Key Points
The median overall survival for older patients with AML treated with frontline HMA monotherapy is 7 to 8 months.
No substantial differences in survival or TI were observed between azacitidine- and decitabine-treated patients.
Abstract
The hypomethylating agents (HMAs) azacitidine and decitabine have been the de facto standard of care for patients with acute myeloid leukemia (AML) who are unfit for intensive therapy. Using the Surveillance, Epidemiology, and End Results-Medicare linked database, we identified 2263 older adults (age ≥66 years) diagnosed with AML during 2005-2015 who received a first-line HMA; 1154 (51%) received azacitidine, and 1109 (49%) received decitabine. Median survival from diagnosis was 7.1 and 8.2 months (P < .01) for azacitidine- and decitabine-treated patients, respectively. Mortality risk was higher with azacitidine vs decitabine (hazard ratio [HR], 1.11; 95% confidence interval [CI], 1.01-1.21; P = .02). The findings were similar when evaluating only patients completing ≥4 cycles (42% of patients treated with either azacitidine or decitabine). These findings lost significance when evaluating those completing a standard 7-day schedule of azacitidine (34%) vs 5-day schedule for decitabine (66%) (HR, 0.95; 95% CI, 0.83-1.08; P = .43). Red blood cell (RBC) transfusion independence (TI) was achieved in one-third of patients with no difference between the 2 HMAs. In conclusion, the majority of older AML patients did not receive the minimum of 4 cycles of HMA often needed to elicit clinical benefit. We observed no clinically meaningful differences between azacitidine- and decitabine-treated patients in their achievement of RBC TI or survival.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of older adults, with a median age of 67 years at diagnosis.1 Older age at AML diagnosis is associated with greater prevalence of comorbidity and inferior performance status, both of which increase the risk of toxicity and death with intensive induction therapy.2 Furthermore, age is associated with adverse molecular, cytogenetic, and biologic features that confer chemoresistance and predict inferior outcomes.3,4 Almost half of older AML patients in the United States diagnosed in 2001-2013 did not receive active therapy, including 42% of those diagnosed as recently as 2013.5 The median survival for all AML patients diagnosed at age ≥65 years in the United States is ∼2.7 months, and 80% of these patients die within 1 year.1
The availability of the hypomethylating agents (HMAs) after 2004 represented an important expansion in treatment options for older patients with AML. The US Food and Drug Administration (FDA) approved azacitidine and decitabine for treatment of myelodysplastic syndrome in 2004 and 2006, respectively, based on clinical trial evidence of increased red blood cell (RBC) transfusion independence (TI) and improved overall survival (OS) in the case of azacitidine.6-9 In AML, large randomized clinical trials supported a clinical benefit of HMAs for AML patients (complete remission and TI rates ranging from 15% to 20% and 30% to 40%, respectively) but did not demonstrate a definite improvement in OS.10-13 Results from controlled retrospective analyses suggested that OS for some older AML patients who received HMAs might be similar, or even superior, to those who received intensive therapy.14 The toxicity and mortality associated with intensive chemotherapy and the lack of alternative nonintensive therapies for older AML patients resulted in the adoption of HMAs as the de facto standard of care for this population.10-13,15 A prior Surveillance, Epidemiology and End Results (SEER)–Medicare study reported that among older AML patients diagnosed in 2005-2007, 10.7% patients were treated with a frontline HMA.11 More recent studies reported that 40% to 60% of newly diagnosed older AML patients receive an HMA.16-18
Real-world evidence for the clinical benefits of HMAs in older AML patients is limited, and the 2 approved HMAs have not been directly compared in large clinical trials. This gap in evidence is increasingly problematic, as HMAs have become the backbone for combination regimens (eg, with venetoclax), with approval based on single-arm studies without an HMA monotherapy control arm.19 This population-based study fills that gap by examining the real-world performance of HMA monotherapy in older AML patients, establishing benchmark estimates for survival and RBC TI rates for the 2 agents.
