Abstract
Imatinib, the first tyrosine kinase inhibitor (TKI) for the treatment of chronic myeloid leukemia (CML), improves overall survival (OS), but the introduction of newer TKIs requires the definition of the optimal first-line TKI for newly diagnosed Philadelphia chromosome–positive (Ph+) chronic-phase (CP) CML. This systematic review of randomized controlled trials (RCTs) compares the efficacy and safety of imatinib vs second-generation (dasatinib, nilotinib, bosutinib) and third-generation TKIs (ponatinib) in adults with newly diagnosed Ph+ CP CML, concentrating on OS, progression-free survival (PFS), and hematological and nonhematological adverse events. The quality of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method. Seven RCTs published between 1990 and 2019 (involving 3262 participants) satisfied the eligibility criteria. Two RCTs (imatinib vs nilotinib and imatinib vs dasatinib) found no difference in 5-year OS or PFS. Second- and third-generation TKIs improved 3-month major molecular responses (relative risk [RR], 4.28; 95% confidence interval [CI], 2.20-8.32) and other efficacy outcomes, decreased accelerated/blastic-phase transformations (RR, 0.44; 95% CI, 0.26-0.74), but were associated with more cases of thrombocytopenia (RR, 1.57; 95% CI, 1.20-2.05), cardiovascular events (RR, 2.54; 95% CI, 1.49-4.33), and pancreatic (RR, 2.29; 95% CI, 1.32-3.96) and hepatic effects (RR, 3.51; 95% CI 1.55-7.92). GRADE showed that the certainty of the evidence ranged from high to moderate. This study shows that, in comparison with imatinib, second- and third-generation TKIs improve clinical responses, but the safer toxicity profile of imatinib may make it a better option for patients with comorbidities.
Introduction
The overall incidence of chronic myeloid leukemia (CML) in Europe was 1.10 per 100 000 in 2000 to 2002 (∼4 per 100 000 among patients aged 75-99 years)1,2 and, according to the population-based registry of the European Treatment and Outcome Study (EUTOS), accelerated-phase (AP) and blastic-phase (BP) CML, respectively, account for ∼4% and 2% of diagnoses.3
Imatinib (Glivec; Novartis), the first tyrosine kinase inhibitor (TKI), completely changed patients’ life expectancy.4 It was approved in 2001 (in Europe and the United States) for all CML phases and, as its patent has expired, is now available as a generic drug.5
Two second-generation TKIs, dasatinib (Sprycel; Bristol-Myers Squibb) and nilotinib (Tasigna; Novartis), were approved in the United States and Europe in 2006 to 2007 as second-line treatment of patients resistant to, or intolerant of, previous treatment (including imatinib): dasatinib was approved in all CML phases and nilotinib was only approved in the chronic phase (CP) or AP.6-9 Since 2010 and 2011, both have been authorized for the first-line treatment of newly diagnosed Philadelphia chromosome–positive (Ph+) adult CP CML.10,11 Another second-generation TKI, bosutinib (Bosulif; Pfizer), was licensed in the United States in 2012 and in Europe in 2013 for the treatment of adults with CP, AP, or BP Ph+ CML who are resistant to, or intolerant of, previous treatment with 1 or more TKIs12-14 ; in December 2017, the indication was extended in the United States to include newly diagnosed adult Ph+ CP CML.
The third-generation TKI, ponatinib (Iclusig; ARIAD), was approved in the United States in 2012 and in Europe in 2013 for the treatment of adults with CP, AP, or BP Ph+ CML who are resistant to, or intolerant of, other TKIs; it was also approved for those with CP, AP, or BP Ph+ CML and the T315I mutation, which is known to be involved in resistance to imatinib.15,16
The introduction of TKIs has dramatically improved patient survival from a median of 3 to 6 years before imatinib; now, CML is considered a chronic disease. An update of the International Randomized Study of Interferon and STI571 (IRIS) showed that the estimated 10-year overall survival (OS) of imatinib-treated patients was 83.3%,17 and most studies based on population cancer registries have shown that 5-year survival has increased since TKIs became available. The Girona population-based cancer registry showed that 5-year relative survival in 1994 to 2008 was 80% among CML patients treated with TKIs, and only 44% among those not receiving TKIs.18 The European Cancer Registry-based Study on Survival and Care of Cancer Patients 5 (EUROCARE-5) showed that 5-year relative survival increased throughout Europe between 1997 and 2008 (particularly after 2000), although there were considerable differences between countries.19 Survival increased in all age groups, particularly among patients aged <65 years, but there was also a 10% increase among patients aged ≥75 years.19 However, other authors have found a smaller increase among the elderly, possibly because of the underuse of imatinib and second-generation TKIs (imatinib was received by 89.7% of the patients aged 20-59 years, 75.0% of those aged 60-79 years, and 46.0% of those aged ≥80 years).20
It is very difficult to define the best first-line TKI for treating adults with newly diagnosed Ph+ CP CML. According to the 2017 European Society for Medical Oncology (ESMO) guidelines, the decision should be based on treatment goals, age, comorbidities, and the adverse event (AE) profile of the available drugs: 5-year OS is similar among patients receiving first-line imatinib, dasatinib, or nilotinib (85% to 95%) [I, A],21 and therapeutic goals should be discussed with patients and defined before first-line drug selection, taking age, comorbidities, and drug toxicity into account [V, B].21 This is also in line with the 2018 National Comprehensive Cancer Network (NCCN) guidelines, which include bosutinib as an option for first-line treatment.22 However, if it is not possible to demonstrate the advantage of 1 drug over another in a superiority study, that does not mean that the 2 drugs are equal.
The aim of this study is to provide comprehensive, updated, and precise information regarding the comparative efficacy and safety of TKIs (imatinib vs dasatinib, nilotinib, bosutinib, ponatinib), with particular emphasis on drug-related AEs.
Methods
Objectives
This systematic review and meta-analysis of randomized controlled trials (RCTs) compares the efficacy and safety of imatinib vs second-generation (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs in adults with newly diagnosed Ph+ CP CML. The considered outcomes were OS, progression-free survival (PFS), response, and safety (hematological and nonhematological AEs).
Study design
The review protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD42016032903),23 and the study was carried out in accordance with Cochrane collaboration procedures and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.24,25
Eligibility criteria
We considered published and unpublished RCTs comparing imatinib at any dose with second-generation (dasatinib, nilotinib, or bosutinib at any dose) or third-generation (ponatinib at any dose) TKIs in adults aged ≥18 years old with newly diagnosed (within the previous 6 months) CP CML and the Ph chromosome in peripheral blood or bone marrow detected by means of cytogenetics or fluorescent in situ hybridization, or the Abelson 1 (Abl1) oncogene with a breakpoint cluster region (Bcr) translocation (Bcr-Abl) detected by means of reverse transcription polymerase chain reaction.1 The studies had to exclude patients previously treated with a TKI (except those treated with imatinib for no more than 2 weeks) and those receiving any other CML medical treatment, except hydroxyurea and/or anagrelide, for >2 weeks before study entry. Studies comparing imatinib with any treatment other than TKI (eg, interferon-α, chemotherapy, stem cell transplantation, or best supportive care) were excluded, as were nonrandomized studies.
Trials for which it was unclear whether the method of randomization provided adequate allocation concealment (quasi-RCTs) and open-label RCTs were considered, but their quality was taken into account.
Primary outcomes
OS and PFS were primary outcomes.
Secondary outcomes
Efficacy.
Efficacy was determined by the following end points.
