Abstract
After deep vein thrombosis (DVT) is diagnosed, prompt evaluation and therapeutic intervention are of paramount importance for improvement in patient-important outcomes. We systematically reviewed patient-important outcomes in patients with suspected DVT, including mortality, incidence of pulmonary embolism (PE) and DVT, major bleeding, intracranial hemorrhage, and postthrombotic sequelae. We searched the Cochrane Central Register of Controlled Trials, Ovid Medline, Embase for eligible studies, references lists of relevant reviews, registered trials, and relevant conference proceedings. Two investigators screened and abstracted data. Nine studies with 5126 patients were included for lower extremity DVT. Three studies with 500 patients were included for upper extremity DVT. Among patients with lower extremity DVT, 0.85% (95% confidence interval [CI], 0% to 2.10%) and 0% developed recurrent DVT and PE, respectively, at 3 months. Among patients with upper extremity DVT, 0.49% (95% CI, 0% to 1.16%) and 1.98% (95% CI, 0.62% to 3.33%) developed recurrent DVT and PE, respectively, at 3 months. No major bleeding events were reported for those anticoagulated, which is lower than in other systematic reviews. For both upper and lower extremity DVT, low pretest probability patients with a negative D-dimer had a comparable incidence of VTE at 3 months (∼1%) as patients with a negative ultrasound (US). At higher pretest probabilities, negative US testing with or without serial US appears to be the safer option. In this review, we summarized the outcomes of patients evaluated by various diagnostic pathways. In most instances, there was significant limitation due to small population size or lack of direct evidence of effects of using a specific pathway. This systematic review was registered at PROSPERO as CRD42018100502.
Introduction
The annual incidence of deep vein thrombosis (DVT) in the general population is 48 per 100 000 and can be associated with significant morbidity and mortality.1 Prompt evaluation and expeditious therapeutic intervention when DVT is confirmed is of paramount importance for optimal patient management. Various strategies are currently used for the evaluation of suspected DVT. The first step involves determining the pretest probability (PTP) of DVT, either formally using a clinical decision rule or informally through clinical judgment, prior to diagnostic testing. Following this, options for diagnostic tests include compression ultrasound (US) with or without Doppler US of the proximal leg veins (duplex US), whole-leg US, serial US, and D-dimer assays. These tests can be used alone or in sequence, depending on the pre-test probability.
While the diagnostic pathway is an important consideration in determining the optimal strategy for the evaluation of suspected DVT, this review focuses on patient-important outcomes. These outcomes assess the consequences of missed or incorrect diagnoses when anticoagulant treatments are mistakenly withheld or administered unnecessarily. Anticoagulant treatment of DVT is associated with risks of bleeding, with major bleeding (bleeding requiring red cell transfusion or intervention to stop bleeding or bleeding into a critical area, such as intracranial hemorrhage) being the most clinically relevant. Missed diagnoses can be associated with an increased risk of recurrent DVT, development of pulmonary embolism (PE), and postthrombotic syndrome. We conducted a systematic review and meta-analysis to evaluate the outcomes of patients with suspected DVT evaluated by various diagnostic pathways to determine the frequency of such outcomes. This systematic review was performed in conjunction with upper and lower extremity DVT test accuracy reviews that evaluated optimal diagnostic pathways based on PTP to inform an overall guideline on management of venous thromboembolism, detailed further in “Methods.”2-4
Methods
Determining outcomes of interest
This systematic review was undertaken for the purposes of informing the American Society of Hematology Guidelines on Management of Venous Thromboembolism, specifically diagnosis of venous thromboembolism (VTE). The review process began with a multidisciplinary panel coordinated by the American Society of Hematology Venous Thromboembolism Guideline Coordination Committee consisting of physicians with clinical and research expertise on the guideline topic, methodologists with expertise in evidence appraisal and guideline development, and patient representatives. These panel members developed clinical questions of interest regarding the diagnosis of VTE. The process is briefly described below; however, for detailed information, refer to the original guideline publication.2
After the primary questions were developed, the panel chairs developed diagnostic pathways that were refined through an iterative process with input from the panel (supplemental Material 1). The diagnostic strategies for DVT are based on the PTPs for individual patients, which provide an estimate of the expected prevalence of DVT at a population level. PTP can be determined using validated clinical decision rules, such as the Wells criteria.5 The original Wells criteria divided outpatients into 3 categories (low, intermediate, and high), and the dichotomized Wells criteria divided patients into 2 categories (unlikely and likely). In patients with suspected lower extremity DVT, the guideline assumed the prevalence in patients with low, intermediate, and high PTP to be 10%, 25% to 35%, and >50%, respectively. In patients with suspected upper extremity DVT using the Constans score,6 the guideline assumed the prevalence in patients with unlikely and likely PTP to be 10% and 40%, respectively. Therefore, when possible, outcomes were also further classified by PTP.
The panel then selected outcomes of interest for each question a priori, following the approach described in detail elsewhere.7 The panel brainstormed all possible outcomes and then rated their relative importance for decision making following the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) approach.8 During this rating process, the panel used definitions of the outcomes (“marker states”) that were developed for these guidelines by the McMaster Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Center. Rating outcomes by their relative importance can focus attention on those outcomes that are considered most important and help to resolve or clarify potential disagreements. The panel rated the following outcomes as critical for decision making across the DVT diagnosis questions: all-cause mortality, mortality from VTE, development of PE, development of DVT, development of recurrent DVT, development of postthrombotic sequelae, major bleeding, and intracranial hemorrhage; in addition to the diagnostic accuracy outcomes (false positive [FP], false negative [FN], true positive [TP], and true negative [TN] test results).
