocytes was tested in vitro, only product IV at supraphysiological concentrations could induce some complement deposition, but this was not observed for the other products tested (supplemental Figure 1).
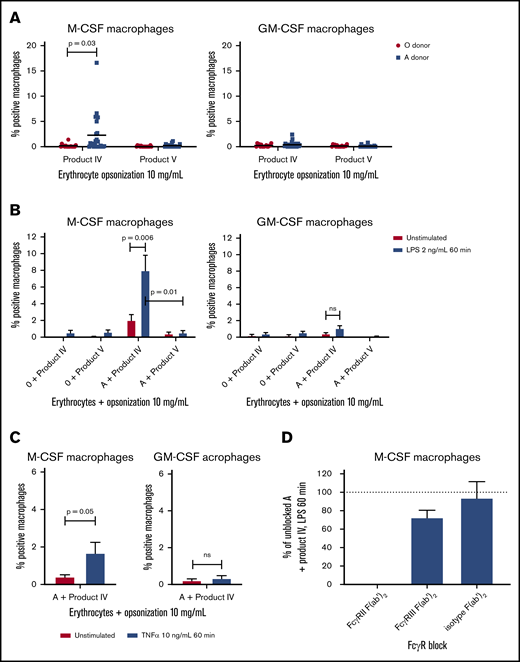
We then performed a phagocytosis assay in which opsonized erythrocytes were phagocytosed by monocyte-derived macrophages either cultured with M-CSF or GM-CSF to generate anti-inflammatory and proinflammatory macrophages.27 We compared product IV and product V because product V showed relatively low anti-A and anti-B titers and opsonization, whereas product IV showed higher anti-A and anti-B titers and the highest opsonization rate. The phagocytosis assay showed phagocytosis of product IV–opsonized blood group A erythrocytes in some cases, whereas there was neither phagocytosis of product IV–opsonized blood group O erythrocytes, nor any uptake of product V–opsonized blood group A erythrocytes (Figure 3A). These findings collectively indicate that the IVIG-related anemia in the patients was dependent on FcγR-mediated clearance of erythrocytes, whereas complement-mediated intravascular hemolysis did not contribute.
Product IV-opsonized erythrocytes are phagocytosed by LPS-stimulated M-CSF–cultured monocyte-derived macrophages in an FcγRIIa-dependent manner. (A) Phagocytosis of product IV and product V-opsonized O and A erythrocytes by monocyte-derived macrophages cultured with M-CSF (left) and GM-CSF (right). The percentage of positive macrophages is shown, corrected for unopsonized erythrocytes (n = 16-25 monocyte donors of n = 8-14 independent experiments). (B) Phagocytosis of product IV– and product V–opsonized erythrocytes by unstimulated and LPS-stimulated (2 ng/mL for 60 minutes) monocyte-derived macrophages, cultured in the presence of M-CSF (left) and GM-CSF (right). The percentage of positive macrophages, corrected for unopsonized erythrocytes, is shown. Data represent means and standard error of the mean (n = 6-12 monocyte donors of n = 3-6 independent experiments). (C) Phagocytosis of product IV– and product V–opsonized erythrocytes by unstimulated and TNFα-stimulated monocyte-derived macrophages, cultured in the presence of M-CSF (left) and GM-CSF (right). The percentage of positive macrophages, corrected for unopsonized erythrocytes, is shown. Data represent means and standard error of the mean (n = 6-7). (D) Phagocytosis of product IV–opsonized erythrocytes positive for the A blood group by LPS-stimulated monocyte-derived macrophages cultured in the presence of M-CSF. FcγRII and FcγRIII were blocked by F(ab′)2 fragments. The phagocytosis of product IV–opsonized erythrocytes by unblocked macrophages stimulated with LPS was set as 100%. Data represent means and standard error of the mean (n = 4).