A complete cytogenetic response (CCyR) (ie, the absence of Ph+ metaphases determined on the basis of G-banding in at least 20 cells in metaphase per BM sample), and a confirmed complete cytogenetic response (cCCyR) (ie, a documented CCyR on 2 consecutive occasions separated by an interval of at least 28 days), after 3, 12, 24, 36, 48, and 60 months of treatment.
An early molecular response (EMR; Bcr-Abl International Scale [IS] = 10%) after 3 months of treatment.
A major molecular response (MMR; Bcr-Abl IS = 0.1%) after 3, 12, 24, 36, 48, and 60 months of therapy.
A molecular response 4 (MR4; Bcr-Abl IS = 0.01%) and molecular response 4.5 (MR4.5; Bcr-Abl IS = 0.0032%) at any time during treatment.
Transformation to AP and BP CML (excluding clonal evolution) at any time during treatment.26
Treatment discontinuation at any time.
Safety.
Safety was evaluated by grade 3-4 hematological AEs (anemia, neutropenia, thrombocytopenia) at any time or by grade 3-4 nonhematological AEs (hypertension; cardiovascular events; pulmonary arterial hypertension; pancreatic, hepatic, cutaneous and gastrointestinal effects; fluid retention [pleural or pericardial effusion]; bleeding; musculoskeletal disorders; neuropathy; ocular toxicity; infectious events; metabolic syndrome; diabetes) at any time.
Searches
The PUBMED, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.Gov databases were systematically searched for published or unpublished reports in any language concerning RCTs or quasi-RCTs conducted between 1990 and 28 May 2019, and their reference lists, recent systematic reviews, meta-analyses, and guidelines were examined for any other pertinent studies. We also screened the abstracts of the main conferences in the field (the American Society of Hematology [ASH], the American Society of Clinical Oncology [ASCO], the European Haematology Association [EHA], the Italian Society of Hematology [SIE]), and invited all of the manufacturers of the TKIs in question to provide any unpublished material.
The full electronic search strategy is described in the “Pubmed” and “Embase Session Results” sections of the supplemental Data.
Study selection and data extraction
Two authors (C.V. and A.F.) independently screened the titles and abstracts to identify potentially eligible papers, and confirmed their eligibility by reading the full text. Publications concerning the same RCT were collected, and the same authors independently extracted the following information about each RCT: study year, study design, and outcomes in the intervention and control arms. Any disagreement was resolved by means of discussion with a third reviewer (R.B.).
Risk-of-bias assessment and quality-of-evidence grading
C.V. and A.F. independently assessed the studies using the Cochrane risk-of-bias tool.27 The considered domains were selection bias (sequence generation, allocation concealment), detection bias (blinding of outcome assessors), performance bias (blinding of participants and personnel), attrition bias (incomplete outcome data), and reporting bias. The results were described using the risk-of-bias summary (ie, authors’ judgement of each risk of bias for each study) and the risk-of-bias graph (ie, authors’ judgements of each risk-of-bias item presented as percentages of all studies), with the for-profit bias under “other bias.”
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) method was used to assess the quality of the evidence: that is, the extent to which we were confident that the estimate of effect was correct.28 The evaluation considered 5 dimensions: study limitations (eg, the risk of bias), inconsistency, indirectness, imprecision, and publication bias.28 The grading was decreased by 1 (serious [−1]) or 2 levels (very serious [−2]) in the case of a risk of bias, inconsistent study results, uncertain directness (the correspondence between population, intervention, or outcomes measured in the studies actually found and those under consideration in our systematic review),23 imprecise pooled estimates, and strongly suspected publication bias (−1).28
Strategy for data synthesis
Whenever available, we extracted data at different time points (eg, 3, 12, 18, 24, 36, 48, 60, and 72 months) from the intention-to-treat analyses. Only aggregate qualitative and quantitative data were summarized. For each study and outcome, a record was made of the number of subjects in the control (imatinib) and intervention group (dasatinib, nilotinib, bosutinib, ponatinib) and the number of events. We used the preposition “by” to indicate cumulative responses (the sum of events occurring between starting treatment and the specified time point), and “at” to indicate the number of events observed at the specified time point.
The data were pooled using the random-effects method (because of the heterogeneity of the studies) and the Mantel-Haenszel (M-H) method (because some studies had a small number of patients and events). Hazard ratios (HRs) were used for OS and PFS (Kaplan-Meier survival curves), and relative risk (RR), or the risk ratio, for other dichotomous variables. Forest and funnel plots were provided for each outcome included in the meta-analysis.
Sensitivity analyses were used to establish the best threshold for including secondary outcomes in the meta-analysis (the minimum number of RCTs reporting a given outcome) on the basis of the completeness of the information concerning each outcome, and the meta-analysis value after removing that outcome (data not shown). It was decided that a threshold of 5 should be used, which was applied to all outcomes before pooling the data.