Patients and methods
Data source
We used the SEER-Medicare linked database to assemble a population-based cohort of older adults newly diagnosed with AML. The SEER-Medicare database links patient-level information on incident cancer diagnoses reported to the SEER registries with a master file of Medicare enrollment and claims for inpatient, outpatient, and professional services and prescription medications and accounts for ∼30% of the US population.20,21 The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Study population
The study cohort included individuals (1) newly diagnosed with AML in 2005-2015 at the age of 66 to 99 years (minimum age of 66 years was chosen to allow a 1-year lookback period within the database); (2) with known diagnosis month; (3) with diagnosis not reported on autopsy or death certificate only; (4) who had continuous Medicare fee-for-service coverage (Parts A and B) and were not enrolled in Medicare Advantage (health maintenance organizations or preferred provider organizations) from 12 months before diagnosis through death or the end of study (12/31/2016); and (5) who received no HMA prior to AML diagnosis and initiated azacitidine or decitabine monotherapy (at least 1 day of treatment) within 6 months of AML diagnosis. To ensure the captured outpatient HMA was the first AML therapy received, we further excluded patients with brief (≤7-day) chemotherapy-related hospitalization within 2 months before HMA initiation (supplemental Figure 1). In an analysis examining HMA receipt and RBC TI, we limited to individuals who were transfusion dependent (TD) at HMA initiation (see "Ascertainment of HMAs").
Ascertainment of HMAs
Azacitidine and decitabine were identified via the Healthcare Common Procedure Coding System codes (J9025, C9218 for azacitidine and J0894, C9231 for decitabine on hospital outpatient or carrier claims). A cycle of HMA was defined as (1) at least 3 days with HMA treatment within 14 days and (2) a new cycle started if 2 days with HMA were ≥10 days apart. We generated a count of cycles completed. After the initial cycle, a new cycle started if 2 days with HMA were ≥10 days apart. We stopped counting HMA cycles if the gap was >90 days. We also assessed whether a patient received a standard HMA schedule based on their first complete cycle, defined as receiving 7 doses of azacitidine within 7 to 9 consecutive days or 5 doses of decitabine within 5 consecutive days.
Outcome measures
The study had 2 outcomes: OS and RBC TI. OS was defined as all-cause mortality with days counted from the first day of the diagnosis month. We measured transfusion status using the same approach as in our previous studies.22-25 In brief, we created a longitudinal person-week file with indicators for RBC transfusions received each week, beginning 18 weeks before diagnosis. To determine transfusion status in a specific week, we assessed for transfusions in that week and the prior 7 weeks. Transfusion status was assigned as follows: transfusion naive, no prior history of transfusion; transfusion user, 1 week with transfusions during an 8-week period; TD, ≥2 weeks during the 8-week period with transfusions, requiring a gap of at least 2 weeks between 2 transfusions; and TI, no transfusions during the 8-week period after a history of transfusion use. To avoid mismeasurement associated with transfusion discontinuation at end-of-life, transfusion status was censored 28 days before death.
Covariates
We obtained information on the following sociodemographic characteristics: sex, age at diagnosis (66-69, 70-74, 75-79, or ≥80 years), race (white, other), marital status at diagnosis (single, married, or unknown), median household income at the zip code level (in quartiles), dual Medicaid enrollment within 12 months before diagnosis, metropolitan statistical area status of residence (big metro, metro, or other), and census region (Northeast, Midwest, South, or West). Zip code–level median household income and dual Medicaid enrollment were used as proxies for neighborhood- and individual-level socioeconomic status, respectively. To assess comorbidity, we used the approach developed by Elixhauser et al, searching for diagnosis codes in the 12 months prior to AML diagnosis that appeared on any inpatient claims or ≥2 outpatient/physician claims >30 days apart.26,27 We included a claims-based proxy for poor performance status (“disability status”).28
Statistical analysis
We report univariate descriptive statistics (percentages, median, and interquartile range [IQR]); Student t test and Pearson’s χ2 test were used to evaluate the association between treatment type (azacitidine vs decitabine) and study covariates. The Kaplan-Meier method and corresponding log-rank tests were used to examine OS by HMA agent, with multivariable Cox proportional hazards regression providing estimates adjusted for patient and area characteristics. We used Kaplan-Meier statistics and Cox proportional hazards regression to examine effects of HMA type on RBC TI among patients who were TD at HMA initiation. Death was treated as a competing risk for TI. Comparisons of cumulative incidence of TI across treatment groups were performed using Gray’s test.29 Multivariable competing risks regression models were performed to evaluate associations between HMA and RBC TI.30 All multivariable models included patient’s sex, age at diagnosis, race, marital status, Elixhauser comorbidity index, disability status, zip code–level median household income, dual Medicaid enrollment, MSA size, and census region as covariates. All significance tests were 2 sided with an α level of 0.05. All analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC).