Data sources and searches
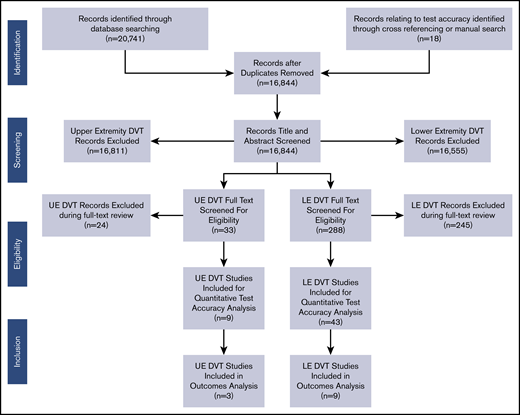
We conducted this systematic review in accordance with a prespecified registered protocol available on the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42018100502). We reported the results according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.9 The primary source of information was obtained from the studies that were included for the analysis of test accuracy, represented in Figure 1 and discussed in a separate paper.3,4 The secondary source included studies identified as having potential information for outcomes when the initial search was completed, regardless of whether the study contained test accuracy information. The final source was any additional studies suggested by experts in the field or other guidelines.
We performed an electronic search of CENTRAL (until May 2019), Ovid Medline (from 1976 to May 2019), and EMBASE (from 1974 to May 2019). A methodological filter was applied to limit retrieval of studies with data for test accuracy (prospective studies, cross-sectional studies, cohort studies, or abstracts and conference posters after 2014, limited to humans); a detailed search strategy provided in supplemental Material 2. We also reviewed the reference lists of relevant articles and reviews.
Study selection
We used the following eligibility criteria for the outcomes studies:
Study design.
Prospective studies, cross-sectional studies, cohort studies from January 1974 to May of 2019, or abstracts and conference posters after 2014 were used.
Participants.
All adult patients (age ≥18 years) suspected of having a symptomatic first or recurrent DVT were included.
Outcomes.
Studies assessing all-cause mortality, mortality from VTE, development of PE or DVT, development of recurrent DVT, or postthrombotic sequelae, major bleeding, and intracranial hemorrhage in patients with suspected first or recurrent episode of symptomatic DVT were included.
Language.
We included studies published in any language.
Publication status.
We reviewed all published and unpublished studies. Abstracts with relevant information were also reviewed.
Exclusion criteria.
Studies that did not assess or provide information on the outcomes of interest (eg, narratives, letters to editor without primary data), abstracts before 2014, duplicate populations, and studies with missing or incomplete outcomes results were excluded.
We used the following eligibility criteria for the studies with test accuracy information3,4 :
Study design.
Prospective studies, cross-sectional studies, cohort studies from January 1974 to May 2019, or abstracts and conference posters after 2014 were included.
Participants.
All adult patients (age ≥18 years) suspected of having a symptomatic first or recurrent DVT were included.
Outcomes.
Studies assessing test accuracy of whole leg US, compression US, serial US, and high-sensitivity quantitative D-dimer (Vidas, STA Liatest, TinaQuant, Innovance, and HemoSIL) to diagnose a first or recurrent symptomatic DVT were included.
Language.
We included studies published in any language.
Publication status.
We reviewed all published and unpublished studies. Abstracts with relevant information were also reviewed.
Exclusion criteria.
We excluded studies that did not assess test accuracy or had missing data (eg, narrative reviews, letters to the editor without primary data), abstracts before 2014, duplicate populations, and studies that included <100 patients, patients who were asymptomatic, pregnant, had superficial venous thrombosis, had atrial fibrillation, or were in the pediatric age group (age <18 years or if >80% of the population was <18 years or if mean age of group was <25 years). We also excluded studies using impedance plethysmography, Doppler US without compression, US of calf veins without examination of proximal veins, contrast computed tomography for upper extremity DVT, unacceptable reference standards (D-dimer alone, impedance plethysmography), as well as studies that did not use a standard D-dimer cutoff for lower extremity DVT or used D-dimer assays that are no longer in use or not highly sensitive for lower extremity DVT (SimpliRed, MDA, Asserachrom, Dimertest I, Enzygnost, Fibrinostika FbDP, Acculot, Wellcotest, and Minutex).
Two investigators (M. Bhatt or C.B., and Parth Patel.) independently screened the search results for articles based on title or title and abstract. Each of the investigators then independently assessed the eligibility of each article by using a pilot-tested, standardized form with written instructions. Disagreements were resolved by a third investigator (Payal Patel).
Data extraction and quality assessment
Two investigators (Parth Patel or M. Bhatt, and Payal Patel) independently extracted data using a pilot-tested, standardized form. Results of data extraction were then compared, and any discrepancy was resolved by discussion. A third reviewer additionally reviewed all study extractions and assessments (R.A.M.). When the same results were presented in >1 publication, we included the publication with the most complete results. If results were incomplete or unclear, we contacted study authors for additional information. We collected the following information from each study: study characteristics (author name, year of publication, country, language, number of centers, number of countries, and inclusion and exclusion criteria), patient characteristics (number, patients completing follow up, age, and comorbidities), diagnostic tests used and comparison characteristics (how the test was performed and interpreted), codiagnostic test(s) used, and outcomes. We collected information about funding sources, conflict-of-interest statements, consent, and ethics approval.