Study heterogeneity was evaluated by calculating the I2 statistic (I2), with little, moderate, and substantial heterogeneity being, respectively, indicated by I2 values of <50%, 50% to 75%, and >75%. Ninety-five percent confidence interval (CI) and 2-sided P values were calculated for each result.
All of the analyses were made using Review Manager 5.3 statistical software; the GRADE evidence profile was created on a GRADEpro GDT platform.28
Results
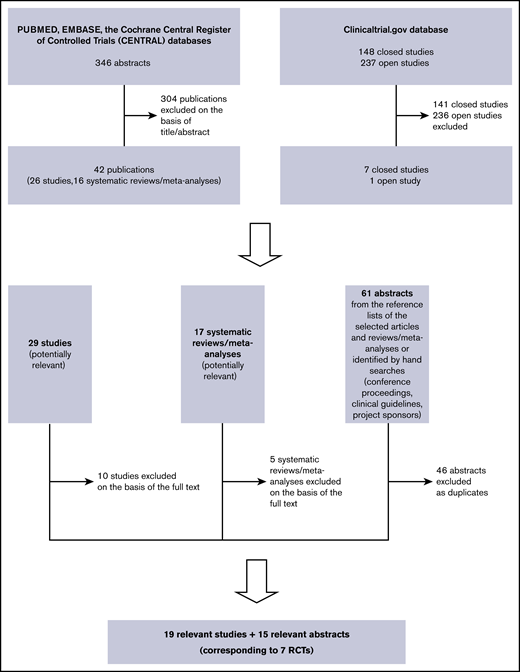
Figure 1 shows the search strategy flowchart and the included and excluded studies. Table 1 descriptively summarizes the included studies.6-9,13,14,16,29-55 Only 2 RCTs reported OS and PFS up to 60 months (data not pooled), and only 1 reported OS up to 72 months.7,9,37,47-49 Five-year OS in the ENEST study was similar in the imatinib and nilotinib groups (91.7% vs 93.7% for 300 mg of nilotinib [HR, 0.80; 95% CI, 0.43-1.50], and 96.2% for 400 mg of nilotinib [HR, 0.44; 95% CI, 0.21-0.93]), and the same was true of 5-year PFS (91.0% vs, respectively, 92.2% [HR, 0.89; 95% CI, 0.50-1.58] and 95.8% [HR, 0.44; 95% CI, 0.22-0.90]).9,47 Similar results were obtained in the DASISION study comparing imatinib with dasatinib: 5-year OS, 90.0% vs 91.0% (HR, 1.01; 95% CI, 0.58-1.73), and 5-year PFS, 86.0% vs 85.0% (HR, 1.06; 95% CI, 0.68-1.66).7,37 The first follow-up (FU) time point at which it would have been possible to analyze pooled OS and PFS was 36 months (data available from 3 RCTs), but it was not clinically relevant.6,7,9,34,44 Consequently, on the basis of the proportional hazards assumption, we pooled the HRs roughly extracted from the printed OS and PFS curves of Radich et al (36-month FU) and the ENEST and DASISION HRs (60-month FU), but the result was not statistically significant (OS [HR, 0.78; 95% CI, 0.54-1.11]; PFS [HR, 0.85; 95% CI, 0.62-1.16]).6,7,9,37,47,56
Summary of the findings of the included studies
| Follow-up, mo*,† . | Year/ref. . | Journal/full paper or abstract . | Outcome evaluated . |
|---|---|---|---|
| RCT: DASISION (D); 519 patients | |||
| Imatinib: 49 (18-78) y; Male: 163 (63%) Dasatinib: 46 (18-84) y; Male: 144 (56%) | |||
| 12 | 2010/7 | N Engl J Med/Full paper | PFS 12 mo; OS 12 mo; CCyR by 3 mo, by 12 mo; cCCyR by 12 mo; MMR by 3 mo, by 12 mo; AP/BP; DIS |
| 18 | 2010/29 | Blood/Abstract | PFS 18 mo; OS 18 mo; AP/BP |
| 24 | 2011/30 | J Clin Oncol/Abstract | PFS 18 mo; OS 18 mo; MR4.5; AP/BP |
| 24 | 2011/31 | Blood/Abstract | EMR at 3 mo; MMR by 3 mo, by 24 mo; MR4; MR4.5 |
| 24 | 2012/32 | J Clin Oncol/Abstract | MR4 |
| 24 | 2012/33 | Blood/Full paper | PFS 24 mo; OS 24 mo; CCyR at 12 mo, by 12 mo, at 24 mo, by 24 mo; cCCyR by 24 mo; MMR at 12 mo, by 12 mo, at 24 mo, by 24 mo; MR4.5; AP/BP; DIS |
| 36 | 2014/34 | Blood/Full paper | PFS 36 mo; OS 36 mo; CCyR at 3 mo, at 12 mo, by 36 mo; cCCyR by 36 mo; EMR at 3 mo; MMR at 12 mo, by 36; MR4; MR4.5; AP/BP; DIS |
| 48 | 2013/35 | Blood/Abstract | PFS 48 mo; OS 48 mo; EMR at 3 mo; MMR at 12 mo, by 48 mo; MR4; MR4.5, AP/BP |
| 60 | 2014/36 | Blood/Abstract | PFS 60 mo; OS 60 mo; cCCyR by 60 mo; EMR at 3 mo; MMR by 60 mo; MR4.5; AP/BP |
| 60 | 2016/37 | J Clin Oncol/Full paper | PFS 60 mo; OS 60 mo; cCCyR at 60 mo; EMR at 3 mo; MMR by 12 mo, by 24 mo, by 36 mo, by 48 mo, by 60 mo, at 60 mo; MR4; MR4.5; DIS |
| Quasi-RCT: NCT00070499 (D); 253 patients | |||
| Imatinib: 50 (19-89) y; Male: 72 (59%) Dasatinib: 47 (18-90) y; Male: 74 (60%) | |||
| 12 (36 mo for PFS, OS) | 2012/6 | Blood/Full paper | PFS 36 mo; OS 36 mo; CCyR by 12 mo; MMR at 12 mo; MR4; MR4.5; AP/BP; DIS |
| RCT: NordCML006 (D); 46 patients | |||
| Imatinib: 60 (38-77) y; Male: 15 (63%) Dasatinib: 54 (29-71) y; Male: 7 (32%) | |||
| 18 | 2013/38 | Leukemia/Full paper | CCyr at 12 mo |
| 24 | 2013/39 | Blood/Abstract | MMR at 3 mo, by 12 mo, by 24 mo; MR4; MR4.5; AP/BP; DIS |
| 36 | 2015/8 | Eur J Haematol/Full paper | CCyR at 3 mo, at 12 mo; EMR at 3mo; MR4; AP/BP; DIS |
| RCT: ENEST (N); 846 patients | |||
| Imatinib: 46 (18-80) y; Male: 158 (56%) Nilotinib 300 mg: 47 (18-85) y; Male: 158 (56%) Nilotinib 400 mg: 47 (18-81) y; Male: 175 (62%) | |||
| 12 | 2010/40 | J Clin Oncol/Abstract | CCyR by 12 mo; MMR at 12 mo; MR4; MR4.5 |
| 12 | 2010/9 | N Engl J Med/Full paper | CCyR at 12 mo, by 12 mo; MMR at 3 mo, at 12 mo, by 12 mo; MR4; MR4.5; AP/BP; DIS |
| 18 | 2010/41 | Blood/Abstract | OS 18 mo; MR4.5; AP/BP |
| 24 | 2011/42 | Lancet Oncol/Full paper | PFS 24 mo; OS 24 mo; CCyR by 12 mo, by 24 mo; MMR by 12 mo, by 24 mo; MR4; MR4.5; AP/BP; DIS |
| 36 | 2012/43 | Blood/Abstract | PFS 36 mo; OS 36 mo; MMR by 36 mo; MR4; MR4.5 |
| 36 | 2012/44 | Leukemia/Full paper | PFS 36 mo; OS 36 mo; MMR at 36 mo, by 36 mo; MR4; MR4.5; AP/BP; DIS |
| 36 | 2013/45 | Blood/Full paper | AP/BP |
| 48 | 2014/46 | Blood/Full paper | PFS 48 mo; OS 48 mo; EMR at 3 mo; MMR by 48 mo; MR4; MR4.5; AP/BP |
| 60 | 2016/47 | Leukemia/Full paper | PFS 60 mo; OS 60 mo; EMR at 3 mo; MMR by 12 mo, by 24 mo, by 36 mo, by 48 mo, by 60 mo; MR4; MR4.5; AP/BP; DIS |
| 72 | 2015/48 | Blood/Abstract | MR4; MR4.5 |
| 72 | 2015/49 | Haematologica/Abstract | OS 72 mo; MMR by 72 mo; MR4.5 |
| Quasi-RCT: BELA (B); 502 patients | |||
| Imatinib: 47 (18-89) y; Male: 135 (54%) Bosutinib: 48 (19-91) y; Male: 149 (60%) | |||
| 12 | 2012/13 | J Clin Oncol/Full paper | OS 12 mo; CCyR at 3 mo, at 12 mo, by 12 mo; MMR at 3 mo, at 12 mo, by 12 mo; AP/BP; DIS |