Results
Demographics and baseline characteristics
Our study sample (N = 2263) had a median age of 77 (IQR, 72-82) years; 58.6% were males, and 89.7% were white. This was a mature dataset with only 150 patients (6.6%) alive at the end of the study. Approximately half (51%) of patients initiated azacitidine (n = 1154). Compared with patients who were treated with azacitidine, those treated with decitabine were younger, had fewer comorbid conditions, and were less likely to have poor disability status (Table 1).
Characteristics of 2263 older adults with AML by type of HMA therapy, 2005-2015
| . | Overall . | Azacitidine . | Decitabine . | P . |
|---|---|---|---|---|
| Total | 2263 | 1154 | 1109 | |
| Female | 937 (41.4) | 492 (42.6) | 445 (40.1) | .23 |
| Male | 1326 (58.6) | 662 (57.4) | 664 (59.9) | |
| Age, y | ||||
| Median (IQR) | 77 (72-82) | 78 (73-82) | 76 (72-81) | |
| 66-69 | 288 (12.7) | 133 (11.5) | 155 (14.0) | .01 |
| 70-74 | 548 (24.2) | 258 (22.4) | 290 (26.1) | |
| 75-79 | 587 (25.9) | 298 (25.8) | 289 (26.1) | |
| ≥80 | 840 (37.1) | 465 (40.3) | 375 (33.8) | |
| Race | ||||
| White | 2029 (89.7) | 1039 (90.0) | 990 (89.3) | .55 |
| Other | 234 (10.3) | 115 (10.0) | 119 (10.7) | |
| Marital status | ||||
| Single | 768 (33.9) | 394 (34.1) | 374 (33.7) | .84 |
| Married | 1345 (59.4) | 684 (59.3) | 661 (59.6) | |
| Unknown | 150 (6.6) | 76 (6.6) | 74 (6.7) | |
| Elixhauser comorbidity index | ||||
| 0 | 576 (25.4) | 268 (23.2) | 308 (27.8) | .03 |
| 1-2 | 927 (41.0) | 479 (41.5) | 448 (40.4) | |
| ≥3 | 760 (33.6) | 407 (35.3) | 353 (31.8) | |
| Disabled | ||||
| No | 2129 (94.1) | 1073 (93.0) | 1056 (95.2) | .02 |
| Yes | 134 (5.9) | 91 (7.0) | 53 (4.8) | |
| Zip code median household income | ||||
| First quartile (lowest) | 552 (24.4) | 275 (23.8) | 277 (25.0) | .92 |
| Second quartile | 554 (24.5) | 287 (24.9) | 267 (24.1) | |
| Third quartile | 554 (24.5) | 284 (24.6) | 270 (24.3) | |
| Fourth quartile (highest) | 554 (24.5) | 284 (24.6) | 270 (24.3) | |
| Unknown | 49 (2.2) | 24 (2.1) | 25 (2.3) | |
| Dual Medicaid enrollment within 12 mo before diagnosis | ||||
| No | 2000 (88.4) | 1012 (87.7) | 988 (89.1) | .30 |
| Yes | 263 (11.6) | 142 (12.3) | 121 (10.9) | |
| Residential area | ||||
| Big metro | 1264 (55.9) | 626 (54.2) | 638 (57.5) | .26 |
| Metro | 682 (30.1) | 364 (31.5) | 318 (28.7) | |