Data synthesis and analysis
The outcomes information from each study was combined quantitatively from different studies and reviews. Information was abstracted with respect to those diagnostic pathways determined to be of interest by the panel in the primary test accuracy systematic review (Tables 1 and 2). The data were further stratified by PTP and patients anticoagulated (TP/FP) compared with those not anticoagulated (TN/FN). This information was compared with the information abstracted from additional resources such as systematic reviews, treatment guidelines that reviewed outcomes, a targeted search of general outcomes studies, and a survey of panel opinion (Table 3; supplemental Material 4).10-12
Outcomes in patients with suspected lower extremity DVT from test accuracy studies
| Pathway* . | PTP . | Studies . | All-cause mortality . | Mortality from VTE at 3 mo . | Incidence of PE at 3 mo . | Incidence of DVT at 3 mo . | Overall VTE at 3 mo . | Postthrombotic syndrome . | Major bleeding . | Intracranial hemorrhage . |
|---|---|---|---|---|---|---|---|---|---|---|
| Anticoagulated (TP/FP population) | ||||||||||
| B | Low | Positive compression US | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) |
| A, E | Low | Positive compression US alone or positive serial US after initial compression US | 7/84 (8.33%; 95% CI, 2.42%-14.24%) | 0/84 (0%) | 0/79 (0%) | 4/79 (5.06%; 95% CI, 0.23%-9.90%) | 4/79 (5.06%; 95% CI, 0.23%-9.90%) | 0/14 (0%) | 0/14 (0%) | 0/14 (0%) |
| H | Low | Positive DD → positive US | 5/80 (6.25%; 95% CI, 0.95%-11.55%) | 0/80 (0%) | 0/80 (0%) | 0/80 (0%) | 0/80 (0%) | NR | NR | NR |
| G | Low | Positive DD | NR | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) |
| B | High | Positive compression US | 2/24 (8.33%; 95% CI, 0%-19.39%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) |
| G | High | Positive DD | NR | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) |
| Overall positive for DVT and initiated on anticoagulation | 6.79%; 95% CI, 3.36%-10.23% (14/206) | 0% (0/476) | 0% (0/471) | 0.85% 95% CI, 0%-2.10%) (4/471) | 0.85%; 95% CI, 0%-2.10%) (4/471) | 0% (0/326) | 0% (0/326) | 0% (0/326) | ||
| Not anticoagulated (TN/FN population) | ||||||||||
| B | Low | Negative compression US | 10/612 (1.63%; 95% CI 0.63%-2.64%) | 0/884 (0%) | 5/884 (0.57%; 95% CI, 0.07%-1.06%) | 10/884 (1.13%; 95% CI, 0.43%-1.83%) | 15/884 (1.70%; 95% CI, 0.89%-2.67%) | 0/277 (0%) | 0/277 (0%) | 0/277 (0%) |
| A, E | Low | Negative compression US or negative serial US after initial compression US | 8/426 (1.88%; 95% CI, 0.59%-3.17%) | 0/1212 (0%) | 3/1212 (0.25%; 95% CI, 0%-0.53%) | 6/1212 (0.50%; 95% CI, 0.10%-0.89%) | 9/1212 (0.74%; 95% CI, 0.26%-1.23%) | 0/91 (0%) | 0/91 (0%) | 0/91 (0%) |
| G | Low | Negative DD | 0/120 (0%) | 0/338 (0%) | 0/338 (0%) | 2/338 (1.03%; 95% CI 0%-1.41%) | 2/338 (1.03%; 95% CI 0%-1.41%) | 0/120 (0%) | 0/120 (0%) | 0/120 (0%) |
| H | Low | Positive DD → negative compression US | NR | 0/85 | 0/85 (0%) | 0/85 (0%) | 0/85 (0%) | NR | NR | NR |
| B | Intermediate | Negative compression US | NR | 0/363 (0%) | 2/363 (0.55%; 95% CI, 0%-1.31%) | 4/363 (1.10%; 95% CI 0.03%-2.18%) | 6/363 (1.65%; 95% CI, 0.34%-2.96%) | NR | NR | NR |
| A | Intermediate | Negative whole leg US | 2/154 (1.30%; 95% CI, 0%-2.18%) | 0/148 (0%) | 0/148 (0%) | 1/148 (0.68%; 95% CI, 0%-2.0%) | 1/148 (0.68%; 95% CI, 0%-2.0%) | NR | NR | NR |
| G | Intermediate | Negative DD | 0/49 (0%) | 0/49 (0%) | 0/49 (0%) | 2/49 (4.08%; 95% CI 0%-9.62%) | 2/49 (4.08%; 95% CI 0%-9.62%) | NR | NR | NR |
| I | Intermediate | Positive DD → negative whole leg US | NR | NR | 0/464 (0%) | 13/464 (2.80%; 95% CI, 1.30%-4.30%) | 13/464 (2.80%; 95% CI, 1.30%-4.30%) | NR | NR | NR |
| C, D | Intermediate | Negative compression US → negative DD | 4/598 (0.67%; 95% CI, 0.02%-1.32%) | 0/679 (0%) | 0/679 (0%) | 1/679 (0.15%; 95% CI, 0%-0.44%) | 1/679 (0.15%; 95% CI, 0%-0.44%) | NR | NR | NR |
| D | Intermediate | Negative compression US → positive DD → negative serial US | 1/83 (1.20%; 95% CI, 0%-3.55%) | 1/180 (0.56%; 95% CI, 0%-1.64%) | 2/180 (1.11%; 95% CI; 0%-2.64%) | 0/180 (0%) | 2/180 (1.11%; 95% CI; 0%-2.64%) | NR | NR | NR |