| 18 | 2011/50 | J Clin Oncol/Abstract | CCyR at 12 mo, by 12 mo; MMR at 12 mo, by 12 mo; AP/BP |
| 24 | 2014/51 | Br J Haematol/Full paper | OS 24 mo; CCyR at 24 mo, by 24; EMR at 3 mo; MMR at 24 mo, by 24 mo; MR4; AP/BP; DIS |
| 30 | 2012/52 | Haematologica/Abstract | OS 24 mo; CCyR by 24 mo; MMR by 24 mo; AP/BP |
| 30 | 2014/53 | Am J Hematol/Full paper | — |
| 48 | 2016/54 | Am J Hematol/Full paper | Only safety |
| RCT: BFORE (B); 536 patients | |||
| Imatinib: 53 (19-84) y; Male: 135 (56%) Bosutinib: 52 (18-84) y; Male: 142 (58%) | |||
| 12 | 2018/14 | J Clin Oncol/Full paper | OS 12 mo; CCyR by 12 mo; MMR at 3 mo, at 12 mo; MR4; MR4.5; AP/BP; DIS |
| 18 | 2017/55 | Blood/Abstract | OS 18 mo; AP/BP |
| Quasi-RCT: EPIC (P); 307 patients | |||
| Imatinib: 52 (18-86) y; Male: 92 (61%) Ponatinib: 55 (18-89) y; Male: 97 (63%) | |||
| 12 | 2016/16 | Lancet Oncol/Full paper | CCyR at 12 mo; EMR at 3 mo; MMR at 3 mo, at 12 mo; MR4; MR4.5; DIS |
| Follow-up, mo*,† . | Year/ref. . | Journal/full paper or abstract . | Outcome evaluated . |
|---|---|---|---|
| RCT: DASISION (D); 519 patients | |||
| Imatinib: 49 (18-78) y; Male: 163 (63%) Dasatinib: 46 (18-84) y; Male: 144 (56%) | |||
| 12 | 2010/7 | N Engl J Med/Full paper | PFS 12 mo; OS 12 mo; CCyR by 3 mo, by 12 mo; cCCyR by 12 mo; MMR by 3 mo, by 12 mo; AP/BP; DIS |
| 18 | 2010/29 | Blood/Abstract | PFS 18 mo; OS 18 mo; AP/BP |
| 24 | 2011/30 | J Clin Oncol/Abstract | PFS 18 mo; OS 18 mo; MR4.5; AP/BP |
| 24 | 2011/31 | Blood/Abstract | EMR at 3 mo; MMR by 3 mo, by 24 mo; MR4; MR4.5 |
| 24 | 2012/32 | J Clin Oncol/Abstract | MR4 |
| 24 | 2012/33 | Blood/Full paper | PFS 24 mo; OS 24 mo; CCyR at 12 mo, by 12 mo, at 24 mo, by 24 mo; cCCyR by 24 mo; MMR at 12 mo, by 12 mo, at 24 mo, by 24 mo; MR4.5; AP/BP; DIS |
| 36 | 2014/34 | Blood/Full paper | PFS 36 mo; OS 36 mo; CCyR at 3 mo, at 12 mo, by 36 mo; cCCyR by 36 mo; EMR at 3 mo; MMR at 12 mo, by 36; MR4; MR4.5; AP/BP; DIS |
| 48 | 2013/35 | Blood/Abstract | PFS 48 mo; OS 48 mo; EMR at 3 mo; MMR at 12 mo, by 48 mo; MR4; MR4.5, AP/BP |
| 60 | 2014/36 | Blood/Abstract | PFS 60 mo; OS 60 mo; cCCyR by 60 mo; EMR at 3 mo; MMR by 60 mo; MR4.5; AP/BP |
| 60 | 2016/37 | J Clin Oncol/Full paper | PFS 60 mo; OS 60 mo; cCCyR at 60 mo; EMR at 3 mo; MMR by 12 mo, by 24 mo, by 36 mo, by 48 mo, by 60 mo, at 60 mo; MR4; MR4.5; DIS |
| Quasi-RCT: NCT00070499 (D); 253 patients | |||
| Imatinib: 50 (19-89) y; Male: 72 (59%) Dasatinib: 47 (18-90) y; Male: 74 (60%) | |||
| 12 (36 mo for PFS, OS) | 2012/6 | Blood/Full paper | PFS 36 mo; OS 36 mo; CCyR by 12 mo; MMR at 12 mo; MR4; MR4.5; AP/BP; DIS |
| RCT: NordCML006 (D); 46 patients | |||
| Imatinib: 60 (38-77) y; Male: 15 (63%) Dasatinib: 54 (29-71) y; Male: 7 (32%) | |||
| 18 | 2013/38 | Leukemia/Full paper | CCyr at 12 mo |
| 24 | 2013/39 | Blood/Abstract | MMR at 3 mo, by 12 mo, by 24 mo; MR4; MR4.5; AP/BP; DIS |
| 36 | 2015/8 | Eur J Haematol/Full paper | CCyR at 3 mo, at 12 mo; EMR at 3mo; MR4; AP/BP; DIS |
| RCT: ENEST (N); 846 patients | |||
| Imatinib: 46 (18-80) y; Male: 158 (56%) Nilotinib 300 mg: 47 (18-85) y; Male: 158 (56%) Nilotinib 400 mg: 47 (18-81) y; Male: 175 (62%) | |||
| 12 | 2010/40 | J Clin Oncol/Abstract | CCyR by 12 mo; MMR at 12 mo; MR4; MR4.5 |
| 12 | 2010/9 | N Engl J Med/Full paper | CCyR at 12 mo, by 12 mo; MMR at 3 mo, at 12 mo, by 12 mo; MR4; MR4.5; AP/BP; DIS |
| 18 | 2010/41 | Blood/Abstract | OS 18 mo; MR4.5; AP/BP |
| 24 | 2011/42 | Lancet Oncol/Full paper | PFS 24 mo; OS 24 mo; CCyR by 12 mo, by 24 mo; MMR by 12 mo, by 24 mo; MR4; MR4.5; AP/BP; DIS |
| 36 | 2012/43 | Blood/Abstract | PFS 36 mo; OS 36 mo; MMR by 36 mo; MR4; MR4.5 |
| 36 | 2012/44 | Leukemia/Full paper | PFS 36 mo; OS 36 mo; MMR at 36 mo, by 36 mo; MR4; MR4.5; AP/BP; DIS |
| 36 | 2013/45 | Blood/Full paper | AP/BP |
| 48 | 2014/46 | Blood/Full paper | PFS 48 mo; OS 48 mo; EMR at 3 mo; MMR by 48 mo; MR4; MR4.5; AP/BP |
| 60 | 2016/47 | Leukemia/Full paper | PFS 60 mo; OS 60 mo; EMR at 3 mo; MMR by 12 mo, by 24 mo, by 36 mo, by 48 mo, by 60 mo; MR4; MR4.5; AP/BP; DIS |
| 72 | 2015/48 | Blood/Abstract | MR4; MR4.5 |
| 72 | 2015/49 | Haematologica/Abstract | OS 72 mo; MMR by 72 mo; MR4.5 |
| Quasi-RCT: BELA (B); 502 patients | |||
| Imatinib: 47 (18-89) y; Male: 135 (54%) Bosutinib: 48 (19-91) y; Male: 149 (60%) | |||
| 12 | 2012/13 | J Clin Oncol/Full paper | OS 12 mo; CCyR at 3 mo, at 12 mo, by 12 mo; MMR at 3 mo, at 12 mo, by 12 mo; AP/BP; DIS |
| 18 | 2011/50 | J Clin Oncol/Abstract | CCyR at 12 mo, by 12 mo; MMR at 12 mo, by 12 mo; AP/BP |
| 24 | 2014/51 | Br J Haematol/Full paper | OS 24 mo; CCyR at 24 mo, by 24; EMR at 3 mo; MMR at 24 mo, by 24 mo; MR4; AP/BP; DIS |
| 30 | 2012/52 | Haematologica/Abstract | OS 24 mo; CCyR by 24 mo; MMR by 24 mo; AP/BP |
| 30 | 2014/53 | Am J Hematol/Full paper | — |
| 48 | 2016/54 | Am J Hematol/Full paper | Only safety |
| RCT: BFORE (B); 536 patients | |||
| Imatinib: 53 (19-84) y; Male: 135 (56%) Bosutinib: 52 (18-84) y; Male: 142 (58%) | |||
| 12 | 2018/14 | J Clin Oncol/Full paper | OS 12 mo; CCyR by 12 mo; MMR at 3 mo, at 12 mo; MR4; MR4.5; AP/BP; DIS |
| 18 | 2017/55 | Blood/Abstract | OS 18 mo; AP/BP |
| Quasi-RCT: EPIC (P); 307 patients | |||
| Imatinib: 52 (18-86) y; Male: 92 (61%) Ponatinib: 55 (18-89) y; Male: 97 (63%) | |||
| 12 | 2016/16 | Lancet Oncol/Full paper | CCyR at 12 mo; EMR at 3 mo; MMR at 3 mo, at 12 mo; MR4; MR4.5; DIS |
—, no data available; B, bosutinib; D, dasatinib; DIS, discontinued treatment; I, imatinib; N, nilotinib; P, ponatinib.