| Nonmetro | 317 (14.0) | 164 (14.2) | 153 (13.8) | |
| Region | ||||
| Northeast | 519 (22.9) | 258 (22.4) | 261 (23.5) | .25 |
| Midwest | 222 (9.8) | 112 (9.7) | 110 (9.9) | |
| South | 541 (23.9) | 261 (22.6) | 280 (25.2) | |
| West | 981 (43.3) | 523 (45.3) | 458 (41.3) |
| . | Overall . | Azacitidine . | Decitabine . | P . |
|---|---|---|---|---|
| Total | 2263 | 1154 | 1109 | |
| Female | 937 (41.4) | 492 (42.6) | 445 (40.1) | .23 |
| Male | 1326 (58.6) | 662 (57.4) | 664 (59.9) | |
| Age, y | ||||
| Median (IQR) | 77 (72-82) | 78 (73-82) | 76 (72-81) | |
| 66-69 | 288 (12.7) | 133 (11.5) | 155 (14.0) | .01 |
| 70-74 | 548 (24.2) | 258 (22.4) | 290 (26.1) | |
| 75-79 | 587 (25.9) | 298 (25.8) | 289 (26.1) | |
| ≥80 | 840 (37.1) | 465 (40.3) | 375 (33.8) | |
| Race | ||||
| White | 2029 (89.7) | 1039 (90.0) | 990 (89.3) | .55 |
| Other | 234 (10.3) | 115 (10.0) | 119 (10.7) | |
| Marital status | ||||
| Single | 768 (33.9) | 394 (34.1) | 374 (33.7) | .84 |
| Married | 1345 (59.4) | 684 (59.3) | 661 (59.6) | |
| Unknown | 150 (6.6) | 76 (6.6) | 74 (6.7) | |
| Elixhauser comorbidity index | ||||
| 0 | 576 (25.4) | 268 (23.2) | 308 (27.8) | .03 |
| 1-2 | 927 (41.0) | 479 (41.5) | 448 (40.4) | |
| ≥3 | 760 (33.6) | 407 (35.3) | 353 (31.8) | |
| Disabled | ||||
| No | 2129 (94.1) | 1073 (93.0) | 1056 (95.2) | .02 |
| Yes | 134 (5.9) | 91 (7.0) | 53 (4.8) | |
| Zip code median household income | ||||
| First quartile (lowest) | 552 (24.4) | 275 (23.8) | 277 (25.0) | .92 |
| Second quartile | 554 (24.5) | 287 (24.9) | 267 (24.1) | |
| Third quartile | 554 (24.5) | 284 (24.6) | 270 (24.3) | |
| Fourth quartile (highest) | 554 (24.5) | 284 (24.6) | 270 (24.3) | |
| Unknown | 49 (2.2) | 24 (2.1) | 25 (2.3) | |
| Dual Medicaid enrollment within 12 mo before diagnosis | ||||
| No | 2000 (88.4) | 1012 (87.7) | 988 (89.1) | .30 |
| Yes | 263 (11.6) | 142 (12.3) | 121 (10.9) | |
| Residential area | ||||
| Big metro | 1264 (55.9) | 626 (54.2) | 638 (57.5) | .26 |
| Metro | 682 (30.1) | 364 (31.5) | 318 (28.7) | |
| Nonmetro | 317 (14.0) | 164 (14.2) | 153 (13.8) | |
| Region | ||||
| Northeast | 519 (22.9) | 258 (22.4) | 261 (23.5) | .25 |
| Midwest | 222 (9.8) | 112 (9.7) | 110 (9.9) | |
| South | 541 (23.9) | 261 (22.6) | 280 (25.2) | |
| West | 981 (43.3) | 523 (45.3) | 458 (41.3) |
Values are reported as n (%) of patients unless otherwise specified.