| B | High | Negative compression US | 0/25 (0%) | 0/25 (0%) | 0/29 (0%) | 4/29 (13.8%; 95% CI 1.24%-26.34%) | 4/29 (13.8%; 95% CI 1.24%-26.34%) | 0/29 (0%) | 0/29 (0%) | 0/29 (0%) |
| G | High | Negative DD | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) |
| Pathway* . | PTP . | Studies . | All-cause mortality . | Mortality from VTE at 3 mo . | Incidence of PE at 3 mo . | Incidence of DVT at 3 mo . | Overall VTE at 3 mo . | Postthrombotic syndrome . | Major bleeding . | Intracranial hemorrhage . |
|---|---|---|---|---|---|---|---|---|---|---|
| Anticoagulated (TP/FP population) | ||||||||||
| B | Low | Positive compression US | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) | 0/18 (0%) |
| A, E | Low | Positive compression US alone or positive serial US after initial compression US | 7/84 (8.33%; 95% CI, 2.42%-14.24%) | 0/84 (0%) | 0/79 (0%) | 4/79 (5.06%; 95% CI, 0.23%-9.90%) | 4/79 (5.06%; 95% CI, 0.23%-9.90%) | 0/14 (0%) | 0/14 (0%) | 0/14 (0%) |
| H | Low | Positive DD → positive US | 5/80 (6.25%; 95% CI, 0.95%-11.55%) | 0/80 (0%) | 0/80 (0%) | 0/80 (0%) | 0/80 (0%) | NR | NR | NR |
| G | Low | Positive DD | NR | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) | 0/202 (0%) |
| B | High | Positive compression US | 2/24 (8.33%; 95% CI, 0%-19.39%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) |
| G | High | Positive DD | NR | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) | 0/68 (0%) |
| Overall positive for DVT and initiated on anticoagulation | 6.79%; 95% CI, 3.36%-10.23% (14/206) | 0% (0/476) | 0% (0/471) | 0.85% 95% CI, 0%-2.10%) (4/471) | 0.85%; 95% CI, 0%-2.10%) (4/471) | 0% (0/326) | 0% (0/326) | 0% (0/326) | ||
| Not anticoagulated (TN/FN population) | ||||||||||
| B | Low | Negative compression US | 10/612 (1.63%; 95% CI 0.63%-2.64%) | 0/884 (0%) | 5/884 (0.57%; 95% CI, 0.07%-1.06%) | 10/884 (1.13%; 95% CI, 0.43%-1.83%) | 15/884 (1.70%; 95% CI, 0.89%-2.67%) | 0/277 (0%) | 0/277 (0%) | 0/277 (0%) |
| A, E | Low | Negative compression US or negative serial US after initial compression US | 8/426 (1.88%; 95% CI, 0.59%-3.17%) | 0/1212 (0%) | 3/1212 (0.25%; 95% CI, 0%-0.53%) | 6/1212 (0.50%; 95% CI, 0.10%-0.89%) | 9/1212 (0.74%; 95% CI, 0.26%-1.23%) | 0/91 (0%) | 0/91 (0%) | 0/91 (0%) |
| G | Low | Negative DD | 0/120 (0%) | 0/338 (0%) | 0/338 (0%) | 2/338 (1.03%; 95% CI 0%-1.41%) | 2/338 (1.03%; 95% CI 0%-1.41%) | 0/120 (0%) | 0/120 (0%) | 0/120 (0%) |
| H | Low | Positive DD → negative compression US | NR | 0/85 | 0/85 (0%) | 0/85 (0%) | 0/85 (0%) | NR | NR | NR |
| B | Intermediate | Negative compression US | NR | 0/363 (0%) | 2/363 (0.55%; 95% CI, 0%-1.31%) | 4/363 (1.10%; 95% CI 0.03%-2.18%) | 6/363 (1.65%; 95% CI, 0.34%-2.96%) | NR | NR | NR |
| A | Intermediate | Negative whole leg US | 2/154 (1.30%; 95% CI, 0%-2.18%) | 0/148 (0%) | 0/148 (0%) | 1/148 (0.68%; 95% CI, 0%-2.0%) | 1/148 (0.68%; 95% CI, 0%-2.0%) | NR | NR | NR |
| G | Intermediate | Negative DD | 0/49 (0%) | 0/49 (0%) | 0/49 (0%) | 2/49 (4.08%; 95% CI 0%-9.62%) | 2/49 (4.08%; 95% CI 0%-9.62%) | NR | NR | NR |
| I | Intermediate | Positive DD → negative whole leg US | NR | NR | 0/464 (0%) | 13/464 (2.80%; 95% CI, 1.30%-4.30%) | 13/464 (2.80%; 95% CI, 1.30%-4.30%) | NR | NR | NR |
| C, D | Intermediate | Negative compression US → negative DD | 4/598 (0.67%; 95% CI, 0.02%-1.32%) | 0/679 (0%) | 0/679 (0%) | 1/679 (0.15%; 95% CI, 0%-0.44%) | 1/679 (0.15%; 95% CI, 0%-0.44%) | NR | NR | NR |
| D | Intermediate | Negative compression US → positive DD → negative serial US | 1/83 (1.20%; 95% CI, 0%-3.55%) | 1/180 (0.56%; 95% CI, 0%-1.64%) | 2/180 (1.11%; 95% CI; 0%-2.64%) | 0/180 (0%) | 2/180 (1.11%; 95% CI; 0%-2.64%) | NR | NR | NR |
| B | High | Negative compression US | 0/25 (0%) | 0/25 (0%) | 0/29 (0%) | 4/29 (13.8%; 95% CI 1.24%-26.34%) | 4/29 (13.8%; 95% CI 1.24%-26.34%) | 0/29 (0%) | 0/29 (0%) | 0/29 (0%) |
| G | High | Negative DD | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) |
Total of 9 studies,13-21 5126 patients.
DD, D-dimer; NR, not reported.
Pathways labeled by letter available with diagrams in supplemental Material 1.