*In the row headings in column 1, RCTs and Quasi-RCTs included are: B, BEFORE (NCT02130557); B, BELA (NCT00574873); D, DASISION (NCT00481247); D (NCT00070499); D, NordCML006 (NCT00852566); N, ENEST (NCT00471497); P, EPIC (NCT01650805).
In the row subheadings in column 1, data are presented as: median age (range), y; male patients, number (percentage).
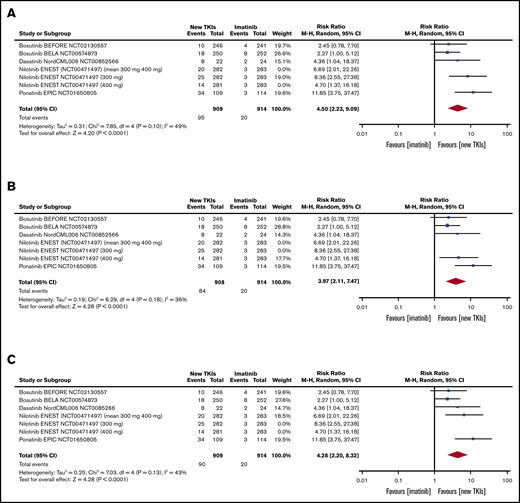
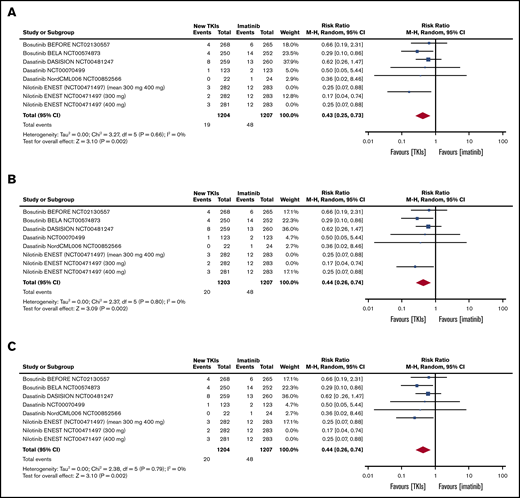
Table 2 shows the pooled RRs of the second- and third-generation TKIs in comparison with imatinib in terms of efficacy.9 As the ENEST study reported the efficacy and toxicity data for each nilotinib dose, we first pooled the 300 mg of nilotinib data relating to all of the outcomes included in the meta-analysis (Table 2), then the 400 mg of nilotinib data (Table 2), and then the mean values of the 2 doses (Table 2) (sensitivity analysis).9 All of the pooled efficacy outcomes except drug discontinuation showed that second- and third-generation TKIs have a clear advantage over imatinib. In particular, the RR of MMR after 3 months was statistically higher than that of the other efficacy outcomes in the patients treated with second- and third-generation TKIs (Figure 2). Moreover, treatment with second- and third-generation TKIs clearly prevented AP and BP transformation (Figure 3). All of the results were confirmed by the sensitivity analyses of the nilotinib doses (supplemental Figures 1-9).
Pooled relative risk of second- and third-generation TKIs in comparison with imatinib by efficacy
| Study . | TKIs ENEST RCT: 300 mg . | TKIs ENEST RCT: 400 mg . | TKIs ENEST RCT: mean values of 300 mg and 400 mg . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome efficacy* . | No. of RCTs . | No. of pts . | RR† . | 95% CI† . | No. of RCTs . | No. of pts . | RR† . | 95% CI† . | No. of RCTs . | No. of pts . | RR† . | 95% CI† . |
| EMR at 3 mo | 6 | 2182 | 1.34 | 1.27-1.41 | 6 | 2184 | 1.33 | 1.27-1.40 | 6 | 2183 | 1.34 | 1.27-1.40 |
| MMR at 12 mo | 6 | 2208 | 1.52 | 1.32-1.75 | 6 | 2207 | 1.50 | 1.32-1.70 | 6 | 2208 | 1.51 | 1.32-1.73 |
| MR4 at any time | 7 | 2331 | 1.67 | 1.32-2.11 | 7 | 2330 | 1.60 | 1.31-1.96 | 7 | 2331 | 1.64 | 1.31-2.04 |
| CCyR at 12 mo | 5 | 1553 | 1.13 | 1.04-1.22 | 5 | 1552 | 1.13 | 1.04-1.22 | 5 | 1553 | 1.13 | 1.04-1.22 |
| CCyR by 12 mo | 5 | 2204 | 1.15 | 1.09-1.22 | 5 | 2203 | 1.15 | 1.09-1.20 | 5 | 2204 | 1.15 | 1.09-1.21 |
| MMR at 3 mo | 5 | 1823 | 4.50 | 2.23-9.09 | 5 | 1822 | 3.97 | 2.11-7.47 | 5 | 1823 | 4.28 | 2.20-8.32 |
| MR4.5 at any time | 6 | 1930 | 2.65 | 1.44-4.88 | 6 | 1929 | 2.58 | 1.42-4.70 | 6 | 1930 | 2.63 | 1.43-4.82 |
| AP/BP during study treatment | 6 | 2411 | 0.43 | 0.25-0.73 | 6 | 2410 | 0.44 | 0.26-0.74 | 6 | 2411 | 0.44 | 0.26-0.74 |
| Discontinued any time | 7 | 2715 | 1.00 | 0.81-1.24 | 7 | 2714 | 1.00 | 0.80-1.25 | 7 | 2715 | 1.00 | 0.80-1.24 |
| Study . | TKIs ENEST RCT: 300 mg . | TKIs ENEST RCT: 400 mg . | TKIs ENEST RCT: mean values of 300 mg and 400 mg . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome efficacy* . | No. of RCTs . | No. of pts . | RR† . | 95% CI† . | No. of RCTs . | No. of pts . | RR† . | 95% CI† . | No. of RCTs . | No. of pts . | RR† . | 95% CI† . |
| EMR at 3 mo | 6 | 2182 | 1.34 | 1.27-1.41 | 6 | 2184 | 1.33 | 1.27-1.40 | 6 | 2183 | 1.34 | 1.27-1.40 |
| MMR at 12 mo | 6 | 2208 | 1.52 | 1.32-1.75 | 6 | 2207 | 1.50 | 1.32-1.70 | 6 | 2208 | 1.51 | 1.32-1.73 |
| MR4 at any time | 7 | 2331 | 1.67 | 1.32-2.11 | 7 | 2330 | 1.60 | 1.31-1.96 | 7 | 2331 | 1.64 | 1.31-2.04 |
| CCyR at 12 mo | 5 | 1553 | 1.13 | 1.04-1.22 | 5 | 1552 | 1.13 | 1.04-1.22 | 5 | 1553 | 1.13 | 1.04-1.22 |
| CCyR by 12 mo | 5 | 2204 | 1.15 | 1.09-1.22 | 5 | 2203 | 1.15 | 1.09-1.20 | 5 | 2204 | 1.15 | 1.09-1.21 |
| MMR at 3 mo | 5 | 1823 | 4.50 | 2.23-9.09 | 5 | 1822 | 3.97 | 2.11-7.47 | 5 | 1823 | 4.28 | 2.20-8.32 |
| MR4.5 at any time | 6 | 1930 | 2.65 | 1.44-4.88 | 6 | 1929 | 2.58 | 1.42-4.70 | 6 | 1930 | 2.63 | 1.43-4.82 |
| AP/BP during study treatment | 6 | 2411 | 0.43 | 0.25-0.73 | 6 | 2410 | 0.44 | 0.26-0.74 | 6 | 2411 | 0.44 | 0.26-0.74 |
| Discontinued any time | 7 | 2715 | 1.00 | 0.81-1.24 | 7 | 2714 | 1.00 | 0.80-1.25 | 7 | 2715 | 1.00 | 0.80-1.24 |
Sensitivity analysis of different nilotinib doses (ENEST RCT: 300 mg, 400 mg, and mean values of 300 mg and 400 mg) (bosutinib BEFORE [NCT02130557], bosutinib BELA [NCT00574873], dasatinib DASISION [NCT00481247], dasatinib [NCT00070499], dasatinib NordCML006 [NCT00852566], nilotinib ENEST [NCT00471497; 300-mg and 400-mg doses], and ponatinib EPIC [NCT01650805]). In ENEST RCT, the patients (pts) were randomly assigned to receive 300 mg of nilotinib (n = 282), 400 mg of nilotinib (n = 281), 400 mg of imatinib (n = 283); the number of events is presented for the 3 groups (300 mg of nilotinib, 400 mg of nilotinib, 400 mg of imatinib). The sensitivity analysis was made by introducing into the meta-analysis: first, the results of 300 mg of nilotinib; second, the results of 400 mg of nilotinib; and third, the arithmetic mean of the results of the 2 nilotinib doses.