Patterns of use of HMA
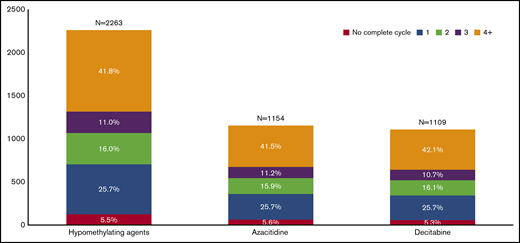
Median time from AML diagnosis to HMA initiation was 41 (IQR, 28-66) days, and patients received a median of 3 (IQR, 1-6) completed cycles without difference between the 2 agents. Only 946 (41.8%) patients completed ≥4 cycles with similar proportions for azacitidine- and decitabine-treated patients (Figure 1). Among these 946 patients, the median time from AML diagnosis to HMA initiation was the same as the overall cohort (41.5 days), with a median of 7 completed cycles (IQR, 5-12) and no difference between the 2 HMAs. Of patients receiving ≥4 cycles of HMA or surviving ≥6 months, the median number of cycles of either azacitidine or decitabine received remained stable by year during the study period. The large majority of patients (95.1%) were hospitalized at some point after their diagnosis, with a median number of 3 hospitalizations (first quartile, 2; fourth quartile, 5) and median 25-day hospitalization without differences between azacitidine- and decitabine-treated patients.
Distribution of older patients with AML by number of completed cycles and type of HMA used.
Distribution of older patients with AML by number of completed cycles and type of HMA used.
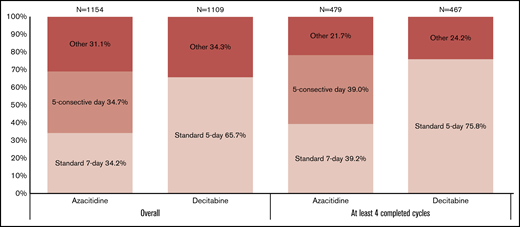
Only 34.3% (n = 395) of patients receiving azacitidine followed a 7-day regimen, with only 169 (42.8%) of those receiving therapy in 7 consecutive days. In fact, 400 patients received 5 days of azacitidine in 5 consecutive days for their first complete cycle. Among patients receiving decitabine, 65.7% (n = 729) followed the standard 5-day regimen (P < .01) (Figure 2). Only 105 (9.5%) of decitabine-treated patients were treated with a >5-day decitabine regimen, including 80 (7.2%) who received 10-day decitabine. Among the patients treated with 10-day decitabine, 50 (62.5%) received decitabine on 10 consecutive days. Patterns were similar when we limited to patients receiving ≥4 cycles, although the exact proportions differed, and smaller percentages of patients received medication on other schedules.
Distribution of older patients with AML by type of HMA dosing schedule used.
Survival outcomes
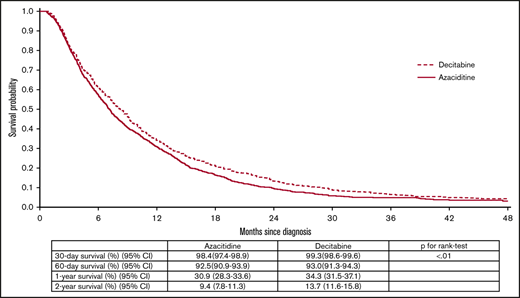
After a maximum of 8.97 (median, 0.64; IQR, 0.32-1.18) years of follow-up, the median survival from diagnosis was 7.6 (95% confidence interval [CI], 7.2-8.0) months. The median OS for azacitidine- and decitabine-treated patients was 7.1 (95% CI, 6.7-7.6) and 8.2 (95% CI, 7.6-8.9) months (P < .01), respectively (Figure 3). The 2-year OS for azacitidine- and decitabine-treated patients was 9.43% (95% CI, 7.76% to 11.29%) and 13.72% (95% CI, 11.70% to 15.91%), respectively. The 3-year OS for azacitidine- and decitabine-treated patients was 4.69% (95% CI, 3.45% to 6.19%) and 6.43% (95% CI, 4.94% to 8.17%), respectively. In multivariable analysis, a slightly higher risk of death was observed for patients treated with azacitidine compared with decitabine (hazard ratio [HR], 1.11; 95% CI, 1.01-1.21; P = .02). In addition, increasing age (P < .01) and Elixhauser comorbidity index ≥3 (P < .01) were independently-associated with worse survival (Figure 4). For those receiving ≥4 cycles of HMA, patients treated with azacitidine still had inferior survival when compared with their counterparts treated with decitabine (HR, 1.17; 95% CI, 1.02-1.35; P = .03) (Figure 5). However, when we restricted our analysis to patients who had received their HMA on a standard dosing schedule, the difference between the 2 HMAs (azacitidine vs decitabine [HR, 0.95; 95% CI, 0.83-1.08; P = .43]) no longer achieved statistical significance (data not shown). There was no statistically significant difference in OS for patients treated with 5- vs 10-day decitabine (HR, 0.94; 95% CI, 0.82-1.07; P = .33), including when only evaluating those patients completing ≥4 cycles (HR, 1.25; 95% CI, 0.98-1.59; P = .07). Among the 2055 (90.8%) patients with an available cause of death, the majority (63.7%) were reported to have died of AML.