Outcomes in patients with suspected upper extremity DVT from test accuracy studies
| Pathway* . | PTP . | Studies . | Mortality: all cause . | Mortality: from UE DVT at 3 mo . | PE at 3 mo . | Mortality: from PE at 3 mo . | UE DVT at 3 mo . | Overall VTE at 3 mo . | Post thrombotic syndrome . | Major bleeding . | Intracranial hemorrhage . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticoagulated (TP/FP population) | |||||||||||
| E | Unlikely | Positive DD | 2/203 (0.99%; 95% CI, 0%-2.34%) | 0/112 (0%) | 0/112 (0%) | 0/112 (0%) | 0/112 (0%) | 0/112 (0%) | NR | NR | NR |
| F | Unlikely | Positive DD → Positive US | 2/203 (0.99%; 95% CI, 0.37%-2.34%) | 0/37 (0%) | 0/37 (0%) | 0/37 (0%) | 0/37 (0%) | 0/37 (0%) | NR | NR | NR |
| A | Likely | Positive US | 10/261 (3.83%; 95% CI,1.50%- 6.16%) | 0/144 (0%) | 8/137 (5.84%; 95% CI, 1.91%-9.76%) | 0/173 (0%) | 2/142 (1.41%; 95% CI,0%-3.34%) | 10/142 (7.04%; 95% CI, 2.83%-11.25%) | 0/58 (0%) | NR | NR |
| D | Likely | Positive US or Negative US → positive DD → positive serial US | 5/203 (2.46%; 95% CI, 0.33%-4.59%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | NR | NR | NR |
| Overall positive for DVT and initiated on anticoagulation | 1.03%; 95% CI, 0.36%- 1.71% (9/870) | 0% (0/412) | 1.98%; 95% CI, 0.62%-3.33% (8/405) | 0% (0/441) | 0.49%; 95% CI, 0%-1.16% (2/410) | 2.44%; 95% CI, 0.95%-3.93% (10/410) | 0% (0/58) | NR | NR | ||
| Not anticoagulated (TN/FN population) | |||||||||||
| E | Unlikely | Negative DD | 0/117 (0%) | 0/204 (0%) | 0/204 (0%) | 0/204 (0%) | 2/204 (0.98%; 95% CI, 0%-2.33%) | 2/204 (0.98%; 95% CI, 0%-2.33%) | 0/117 (0%) | NR | NR |
| A | Unlikely | Negative US | 0/182 (0%) | 0/182 (0%) | 0/182 (0%) | 0/182 (0%) | 3/182 (1.65%;95% CI, 0%-3.49%) | 3/182 (1.65%;95% CI, 0%-3.49%) | 0/182 (0%) | NR | NR |
| B | Unlikely | Negative US → and negative serial US | 0/180 (0%) | 0/180 (0%) | 0/180 (0%) | 0/180 (0%) | 1/180 (0.56%; 95% CI, 0%-1.64%) | 1/180 (0.56%; 95% CI, 0%-1.64%) | 0/180 (0%) | NR | NR |
| F | Unlikely | Negative DD or Positive DD → negative US | 2/203 (0.99%; 95% CI, 0%-2.34%) | 0/162 (0%) | 0/162 (0%) | 0/162 (0%) | 0/162 (0%) | 0/162 (0%) | NR | NR | NR |
| A | Likely | Negative US | 10/261 (3.83%; 95% CI, 1.50%-6.16%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | 5/119 (4.20%; 95% CI, 0.59%-7.80%) | 5/119 (4.20%; 95% CI, 0.59%-7.80%) | 0/58 (0%) | NR | NR |
| D | Likely | Negative US → positive DD → negative serial US | 5/203 (2.46%; 95% CI, 0.33%-4.59%) | 0/84 (0%) | 0/84 (0%) | 0/84 (0%) | 1/84 (1.19%; 95% CI, 0%-3.50%) | 1/84 (1.19%; 95% CI, 0%-3.50%) | NR | NR | NR |
| Pathway* . | PTP . | Studies . | Mortality: all cause . | Mortality: from UE DVT at 3 mo . | PE at 3 mo . | Mortality: from PE at 3 mo . | UE DVT at 3 mo . | Overall VTE at 3 mo . | Post thrombotic syndrome . | Major bleeding . | Intracranial hemorrhage . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticoagulated (TP/FP population) | |||||||||||
| E | Unlikely | Positive DD | 2/203 (0.99%; 95% CI, 0%-2.34%) | 0/112 (0%) | 0/112 (0%) | 0/112 (0%) | 0/112 (0%) | 0/112 (0%) | NR | NR | NR |
| F | Unlikely | Positive DD → Positive US | 2/203 (0.99%; 95% CI, 0.37%-2.34%) | 0/37 (0%) | 0/37 (0%) | 0/37 (0%) | 0/37 (0%) | 0/37 (0%) | NR | NR | NR |
| A | Likely | Positive US | 10/261 (3.83%; 95% CI,1.50%- 6.16%) | 0/144 (0%) | 8/137 (5.84%; 95% CI, 1.91%-9.76%) | 0/173 (0%) | 2/142 (1.41%; 95% CI,0%-3.34%) | 10/142 (7.04%; 95% CI, 2.83%-11.25%) | 0/58 (0%) | NR | NR |
| D | Likely | Positive US or Negative US → positive DD → positive serial US | 5/203 (2.46%; 95% CI, 0.33%-4.59%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | NR | NR | NR |
| Overall positive for DVT and initiated on anticoagulation | 1.03%; 95% CI, 0.36%- 1.71% (9/870) | 0% (0/412) | 1.98%; 95% CI, 0.62%-3.33% (8/405) | 0% (0/441) | 0.49%; 95% CI, 0%-1.16% (2/410) | 2.44%; 95% CI, 0.95%-3.93% (10/410) | 0% (0/58) | NR | NR | ||