M-H method, random-effects method.
Bold values in these columns are statistically significant.
Tables and forest plots of MMR at 3 months. ENEST RCT sensitivity analysis: 300 mg and 400 mg of nilotinib and mean values of 300 mg and 400 mg of nilotinib. Nilotinib ENEST (NCT00471497; 300 mg and 400 mg). In ENEST RCT, the patients were randomly assigned to receive 300 mg of nilotinib (n = 282), 400 mg of nilotinib (n = 281), 400 mg of imatinib (n = 283); the number of events is presented for the 3 groups (300 mg of nilotinib, 400 mg of nilotinib, 400 mg of imatinib). The sensitivity analysis was made by introducing into the meta-analysis: first, the results of 300 mg of nilotinib (A); second, the results of 400 mg of nilotinib (B); and third, the arithmetic mean of the results of the 2 nilotinib doses (C). df, degrees of freedom.
Tables and forest plots of MMR at 3 months. ENEST RCT sensitivity analysis: 300 mg and 400 mg of nilotinib and mean values of 300 mg and 400 mg of nilotinib. Nilotinib ENEST (NCT00471497; 300 mg and 400 mg). In ENEST RCT, the patients were randomly assigned to receive 300 mg of nilotinib (n = 282), 400 mg of nilotinib (n = 281), 400 mg of imatinib (n = 283); the number of events is presented for the 3 groups (300 mg of nilotinib, 400 mg of nilotinib, 400 mg of imatinib). The sensitivity analysis was made by introducing into the meta-analysis: first, the results of 300 mg of nilotinib (A); second, the results of 400 mg of nilotinib (B); and third, the arithmetic mean of the results of the 2 nilotinib doses (C). df, degrees of freedom.
Tables and forest plots of AP and BP transformations during study treatment. ENEST RCT sensitivity analysis: 300 mg of nilotinib, 400 mg of nilotinib, and mean values of 300 mg and 400 mg of nilotinib. Nilotinib ENEST (NCT00471497; 300 mg and 400 mg). In ENEST RCT, the patients were randomly assigned to receive 300 mg of nilotinib (n = 282), 400 mg of nilotinib (n = 281), 400 mg of imatinib (n = 283); the number of events is presented for the 3 groups (300 mg of nilotinib, 400 mg of nilotinib, 400 mg of imatinib). The sensitivity analysis was made by introducing into the meta-analysis: first, the results of 300 mg of nilotinib (A); second, the results of 400 mg of nilotinib (B); and third, the arithmetic mean of the results of the 2 nilotinib doses (C).
Tables and forest plots of AP and BP transformations during study treatment. ENEST RCT sensitivity analysis: 300 mg of nilotinib, 400 mg of nilotinib, and mean values of 300 mg and 400 mg of nilotinib. Nilotinib ENEST (NCT00471497; 300 mg and 400 mg). In ENEST RCT, the patients were randomly assigned to receive 300 mg of nilotinib (n = 282), 400 mg of nilotinib (n = 281), 400 mg of imatinib (n = 283); the number of events is presented for the 3 groups (300 mg of nilotinib, 400 mg of nilotinib, 400 mg of imatinib). The sensitivity analysis was made by introducing into the meta-analysis: first, the results of 300 mg of nilotinib (A); second, the results of 400 mg of nilotinib (B); and third, the arithmetic mean of the results of the 2 nilotinib doses (C).