Kaplan-Meier curves for OS among 2263 older adults with AML by type of the HMA, 2005-2015.
Kaplan-Meier curves for OS among 2263 older adults with AML by type of the HMA, 2005-2015.
Adjusted HRs and 95% CIs for associations between patient characteristics and OS among older adults with AML who received a HMA, 2005-2015 (n = 2263).
Adjusted HRs and 95% CIs for associations between patient characteristics and OS among older adults with AML who received a HMA, 2005-2015 (n = 2263).
Adjusted HRs and 95% CIs for associations between patient characteristics and overall among older adults with AML who received at least 4 cycles of a HMA, 2005-2015 (n = 946).
Adjusted HRs and 95% CIs for associations between patient characteristics and overall among older adults with AML who received at least 4 cycles of a HMA, 2005-2015 (n = 946).
RBC TI outcomes
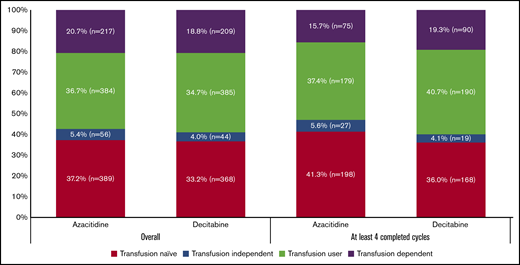
RBC transfusion status at HMA initiation was available for 1046 patients treated with azacitidine and 1006 patients treated with decitabine. We note that 211 patients initiated HMA ≤28 days before death, and their exposure was censored. Approximately 37% of evaluable patients were transfusion naive and 21% of patients were TD with no difference between the 2 agents (P = .71) (Figure 6). Among patients who completed ≥4 cycles, only 19.3% and 15.7% of azacitidine- and decitabine-treated patients, respectively, were TD (P = .15).
RBC transfusion dependence status among older patients with AML by the type of HMA used at time of initiation of therapy.
RBC transfusion dependence status among older patients with AML by the type of HMA used at time of initiation of therapy.
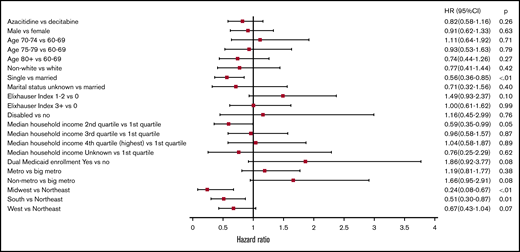
Of patients who were RBC TD at the time of HMA initiation (n = 426), 33.3% of patients (n = 142) eventually achieved TI, with no difference by HMA used (P = .37) (supplemental Figure 2). This increased to 57.0% when evaluating patients receiving ≥4 cycles, again with no difference between the 2 agents (P = .73) (supplemental Figure 3). Among the 142 patients achieving TI after HMAs, median time to TI was 14 (IQR, 10-20) weeks with a median duration of 17 (IQR, 5-38) weeks. Of TD patients completing ≥4 cycles of HMA and reaching TI, median time to and duration of TI was 14 (IQR, 11-20) and 26.5 (IQR, 9-50) weeks, respectively. In multivariable analysis, the choice of HMA agent had no impact on achieving TI among patients who were TD at initiation (azacitidine vs decitabine HR, 0.82; 95% CI, 0.58-1.16; P = .26). Similarly, sex, age, race, comorbidity, disability status, median household income, Medicaid dual enrollment, and urban status had no impact on achieving TI (Figure 7). We observed similar findings for those completing ≥4 cycles of HMA (azacitidine vs decitabine HR, 0.98; 95% CI, 0.61-1.57; P = .93) (supplemental Figure 4) or those with standard dosing schedule only (azacitidine vs decitabine HR, 0.76; 95% CI, 0.45-1.28; P = .30). There was no statistically-significant difference in achieving TI for patients treated with 5- vs 10-day decitabine (HR, 1.21; 95% CI, 0.71-2.08; P = .49), including when only evaluating those patients completing ≥4 cycles (HR, 0.76; 95% CI, 0.37-1.55; P = .45).