Product IV-opsonized erythrocytes are phagocytosed by LPS-stimulated M-CSF–cultured monocyte-derived macrophages in an FcγRIIa-dependent manner. (A) Phagocytosis of product IV and product V-opsonized O and A erythrocytes by monocyte-derived macrophages cultured with M-CSF (left) and GM-CSF (right). The percentage of positive macrophages is shown, corrected for unopsonized erythrocytes (n = 16-25 monocyte donors of n = 8-14 independent experiments). (B) Phagocytosis of product IV– and product V–opsonized erythrocytes by unstimulated and LPS-stimulated (2 ng/mL for 60 minutes) monocyte-derived macrophages, cultured in the presence of M-CSF (left) and GM-CSF (right). The percentage of positive macrophages, corrected for unopsonized erythrocytes, is shown. Data represent means and standard error of the mean (n = 6-12 monocyte donors of n = 3-6 independent experiments). (C) Phagocytosis of product IV– and product V–opsonized erythrocytes by unstimulated and TNFα-stimulated monocyte-derived macrophages, cultured in the presence of M-CSF (left) and GM-CSF (right). The percentage of positive macrophages, corrected for unopsonized erythrocytes, is shown. Data represent means and standard error of the mean (n = 6-7). (D) Phagocytosis of product IV–opsonized erythrocytes positive for the A blood group by LPS-stimulated monocyte-derived macrophages cultured in the presence of M-CSF. FcγRII and FcγRIII were blocked by F(ab′)2 fragments. The phagocytosis of product IV–opsonized erythrocytes by unblocked macrophages stimulated with LPS was set as 100%. Data represent means and standard error of the mean (n = 4).
Phagocytosis by antibodies against blood groups A and B is enhanced by cell activation
Because product IV–opsonized erythrocytes were only phagocytosed in some cases, the inflammatory background of KD was considered relevant in the pathobiology. We recapitulated the danger signals in the in vivo inflammatory milieu by prestimulating the macrophages with LPS. Upon stimulation with LPS, phagocytosis of product IV–opsonized blood group A+ erythrocytes was significantly increased (Figure 3B). A similar increase was observed when the macrophages were preactivated with TNFα (Figure 3C).
We only observed this phenomenon of enhanced IgG-mediated uptake with M-CSF–cultured macrophages, but not with GM-CSF–cultured macrophages. Because GM-CSF has a different effect on the macrophage FcγR expression profile from M-CSF,27,28 we investigated which of the FcγRs was mostly involved in the phagocytosis of product IV–opsonized erythrocytes. The FcγR family consists of 1 high-affinity receptor FcγRI (CD64) and several low-affinity receptors FcγRII (CD32) and FcγRIII (CD16). All activating receptors of this family (FcγRI, FcγRIIa, FcγRIIc, FcγRIIIa) are capable of inducing phagocytosis by monocyte-derived macrophages.27,30 Apart from the activating IgG receptors, FcγRIIb (CD32b) is the only inhibitory receptor of this family, and FcγRIIIb functions as a decoy receptor but is not present on monocytes or macrophages.24,30 We found that blockade of FcγRIIa by F(ab′)2 fragments could completely inhibit the phagocytosis of product IV–opsonized erythrocytes by LPS-activated macrophages (Figure 3D). This confirms that the phagocytosis of product IV–opsonized erythrocytes is strictly mediated by FcγRIIa.
When we performed phagocytosis assays with product VI–opsonized erythrocytes, a product not known to be associated with the SAE of severe anemia, we did not observe any phagocytosis, neither in unstimulated nor in LPS-stimulated macrophages (supplemental Figure 2). The discontinued product I could not be tested. Product VI was in limited supply during the study period and used sparingly at The Hospital for Sick Children, Toronto, and only as a rescue therapy for patients needing retreatment. No additional hemolysis was observed with this product (unpublished observations).
Discussion
IVIG-associated hemolysis is a rare side effect of IVIG treatment and appears to have a relatively high prevalence in KD. Presumably, the high prevalence in KD derives from the fact that 2 important risk factors for developing IVIG-associated hemolysis, a high cumulative dosage of IVIG and underlying nature of the inflammatory disorder are always both present. We now show that the prevalence of IVIG-associated hemolysis in KD patients is associated with the titers of anti-A and anti-B in the preparation of IVIG that is used. The prevalence within our earlier retrospective study period varied greatly, from 0% (0 of 116) in the first years when only product I was used, to ∼6% (20 of 324) in the later years of the retrospective cohort when other preparations were introduced. In our prospective cohort, we found a prevalence of ∼9% (13 of 141). A great variance in the prevalence of IVIG-associated hemolysis in KD is also observed in the literature with prevalence estimates varying from 0.36% to 16%.14,17,18,21 Potentially, this variance reflects a true difference in prevalence that is caused by preparation differences in the brands of IVIG that are used. Of note, the only other retrospective study investigating the incidence of IVIG-associated hemolysis in KD, by Nolan et al,17 found a prevalence of 15%, using a lower cutoff for defining hemolysis (drop in hemoglobin of 1 g/dL vs a drop in hemoglobin of 2 g/dL in the current study), which could also explain the difference in prevalence.