Table 3 shows the pooled RRs of second- and third-generation TKIs in comparison with imatinib in terms of toxicity.9 Thrombocytopenia, cardiovascular events, and pancreatic and hepatic effects were more frequent among the patients treated with second- and third-generation TKIs and, once again, all of the results were confirmed by sensitivity analyses of the different nilotinib doses. Fluid retention was also more frequent in the patients treated with second- and third-generation TKIs, but the sensitivity analyses confirmed the results only in the case of 300 mg of nilotinib (Table 3) and the mean values of the results obtained using the 2 nilotinib doses (Table 3), but not in the case of 400 mg of nilotinib (Table 3) (supplemental Figures 10-21).9
Pooled relative risk of second- and third-generation TKIs, in comparison with imatinib by toxicity
| Study . | TKIs ENEST RCT: 300 mg . | TKIs ENEST RCT: 400 mg . | TKIs ENEST RCT: mean values of 300 and 400 mg . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome toxicity: grade 3-4*,† . | No. of RCTs . | No. of pts . | RR‡ . | 95% CI‡ . | No. of RCTs . | No. of pts . | RR‡ . | 95% CI‡ . | No. of RCTs . | No. of pts . | RR‡ . | 95% CI‡ . |
| Anemia | 7 | 2704 | 1.17 | 0.80-1.72 | 7 | 2702 | 1.20 | 0.86-1.69 | 7 | 2703 | 1.19 | 0.83-1.70 |
| Neutropenia | 7 | 2704 | 0.69 | 0.46-1.02 | 7 | 2702 | 0.68 | 0.45-1.01 | 7 | 2703 | 0.68 | 0.46-1.02 |
| Thrombocytopenia | 7 | 2704 | 1.55 | 1.17-2.05 | 7 | 2702 | 1.58 | 1.22-2.06 | 7 | 2703 | 1.57 | 1.20-2.05 |
| Cardiovascular events | 7 | 2704 | 2.26 | 1.32-3.87 | 7 | 2702 | 2.75 | 1.62-4.67 | 7 | 2703 | 2.54 | 1.49-4.33 |
| Cutaneous effects | 7 | 2704 | 0.73 | 0.21-2.47 | 7 | 2702 | 1.03 | 0.35-2.98 | 7 | 2703 | 0.93 | 0.32-2.75 |
| GI effects | 7 | 2704 | 1.80 | 0.67-4.84 | 7 | 2702 | 2.02 | 0.84-4.86 | 7 | 2703 | 1.94 | 0.77-4.85 |
| Fluid retention§ | 7 | 2704 | 3.21 | 1.09-9.48 | 7 | 2702 | 2.81 | 0.99-7.97 | 7 | 2703 | 3.11 | 1.07-9.00 |
| Infectious events | 7 | 2704 | 1.11 | 0.54-2.28 | 7 | 2702 | 1.12 | 0.49-2.56 | 7 | 2703 | 1.11 | 0.54-2.28 |
| Pancreatic effects | 5 | 2413 | 2.24 | 1.29-3.87 | 5 | 2411 | 2.31 | 1.33-4.02 | 5 | 2412 | 2.29 | 1.32-3.96 |
| Hepatic effects | 6 | 2459 | 3.01 | 1.21-7.51 | 6 | 2457 | 3.89 | 1.81-8.35 | 6 | 2458 | 3.51 | 1.55-7.92 |
| Musculoskeletal disorders | 6 | 2658 | 0.76 | 0.36-1.62 | 6 | 2656 | 0.92 | 0.46-1.83 | 6 | 2657 | 0.85 | 0.42-1.73 |
| QT prolongation | 5 | 2352 | 0.82 | 0.39-1.73 | 5 | 2350 | 0.82 | 0.39-1.74 | 5 | 2351 | 0.82 | 0.39-1.73 |
| Study . | TKIs ENEST RCT: 300 mg . | TKIs ENEST RCT: 400 mg . | TKIs ENEST RCT: mean values of 300 and 400 mg . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome toxicity: grade 3-4*,† . | No. of RCTs . | No. of pts . | RR‡ . | 95% CI‡ . | No. of RCTs . | No. of pts . | RR‡ . | 95% CI‡ . | No. of RCTs . | No. of pts . | RR‡ . | 95% CI‡ . |
| Anemia | 7 | 2704 | 1.17 | 0.80-1.72 | 7 | 2702 | 1.20 | 0.86-1.69 | 7 | 2703 | 1.19 | 0.83-1.70 |
| Neutropenia | 7 | 2704 | 0.69 | 0.46-1.02 | 7 | 2702 | 0.68 | 0.45-1.01 | 7 | 2703 | 0.68 | 0.46-1.02 |
| Thrombocytopenia | 7 | 2704 | 1.55 | 1.17-2.05 | 7 | 2702 | 1.58 | 1.22-2.06 | 7 | 2703 | 1.57 | 1.20-2.05 |
| Cardiovascular events | 7 | 2704 | 2.26 | 1.32-3.87 | 7 | 2702 | 2.75 | 1.62-4.67 | 7 | 2703 | 2.54 | 1.49-4.33 |
| Cutaneous effects | 7 | 2704 | 0.73 | 0.21-2.47 | 7 | 2702 | 1.03 | 0.35-2.98 | 7 | 2703 | 0.93 | 0.32-2.75 |
| GI effects | 7 | 2704 | 1.80 | 0.67-4.84 | 7 | 2702 | 2.02 | 0.84-4.86 | 7 | 2703 | 1.94 | 0.77-4.85 |
| Fluid retention§ | 7 | 2704 | 3.21 | 1.09-9.48 | 7 | 2702 | 2.81 | 0.99-7.97 | 7 | 2703 | 3.11 | 1.07-9.00 |
| Infectious events | 7 | 2704 | 1.11 | 0.54-2.28 | 7 | 2702 | 1.12 | 0.49-2.56 | 7 | 2703 | 1.11 | 0.54-2.28 |
| Pancreatic effects | 5 | 2413 | 2.24 | 1.29-3.87 | 5 | 2411 | 2.31 | 1.33-4.02 | 5 | 2412 | 2.29 | 1.32-3.96 |
| Hepatic effects | 6 | 2459 | 3.01 | 1.21-7.51 | 6 | 2457 | 3.89 | 1.81-8.35 | 6 | 2458 | 3.51 | 1.55-7.92 |
| Musculoskeletal disorders | 6 | 2658 | 0.76 | 0.36-1.62 | 6 | 2656 | 0.92 | 0.46-1.83 | 6 | 2657 | 0.85 | 0.42-1.73 |
| QT prolongation | 5 | 2352 | 0.82 | 0.39-1.73 | 5 | 2350 | 0.82 | 0.39-1.74 | 5 | 2351 | 0.82 | 0.39-1.73 |
Sensitivity analysis of different nilotinib doses (ENEST RCT: 300 mg, 400 mg, and mean values of 300 mg and 400 mg) (bosutinib BEFORE [NCT02130557], bosutinib BELA [NCT00574873], dasatinib DASISION [NCT00481247], dasatinib [NCT00070499], dasatinib NordCML006 [NCT00852566], nilotinib ENEST [NCT00471497; 300-mg and 400-mg doses], ponatinib EPIC [NCT01650805]). In ENEST RCT, the patients were randomly assigned to receive 300 mg of nilotinib (n = 282), 400 mg of nilotinib (n = 281), 400 mg of imatinib (n = 283); the number of events is presented for the 3 groups (300 mg of nilotinib, 400 mg of nilotinib, 400 mg of imatinib). The sensitivity analysis was made by introducing into the meta-analysis: first, the results of 300 mg of nilotinib; second, the results of 400 mg of nilotinib; and third, the arithmetic mean of the results of the 2 nilotinib doses.
National Cancer Institute Common Terminology Criteria for Adverse Events.
M-H method, random-effects method.
Bold values in these columns are statistically significant.
Pleural and pericardial effusion.
Supplemental Figures 22 and 23, respectively, show the risk-of-bias summary and graph, with the for-profit bias under “other bias.” All of the articles were at low risk except for the risk-of-performance bias (although it must be remembered that blinding trial participants and study personnel may not be ethical in an oncological setting) and the risk of “other bias” (6 of the 7 trials [85.7%] were sponsored by a pharmaceutical company).
Supplemental Tables 1 and 2 show the GRADE quality of evidence by efficacy and toxicity.28 The certainty of efficacy (the extent to which we are confident that the estimates of effect are sufficient to support a particular recommendation) was considered to be high (further research is very unlikely to change our confidence in the estimates of effect) in the case of EMR after 3 months, MMR after 3 and 12 months, MR4 at any time, CCyR by 12 months, and AP/BP during study treatment; and it was moderate (further research is likely to have a major impact on our confidence in the estimates of effect and may actually change them) in the case of CCyR after 12 months, MR4.5 at any time, and discontinuation at any time. The certainty of toxicity was high in the case of anemia, thrombocytopenia, musculoskeletal disorders, and QT prolongation; moderate in the case of neutropenia, cardiovascular events, cutaneous effects, fluid retention, infectious events, and pancreatic and hepatic effects; and low in the case of gastrointestinal effects (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change them).