| Not anticoagulated (TN/FN population) | |||||||||||
| E | Unlikely | Negative DD | 0/117 (0%) | 0/204 (0%) | 0/204 (0%) | 0/204 (0%) | 2/204 (0.98%; 95% CI, 0%-2.33%) | 2/204 (0.98%; 95% CI, 0%-2.33%) | 0/117 (0%) | NR | NR |
| A | Unlikely | Negative US | 0/182 (0%) | 0/182 (0%) | 0/182 (0%) | 0/182 (0%) | 3/182 (1.65%;95% CI, 0%-3.49%) | 3/182 (1.65%;95% CI, 0%-3.49%) | 0/182 (0%) | NR | NR |
| B | Unlikely | Negative US → and negative serial US | 0/180 (0%) | 0/180 (0%) | 0/180 (0%) | 0/180 (0%) | 1/180 (0.56%; 95% CI, 0%-1.64%) | 1/180 (0.56%; 95% CI, 0%-1.64%) | 0/180 (0%) | NR | NR |
| F | Unlikely | Negative DD or Positive DD → negative US | 2/203 (0.99%; 95% CI, 0%-2.34%) | 0/162 (0%) | 0/162 (0%) | 0/162 (0%) | 0/162 (0%) | 0/162 (0%) | NR | NR | NR |
| A | Likely | Negative US | 10/261 (3.83%; 95% CI, 1.50%-6.16%) | 0/119 (0%) | 0/119 (0%) | 0/119 (0%) | 5/119 (4.20%; 95% CI, 0.59%-7.80%) | 5/119 (4.20%; 95% CI, 0.59%-7.80%) | 0/58 (0%) | NR | NR |
| D | Likely | Negative US → positive DD → negative serial US | 5/203 (2.46%; 95% CI, 0.33%-4.59%) | 0/84 (0%) | 0/84 (0%) | 0/84 (0%) | 1/84 (1.19%; 95% CI, 0%-3.50%) | 1/84 (1.19%; 95% CI, 0%-3.50%) | NR | NR | NR |
Total of 3 studies,22-24 500 patients.
Pathways labeled by letter available with diagrams in supplemental Material 1.
Outcomes in patients with suspected lower extremity DVT from various sources
| Test accuracy results . | Consequences . | Results from published SR10 . | Results from treatment guideline11 . | Targeted search of outcomes studies12 . | Panel survey results . |
|---|---|---|---|---|---|
| TP | Mortality: all cause | NR | 2.0% (6 mo) | 6% (3 mo) | 8.8% (1 y) |
| Mortality: from DVT at 3-12 mo | 0.4% (PE mortality, 3 mo) | NR | 0% (3 mo) | 4.3% (1 y) | |
| Recurrence of DVT on anticoagulation at 3-12 mo | 3.8% (3 mo) | 1.3% (6 mo) | 4.6% (3 mo) | 5.6% (1 y) | |
| Development of PE | NR | 1.0% (6 mo) | 1.0% (3 mo) | 5.8% (1 y) | |
| Major bleeding | NR | 2.1% (6 mo) | 3.1% (3 mo) | 4.2% (1 y) | |
| Fatal major bleeding | NR | 0.2% (6 mo) | NR | NR | |
| Hemorrhagic stroke | NR | NR | NR | 2.0 (ICH; 1 y) | |
| FP | Mortality: all cause | NR | NR | NR | NR |
| Major bleeding | NR | 2.1% (6 mo) | 3.1% (3 mo) | 4.2% (1 y) | |
| Fatal major bleeding | NR | 0.2% (6 mo) | NR | NR | |
| TN | Mortality: from DVT at 3-12 mo | NR | NR | 0% | NR |
| FN | Mortality: all cause | NR | NR | NR | 18.4% (1 y) |
| Mortality: from DVT at 3-12 mo | NR | NR | NR | 10.5% (1 y) | |
| Recurrence of DVT on at 3-12 mo | NR | NR | NR | 11.0% (1 y) |
| Test accuracy results . | Consequences . | Results from published SR10 . | Results from treatment guideline11 . | Targeted search of outcomes studies12 . | Panel survey results . |
|---|---|---|---|---|---|
| TP | Mortality: all cause | NR | 2.0% (6 mo) | 6% (3 mo) | 8.8% (1 y) |
| Mortality: from DVT at 3-12 mo | 0.4% (PE mortality, 3 mo) | NR | 0% (3 mo) | 4.3% (1 y) | |
| Recurrence of DVT on anticoagulation at 3-12 mo | 3.8% (3 mo) | 1.3% (6 mo) | 4.6% (3 mo) | 5.6% (1 y) | |
| Development of PE | NR | 1.0% (6 mo) | 1.0% (3 mo) | 5.8% (1 y) | |
| Major bleeding | NR | 2.1% (6 mo) | 3.1% (3 mo) | 4.2% (1 y) | |
| Fatal major bleeding | NR | 0.2% (6 mo) | NR | NR | |
| Hemorrhagic stroke | NR | NR | NR | 2.0 (ICH; 1 y) | |
| FP | Mortality: all cause | NR | NR | NR | NR |
| Major bleeding | NR | 2.1% (6 mo) | 3.1% (3 mo) | 4.2% (1 y) | |
| Fatal major bleeding | NR | 0.2% (6 mo) | NR | NR | |
| TN | Mortality: from DVT at 3-12 mo | NR | NR | 0% | NR |
| FN | Mortality: all cause | NR | NR | NR | 18.4% (1 y) |
| Mortality: from DVT at 3-12 mo | NR | NR | NR | 10.5% (1 y) | |
| Recurrence of DVT on at 3-12 mo | NR | NR | NR | 11.0% (1 y) |
SR, systematic review.