Our study confirms the finding that IVIG-associated hemolysis occurs predominantly in recipients with blood groups A, B, or AB, but not O. Moreover, the increased risk of developing IVIG-associated hemolysis when a second dose of IVIG was given is in line with previous reports that show an increased risk in patients that received a high cumulative dose.14,16,17
The DAT showed anti-A or anti-B antibodies bound to the patient cells, but no complement deposition. These IVIG-opsonized erythrocytes were observed to be readily phagocytosed by monocyte-derived macrophages, which was enhanced in the presence of proinflammatory cytokines in a strictly FcγRIIa-dependent manner. Our study provides a 2-hit hypothesis to describe the disease mechanism for serious anemia upon IVIG infusion.
The observation that hemolysis often led to the need for transfusion can also be explained by the fact that patients with KD already suffer from anemia as part of the disease, as studies by Huang and Kao et al have reported anemia as 1 of the most common clinical features of KD.31,32 They reported that anemia in these patients, and patients suffering from other inflammatory diseases, are caused by elevated hepcidin levels. This anemia is unrelated to IVIG treatment, as it already exists prior to treatment, and cannot have influenced our conclusions because hemolysis was defined by a drop in hemoglobin levels. However, it stresses the importance of carefully monitoring for this adverse event in KD patients.
These safety concerns have prompted revisions to the approach to the treatment of children with KD with enhanced surveillance for these potential adverse effects, discussion of potential adverse effects including hemolytic anemia as part of the informed consent process prior to treatment, and incorporation in routine counseling and instructions on discharge from hospital. Because a change back to the old IVIG preparations was not possible at the study hospital, the treatment protocols have now been changed. For children who fail to respond to 1 IVIG treatment (supplemental Methods), a clear review of clinical and laboratory features for evidence of hemolysis is included in the management decisions regarding the next course of therapy. For those who have evidence of hemolysis, further IVIG therapy may be contraindicated and other retreatment options may be preferentially considered, including steroids. For those with no evidence of hemolysis, a preparation of IVIG that is not associated with hemolysis and does not demonstrate phagocytosis of opsonized erythrocytes (product VI) is used. Primary therapy continues with the widely available, but suboptimal IVIG preparations, as IVIG remains the gold standard for therapy of KD. However, the fact that optimal IVIG preparations have not become available again after the study period clearly points to the urgent need for more studies in KD to identify improved therapeutic options for affected children, including TNFα or IL-1 blockade.33-35
In a recent study, a new multisystem inflammatory syndrome with acute heart failure in children was described in the context of severe acute respiratory syndrome coronavirus 2.36 The syndrome resembles KD and all patients recovered with high-dose IVIG and steroids. We presume that the findings described in the present study also apply to patients suffering from the new multisystem inflammatory syndrome, and the same precautions should be taken in those patients.
We found increased titers of anti-A and anti-B antibodies in the preparations that caused IVIG-associated hemolysis, suggesting that a higher titer of the antibodies more easily leads to hemolysis, which is also observed in our in vitro model. This finding is in line with observations by Bellac et al,13 but not with observations by Berg et al.16 Potentially, these differences could be explained by the fact that in the period that was investigated by Berg et al,16 the indirect agglutination assay was used, which is known to be less reliable and to vary by 16-fold when the same samples were investigated in different laboratories,9 whereas our study and the study by Bellac et al used the direct agglutination assay which shows less variation between laboratories and is the current standard.
The current requirements for blood-group–specific antibody titers are <1:64 for anti-A and anti-B, which was satisfied by all IVIG preparations tested.6,37 Although the anti-A and anti-B titers of the product still met the regulatory requirements, the number of hemolysis cases upon treatment with all new IVIG preparations increased considerably. Apparently, the fact that a specific IVIG product meets the criteria does not guarantee that IVIG-associated hemolysis will not occur.6,38 Increased antibody binding resulting from relatively high anti-A or anti-B antibody titers was associated with an increased phagocytosis in vitro. In addition, the products with a higher titer showed a higher likelihood of causing IVIG-associated hemolysis. Therefore, it may be considered to personalize the choice of IVIG preparations on specific patient characteristics at the time of administration. For individuals with clear clinical and laboratory signs of severe inflammation, as observed in KD, it may be required to sharpen the requirements for maximum blood-group–specific antibody titers in IVIG. On the other hand, IVIG preparations with relatively high titers (1:32) do not seem to cause hemolysis in the absence of inflammation and can still be used safely as replacement therapy and as an immunomodulatory treatment in diseases without systemic inflammation at the moment of administration (for instance demyelinating disease or ITP), whereas the products with low titer (preferably 1:16 or less) can then be reserved for patients with known evidence of systemic inflammation.