Discussion
Although many studies have compared the use of imatinib and more recent TKIs in patients with newly diagnosed Ph+ CP CML, they have not established which is the most efficacious because: they have not always considered the same outcomes and often evaluated them at different times, they only some provided complete OS and PFS data, and the large number of AEs is not specific to a single TKI.6-9,13,14,16 Furthermore, the 2017 ESMO and 2018 NCCN CML guidelines do not make any precise recommendations that would help clinicians decide.21,22
Our systematic review and meta-analysis of RCTs provides complete, updated, and precise comparative information concerning the use of TKIs in patients with newly diagnosed adult CP CML in terms of OS and PFS at various time points, clinical and biological response variables, and the most relevant hematological and nonhematological AEs. Biases and the quality of the evidence were assessed using the Cochrane risk-of-bias tool and the GRADE method,27,28 and the 2 databases created are available for purposes of data sharing.
As only 2 RCTs considered OS and PFS for up to 60 months, we could not make a pooled analysis. However, the ENEST study found that 60-month OS and PFS among the patients treated with imatinib were, respectively, 91.7% and 91.0%, whereas the patients treated with 300 mg or 400 mg of nilotinib had OS of, respectively, 93.7% and 96.2%, and PFS of 92.2% and 95.8%. The DASISION study recorded 90% and 86% in imatinib-treated patients and 91% and 85% in dasatinib-treated patints.7,9,37,47
Our meta-analysis clearly showed that, in terms of secondary efficacy outcomes, second and third-generation TKIs were better in patients with newly diagnosed CP CML without comorbidities, whereas imatinib should be preferred for patients with comorbidities because of its toxicity profile. These conclusions were supported by the GRADE assessment of the quality of the evidence.28 However, AEs can also occur in patients without preexisting comorbidities.
The fact that only 2 of the 7 RCTs satisfying our meta-analysis eligibility criteria considered OS and PFS up to 60 months is a major limitation of our study6-9,13,14,16,37,47 because, despite what was laid down in the registered statistical plan,23 our conclusions only take secondary outcomes into account. However, the absence of such strong efficacy outcomes as OS and PFS is frequent in the literature: many trials use surrogate outcomes, which explains why postmarketing studies of cancer drugs have revealed limited benefits in terms of OS or the quality of life.57
Other authors have tried to define the best first-line TKI for treating CP CML by means of systematic reviews and meta-analyses, but their conclusions have been less useful in clinical practice than expected.58-64 Our findings are in line with previously published results but are more complete as they relate to all 5 TKIs currently approved for the first-line treatment of CP CML in Europe or the United States (ie, imatinib, dasatinib, nilotinib, bosutinib, and ponatinib), they are updated to May 2019, they consider multiple outcomes (efficacy and toxicity, particularly individual hematological and nonhematological AEs), the original protocol was registered with PROSPERO to avoid the risks of selection and publication bias,23 and biases and the quality of the evidence were assessed using the Cochrane risk-of-bias tool and GRADE profiles.27,28
The network meta-analysis by Mealing et al compared the results obtained using first-line imatinib with those obtained using high-dose imatinib, nilotinib, or dasatinib in studies published up to March 2011 (including 8 RCTs), and showed an advantage in using second-generation TKIs on the basis of 12-month CCyR and MMR.58 However, they did not evaluate essential outcomes such as AEs, and the selection of patients is not clear.58
The study by Gurion et al comparing the results obtained using first-line imatinib with those obtained using nilotinib, dasatinib or bosutinib in studies published up to July 2011 included only 4 RCTs.59 Although they also showed an advantage in using second-generation TKIs on the basis of 12-month CCyR and MMR, they could not make a meta-analysis of AEs because of the clinical heterogeneity of the different drugs.59
The network meta-analysis by Signorovitch et al compared the results obtained using first-line imatinib with those obtained using nilotinib or dasatinib only on the basis of MMR by 12 months.60 Their 3 RCTs showed an advantage of nilotinib over imatinib and dasatinib; there was no risk-of-bias assessment or quality grading of the evidence.60
The study by Firwana et al compared MMR, MR4.5, OS, and PFS after 12 months and at the time of the last FU in patients receiving first-line imatinib with the results obtained using nilotinib, dasatinib, bosutinib, or ponatinib in studies published up to January 2015, including 6 RCTs.61 Although they showed that second- and third-generation TKIs were better than imatinib in terms of MMR at different time points, the results were not always statistically significant; furthermore, they did not make a meta-analysis of AEs.61
The study by Yun et al compared CCyR and MMR, PFS, OS, and progression to AP/BP CML after 12 months in patients receiving first-line imatinib with the results obtained using nilotinib, dasatinib, bosutinib, ponatinib, or radotinib in studies published up to January 2016, including 8 RCTs.62 They showed an advantage of new-generation TKIs in terms of 12-month MMR and progression to AP/BP CML, but they did not make a meta-analysis of AEs.62
The network meta-analysis by Chen et al compared the results obtained using first-line standard-dose imatinib with those obtained using high-dose imatinib, nilotinib, dasatinib, or bosutinib in studies published up to March 2018, including 14 RCTs. The considered outcomes were 12-month CCyR; MMR after 12, 24 and 36 months; deep molecular responses after 12, 24, 36, and 60 months; OS and PFS; and grade 3 and 4 AEs.63 All of the drugs had an advantage (direct comparison) over standard-dose imatinib in terms of 12-month CCyR (except bosutinib), 12-month MMR, MMR at 24 and 36 months (except high-dose imatinib), and deep molecular responses at all time points (except bosutinib). There was no difference in OS but PFS was better in the patients receiving 400 mg of nilotinib. Standard-dose imatinib had the most favorable toxicity profile. The main limitation of the study is that it did not consider individual toxicities but only AEs as a whole.63
The study by Douxfils et al considered occlusive vascular events as the only primary outcome, and 1-year MMR and OS in studies published up to October 2014.64 The protocol registered with PROSPERO23 said that all patients treated with TKIs were included in the study, with no restriction regarding previous therapy, and that stratification by disease was planned to compare populations at similar baseline risk. In the published paper, it was specified that the analysis was restricted to a CML subgroup, but they did not say whether they only considered newly diagnosed CP CML.64
In conclusion, on the basis of secondary efficacy outcomes, the findings of our meta-analysis, supported by GRADE-assessed quality evidence,28 suggest that patients with newly diagnosed CP CML without comorbidities should receive second- or third-generation TKIs; however, on the basis of toxicity outcomes, patients with comorbidities should preferably be treated with imatinib. The use of imatinib is further supported by the current availability of a cheaper generic imatinib. Our data could be used to implement a health technology assessment, and the updated RCT FU data may be useful for making a meta-analysis of primary efficacy outcomes such as OS and PFS after 60 months or more.
We cannot recommend a specific newer TKI because there are no head-to-head RCTs: a network meta-analysis is required. The definition of the optimal TKI for patients with newly CP CML should consider AEs and comorbidities as well as molecular/cytogenetic responses and transformation rates.
The supplemental data available with the online version of this article include the complete database (database 1) and the reduced database of efficacy and toxicity outcomes reported in at least 5, 6, or 7 RCTs (database 2). Data-sharing requests may be e-mailed to the corresponding author, Claudia Vener, at claudia.vener@unimi.it.
Acknowledgment
The authors thank Kevin Smart for assistance in the preparation of the text.
Authorship
Contribution: C.V. designed and carried out the study, analyzed the data, and wrote the paper; R.B. designed the study and contributed to writing the paper; F.A. analyzed the data and contributed to writing the paper; and A.F., G.S., G.P., and M.S. carried out the study and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudia Vener, Department of Oncology and Hemato-Oncology, University of Milan, Via Santa Sofia 9/1, 20122 Milan, Italy; e-mail: claudia.vener@unimi.it.
References
Author notes
The full-text version of this article contains a data supplement.