Results
Search results
Among the 15 435 nonduplicate records identified from the electronic database search, 320 full-text articles were retrieved after title and abstract screening. After exclusion of articles that were not relevant, a total of 12 studies were included.13-24 A summary of the outcomes is presented below with detailed abstraction available in supplemental Material 3.
Lower extremity DVT.
Patients diagnosed with DVT and initiated on anticoagulation had an overall mortality of 6.79% (14/206, 95% confidence interval [CI], 3.36% to 10.23%), 0% (0/476) mortality from VTE at 3 months, 0% (0/471) incidence of PE at 3 months, 0.85% (4/471, 95% CI, 0% to 2.10%) recurrent DVT at 3 months, 0% (0/326) incidence of postthrombotic sequelae, 0% (0/326) major bleeding events, and 0% (0/326) intracranial hemorrhage. There were no important differences between the various diagnostic pathways (Table 1).
For patients with negative diagnostic testing who did not receive anticoagulant treatment, outcome information must be interpreted with respect to PTP and the diagnostic algorithm assessed. For patients with a low PTP of lower extremity DVT, negative proximal compression US alone had an overall VTE rate of 1.70% (15/844, 95% CI, 0.89% to 2.67%) at 3 months, comparable to the 1.03% (2/338, 95% CI, 0% to 1.41%) of patients with negative D-dimer. The rate is reduced to 0.74% (9/1212, 0.74%; 95% CI, 0.26% to 1.23%) in patients discharged from either initial negative proximal compression US or initial positive proximal compression US with subsequent negative serial US (Table 1).
In patients with intermediate or high PTP, the differences are more important. Patients with intermediate PTP and negative compression US have a VTE rate of 1.65% (6/363, 1.65%; 95 CI, 0.34% to 2.96%) at 3 months compared with 0.68% (1/148, 0.68%; 95% CI, 0% to 2.0%) for negative whole leg US and 4.08% (2/49, 4.08%; 95% CI, 0% to 9.62%) for negative D-dimer. This may indicate that D-dimer has an increased FN rate in this population. The rate of VTE for patients with negative serial US after initial proximal compression US in the intermediate PTP population was determined to be 1.11% (2/180, 1.11%; 95% CI, 0% to 2.64%). This may indicate adding serial US may improve accuracy of proximal compression US to make it comparable to that of whole leg US. Finally, in patients with high PTP, negative compression US had a 13.8% (4/29, 13.8%; 95% CI, 1.24% to 26.34%) incidence of VTE at 3 months. We did not identify test accuracy studies with outcomes reported on serial US testing for high PTP patients.
Upper extremity DVT.
Patients discharged with anticoagulation had an overall mortality of 1.03% (9/870; 95% CI, 0.36% to 1.71%), 0% (0/412) mortality from upper extremity DVT at 3 months, 0% (0/441) mortality from PE at 3 months, 1.98% (8/405; 95% CI, 0.62% to 3.33%) incidence of PE at 3 months, 0.49% (2/410, 95% CI, 0% to 1.16%) incidence of recurrent upper extremity DVT at 3 months, and 0% (0/58) incidence of postthrombotic sequelae at 3 months (Table 2).
For patients discharged without treatment due to negative diagnostic test results, outcome information must be interpreted with respect to PTP and the diagnostic algorithm assessed. For the population with an unlikely probability of upper extremity DVT, the overall VTE rate was 0.98% (2/204; 95% CI, 0% to 2.33%) at 3 months for patients with a negative D-dimer, while the overall VTE rate was 1.65% (3/182; 95% CI, 0% to 3.49%) in patients with negative US. The rate is reduced to 0.56% (1/180; 95% CI, 0% to 1.64%) in patients with negative US followed by negative serial US. In patients with likely PTP, those with a negative US have a VTE rate of 4.20% (5/119; 95% CI, 0.59% to 7.80%) at 3 months compared with 1.19% (1/84; 95% CI, 0% to 3.50%) in patients with negative serial US testing (Table 2).
Additional sources of data.
This information was compared with the information abstracted from additional resources such as suggested systematic reviews, treatment guidelines that reviewed outcomes, a targeted search of general outcomes studies, and a survey of panel opinion (Table 3; supplemental Material 4).
Lower extremity DVT.
Patients discharged with anticoagulation had an all-cause mortality of 2.0% at 6 months from a VTE treatment guideline, 6% at 3 months from a targeted search of outcomes studies, and 8.8% at 1 year from a survey of the panel members. Mortality from DVT was reported at 0% at 3 months from a targeted search of outcomes studies and 4.3% at 1 year from a survey of panel members. Major bleeding was reported at 2.1% at 6 months from the VTE treatment guideline, 3.1% at 3 months from a targeted search of outcomes studies, and 4.2% at 1 year from a survey of panel members.
For patients discharged without treatment due to negative diagnostic test results, all-cause mortality was not reported in multiple sources, with a panel survey reporting 18.4% at 1 year. Mortality from DVT reported at 0% in a targeted search of outcome studies and not reported in the remainder of sources. Recurrence of DVT was reported by panel survey at 11.0% at 1 year, with no report from remainder of sources. Findings are summarized in Table 3.
Upper extremity DVT.
Patients discharged with anticoagulation had an all-cause mortality of 22% at 3 months based on a targeted search of outcomes studies and 14% at 1 year by panel survey results. Mortality from DVT was reported at 10.4% at 1 year by a survey of panel members, with no reporting by other sources. Major bleeding was reported at 6.0% at 3 months by a targeted search of outcomes studies and 4.0% at 1 year by panel survey results.