Adjusted HRs and 95% CIs for associations between patient characteristics and achieving RBC TI among older patients with AML who were RBC TD at time of initiation of the HMA (n = 426).
Adjusted HRs and 95% CIs for associations between patient characteristics and achieving RBC TI among older patients with AML who were RBC TD at time of initiation of the HMA (n = 426).
Discussion
This large, population-based study examined patterns of use and clinical benefits of HMAs for older AML patients in the real-world setting. The unadjusted median OS of azacitidine- and decitabine-treated patients from diagnosis was 7.1 and 8.2 months, respectively. The observed difference between azacitidine and decitabine regarding their impact on AML survival in unadjusted analyses was marginal and likely because decitabine-treated patients were younger, had fewer comorbid conditions, and were more likely to receive the published dosing schedule than azacitidine-treated patients. Indeed, after we adjusted for all these factors in a multivariable analysis, no significant differences in survival remained.
AZA-AML-001 was a multicenter, open-label, randomized, phase 3 trial that studied the standard 7-day azacitidine regimen in newly diagnosed AML patients and established the clinical utility of azacitidine in this population.12,13 Despite primary analysis from AZA-AML-001 demonstrating no improvement in median OS when compared with a “conventional care regimen,” a prespecified sensitivity analysis censoring patients at time of subsequent therapy (given the confounding effect of subsequent therapy on OS) did report improved OS for patients receiving azacitidine (12.1 vs 6.9 months; HR, 0.76; 95% CI, 0.60-0.96; P = .019).12,13 Another multicenter, open-label, randomized, phase 3 trial of decitabine on the standard 5-day schedule in AML patients aged ≥65 years (DACO-016) reported statistically significant improvement in median OS (7.7 vs 5.0 months; P = .108; HR, 0.85; 95% CI, 0.69-1.04) when compared with a composite arm of low-dose cytarabine or best supportive care (no intensive therapy) in primary analysis.10
The study provides new insights regarding patterns of treatment among older adults with AML receiving HMAs. We observed that azacitidine and decitabine were used in nearly identical proportions in the study sample, consistent with recent population studies.16-18 This pattern differs from the real-world treatment of patients with myelodysplastic syndrome, for whom azacitidine is used much more frequently.31 Our study further examines dosing regimens and the association with outcomes. Within the first completed treatment cycle, we observed that only one-third of azacitidine-treated and two-thirds of decitabine-treated AML patients received the standard 7- and 5-day dosing schedules, respectively. A significant proportion of patients receiving azacitidine received a shortened 5-day schedule, a regimen with little clinical trial data in this population with unknown efficacy. With respect to effectiveness, when we included patients with all dosing schedules, decitabine patients demonstrated improved survival compared with those treated with azacitidine; however, when patients receiving <7 days of azacitidine were dropped, we observed no significant difference in OS between the 2 agents. These findings suggest that azacitidine administered on a schedule other than the indicated 7-day schedule might be associated with inferior outcomes. Community-based infrastructure and patient wishes often limit the ability to administer azacitidine on the consecutive 7-day (7-0) or the weekend-interrupted (5-2-2) schedules. Our results suggest that if providers and/or their patients prefer to proceed with a 5-day schedule, they should strongly consider decitabine on the standard 5-day schedule rather than a shortened azacitidine schedule that might potentially compromise its clinical activity. Further research on dosing schedules and outcomes is needed.