This study should be viewed with some potential limitations due to its retrospective study design and that the different IVIG products were given in a sequential manner and not randomized, given the observational nature of this study. Despite these limitations, the clinical and laboratory features including blood group, did not differ between the treatment groups and confounding factors were adjusted for in our regression models lending confidence to our results and conclusions.
The ABO blood-group system has a polysaccharide-based nature. Based on studies with bacterial antigens, class-switching to the IgG2 subclass is to be expected against polysaccharide-based antigens.39 Accordingly, we found the IgG2 subclass to be involved in the observed IVIG-induced hemolytic anemia. However, we should emphasize that macrophages do not phagocytose IgG2-opsonized erythrocytes,28 unless activated as shown in this study to mimic the proinflammatory condition of KD. Although LPS or TNFα stimulation may increase the expression of FcγRs, such increase was not observed within a short time frame of 60 minutes preincubation (supplemental Figure 3), and most likely relates to increased cellular signaling for the IgG2-mediated phagocytosis by FcγRIIa. Previous studies in mice have shown an increase of IgG-opsonized erythrocyte phagocytosis upon LPS injection without any change in FcγR expression levels.39 Furthermore, increased expression levels of FcγRIIa induced by a prolonged inflammatory state may also have contributed to the increased phagocytosis in vivo because the expression level of FcγRIIa in active KD is indeed elevated as shown by increased mRNA levels.29,40
Upon priming macrophages with LPS or TNFα, we only observed an increase in IVIG-opsonized erythrocyte phagocytosis in the M-CSF–cultured but not in the GM-CSF–cultured macrophages. FcγRIIa (CD32a), which is the major activating IgG receptor on M-CSF macrophages,27 is able to recognize IgG2,24 and has been reported to be subject to intrinsic cell activation.39,41-43 GM-CSF macrophages phagocytose opsonized erythrocytes mainly via FcγRI (CD64),27 which does not recognize IgG2.24 Our data therefore indicate that phagocytosis occurred in a strictly FcγRIIa-dependent manner.
In conclusion, our findings show that anti-A and anti-B antibodies present in IVIG are able to opsonize erythrocytes of individuals with the A, B, or AB blood group. These antibodies are of the IgG2 isotype and cause phagocytosis by macrophages via an FcγRIIa-mediated mechanism, but only under inflammatory circumstances, as is the case during acute KD. Furthermore, these blood-group–specific antibodies present in IVIG preparations contribute to development of hemolytic anemia as a serious adverse effect of IVIG treatment. The finding that products with higher titers of anti-A and anti-B antibodies were associated with an increased risk of hemolysis in this patient group should prompt a discussion regarding whether stricter limits for maximum blood-group–specific antibody titers in IVIG, which is currently 1:64, should be defined. Knowing that products with a lower titer appear to be safer, products may be used in a more personalized manner, especially for patients with high levels of inflammation such as KD patients. Efforts to reduce anti-A and anti-B titers in IVIG products should be continued. Alternatively, products shown to be safe in past experiences could be further exclusively used in patients with active systemic inflammation.
Publication-related data will be shared with other investigators upon e-mail contact with the corresponding authors, Sietse Q. Nagelkerke and Christine W. Bruggeman, at s.nagelkerke@sanquin.nl and c.w.bruggeman@vu.nl.
Acknowledgment
C.W.B. was supported by a grant from the Dutch Ministry of Health awarded to T.W.K.: Sweet IVIg: a blend of different tastes (PPOP-12-001).
Authorship
Contribution: C.W.B. and S.Q.N. performed experiments, analyzed data, and wrote the manuscript; W.L. performed experiments and analyzed data; C.M., M.d.H., R.v.B., and B.W.M. discussed results and edited the manuscript; and R.S.M.Y. and T.W.K. designed the research, discussed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine W. Bruggeman, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: c.w.bruggeman@vu.nl; or Sietse Q. Nagelkerke, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: s.nagelkerke@sanquin.nl.
References
Author notes
C.W.B., S.Q.N., and W.L. contributed equally to this work.
R.S.M.Y. and T.W.K. contributed equally to this work.
Parts of this work have been published in chapter 5 of the thesis “Expression and function of Fc-g receptors” by Christine Bruggeman.44
The full-text version of this article contains a data supplement.