For patients discharged without treatment due to negative diagnostic test results, there were no outcomes reported by a published systematic review, VTE treatment guideline, or targeted search of outcomes studies. All reports were from a survey of panel members with all-cause mortality 12.5% at 1 year, mortality from DVT 9.5% at 1 year, and recurrence of DVT 19.25% at 1 year. Findings are summarized in supplemental Material 4.
Discussion
There are a variety of diagnostic tests that can be used in the diagnosis of suspected DVT, and these tests can be used in isolation or in combination in a diagnostic pathway to rule in or exclude a diagnosis. This review is one of the first to provide a systematic overview of patient-important outcomes in patients with suspected DVT, both overall and associated with different diagnostic strategies. The findings can assist decision-makers to estimate impacts on patients, they can assist researchers in identifying gaps and plan for adequately powered studies with outcomes beyond diagnostic accuracy.
For both upper and lower extremity DVT, low-PTP patients with negative D-dimer had a lower incidence of VTE at 3 months than patients with negative US. This suggests that D-dimer is comparable to US in lower PTP patients and can be used to rule out lower extremity DVT in this population. At higher PTP, it may be safer to go directly to US testing rather than starting with D-dimer for both lower and upper extremity DVT. Serial US provides additional benefit. Patients deemed to have DVT, a population composed of TPs and FPs, received anticoagulant treatment often without major bleeding events in the studies included in this review. The lack of major bleedings may relate to the fact that identified studies primarily focused on test accuracy of VTE and thus are typically shorter in duration. Therefore, these studies may not have adequately captured bleeding outcomes and bleeding rates may be underreported. This contrasts with findings from a treatment guideline and targeted search of outcomes studies listed in Table 3, which reported that lower extremity DVT had 2.1% and 3.1% risk of major bleeding events, respectively. Similarly, a targeted search of outcome studies on upper extremity DVT reported a 6.0% risk for major bleeding events (supplemental Material 4). Based on our data, patients with upper extremity DVT had a 0.49% (95% CI, 0% to 1.16%) and 1.98% (95% CI, 0.62% to 3.33%) risk of developing recurrent DVT and PE, respectively, at 3 months. Patients with lower extremity DVT had a 0.85% (95% CI, 0% to 2.10%) and 0% risk of developing recurrent DVT and PE, respectively, at 3 months.
This review has several strengths. The comprehensive search makes it unlikely that relevant studies were missed. All steps, including initial screening, study selection, and data abstraction, were performed independently in duplicate to minimize any potential biases. Additionally, we did not limit our review by language and we translated non–English articles. We analyzed sources of bias and explored reasons for diversity in the published literature. Outcomes were stratified by PTP and diagnostic pathway used to assist in decision-making capacity. When compared with other systematic reviews, which typically reviewed consecutive patients with DVT or PE in the Registry Informatizado de la Enfermedad TromboEmbólica (RIETE), outcomes were reported without stratification.25,26
There are a few limitations of the present review. The sample size of the patients from the test accuracy studies were often too small to accurately assess outcomes. In addition, when outcomes are reported in accuracy studies, they generally focus on the safety among these who were designated as negative; thus, outcomes are primarily reported in patients with negative testing. The bleeding risks in patients with positive testing and treatment with anticoagulation may not have been scrutinized to the same degree. Furthermore, diagnostic accuracy studies are not typically designed to capture outcomes, unlike therapeutic studies. Therefore, definitions of outcomes, methods of measurements, and duration of follow-up may not have been clear or consistent across studies. This may lead to under- or overreporting of events. Finally, in most instances, there was no direct evidence that assessed the effect of using one diagnostic pathway versus another on patient outcomes or directly comparing the accuracy of different diagnostic pathways. In some circumstances where the diagnostic pathway of interest was evaluated, details regarding the specific number of patients for each pathway were not provided. Additionally, in many instances, the review was limited to the outcomes reported in the studies that differed from the prioritized outcomes by the guideline panel. To combat this limitation, the original guideline publication did compare test accuracy of diagnostic tests in sequence to individual diagnostic tests alone (ie, D-dimer followed by computed tomography in contrast to D-dimer alone), further characterized by PTP into low, intermediate, and high PTP. The final recommendations for a diagnostic pathway were based on information provided in this review on patient-centered outcomes and based on information provided in the test accuracy review.2-4 In addition to these reviews, the panel considered information on the overall certainty in the evidence, including certainty in the diagnostic test accuracy results, patients’ values and preferences, balance of desirable and undesirable effects, resource implications, feasibility, acceptability, and equity considerations.
Acknowledgments
This systematic review was conducted to support the development of the American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. The entire guideline development process was funded by the American Society of Hematology. Through the McMaster GRADE Center, some researchers received salary (Payal Patel, C.B., M. Bhatt, W.W., Parth Patel, H.B., and J.V.) or grant support (R.A.M. and H.J.S.), and others participated to fulfill requirements of an academic degree or program or volunteered their time.
Authorship
Contribution: Payal Patel contributed to study design, search strategy, study selection, data extraction, statistical analysis, and drafting the report; Payal Patel, M. Bhatt, C.B., H.B., R.N., and Parth Patel contributed to study design, study selection, data extraction, statistical analysis, and critical revision of the report; R. Khatib, C.C.M., Y.Z., I.E.-I., J.V., H.A., W.B., M. Baig, R. Kehar, A.M., R.P., A.S., M.T., and D.W. contributed to study selection, data extraction, and statistical analysis of data; and W.W., W.L., S.M.B., E.L., G.L.G., M.R., H.J.S., and R.A.M. contributed to the study design, interpretation of the results, and critical revision of the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reem A. Mustafa, Division of Nephrology and Hypertension, Department of Medicine, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS; e-mail: rmustafa@kumc.edu.
References
Author notes
The full-text version of this article contains a data supplement.