The estimated median OS for HMA-treated patients in this study is similar to those reported in clinical trials, single-center studies, and prior population-based studies. The OS of decitabine-treated patients (8.2 months) is not substantially different of that reported in the DACO-016 trial (7.7 months), though azacitidine-treated patients in our study had a median OS (7.1 months) significantly lower than that noted in the AZA-AML-001 trial (10.4 months).10,13 This disparity may be explained by the relatively fewer cycles of HMA received by our population in comparison with those studied on AZA-AML-001. Single-center and small studies of older AML patients treated with HMAs have reported median OS estimates ranging from 8.0 to 14.4 months, which largely encompass the estimates provided in the largest phase 3 trials AZA-AML-001 and DACO-016.10,13,14,32-34 A meta-analysis of decitabine-treated, newly diagnosed AML patients aged ≥60 years reported a pooled median OS estimate of 8.1 months, though it should be noted that the analysis included several of the aforementioned single-center studies and data obtained from patients treated on nonstandard decitabine schedules.35 Our study found an identical median survival estimate for azacitidine-treated patients (7.1 months), though a longer median survival for decitabine-treated patients (8.2 months). Prior retrospective studies, including analysis of SEER-Medicare data, have shown no median survival difference between patients treated with azacitidine and decitabine.18,32 A single-center study also observed improved survival for patients treated with decitabine in comparison with azacitidine (8.3 vs 5.5 months, respectively; P = .03).34
One-third of HMA-treated patients who were TD at baseline achieved RBC TI. A majority (57%) of RBC TD patients who received ≥4 cycles of HMAs achieved TI, with a median duration of 26.5 weeks, regardless of which HMA (azacitidine vs decitabine) was administered. These findings are consistent with prior clinical trial data and suggest that HMAs lead to meaningful clinical benefits in a proportion of patients and therefore should be strongly considered even in the absence of a documented OS advantage in the community setting.10-13
Adequate duration of HMA therapy is critical, with a median time to best response for older AML patients reported to be ∼3 to 4 months.10,36-38 Our study demonstrated that only a minority (42%) of older AML patients treated with an HMA completed at least 4 cycles. Claims data do not permit us to assess the reasons for earlier discontinuation of therapy. However, among the 1317 patients who received <4 complete cycles, 55.7% (n = 733) died within 90 days after initiation of HMA, which indicates that early and rapid disease progression is likely to be a major contributor to early discontinuation. While some of the remaining early HMA discontinuations are appropriate given disease progression, clinical deterioration, or unacceptable toxicity, some cases may reflect management by clinicians with more limited understanding regarding the slow and delayed responses observed with HMAs in comparison with classical chemotherapy.39 Alternatively, patients and caregivers may find the standard schedule burdensome with respect to time and travel.
HMA therapy is being used as a backbone for development of combination regimens, yet the study results (with almost 60% of patients receiving ≤3 cycles) raise concerns about the viability of that strategy. The advent of venetoclax-based combinations represents an exciting step toward establishing a new standard of care for AML patients who are ineligible for intensive therapy. A phase 1b study of newly diagnosed AML patients aged ≥65 years and intensive therapy ineligible treated with venetoclax plus either azacitidine or decitabine reported impressive response rates (in the 70% range, including across several poor-risk subgroups) and low (3%) early mortality (at 30 days) without differences between azacitidine- and decitabine-treated patients.19 Ultimately, the median OS for all patients was not reached; venetoclax-HMA combination therapy was approved by the FDA for this population of patients based on this data.19 Novel HMA-based combinations (eg, venetoclax) might have more success than HMA monotherapy if they induce faster responses.19 While the FDA approved HMA-venetoclax combinations based on high rates and durability of responses in a highly selected phase 2 study population, the lack of an HMA monotherapy comparator arm limits unequivocal conclusions.
Our study faced limitations typically associated with the use of administrative claims data. We lacked information on laboratory results or other clinical parameters needed to accurately quantify patient risk at treatment initiation (including cytogenetic or molecular data) or assess responses to HMA therapy as defined by the International Working Group. Further, the lack of cytogenetic, molecular, and mutational data limits the ability to rectify any systematic bias in the specific HMA allocated to patients given the possibility that providers might prefer decitabine (including on a >5-day schedule) for patients with TP53-mutated disease. Given this limitation, we focused on RBC TI and OS, which reflect clinically meaningful benefits from HMA therapy that can be derived from the available data. While we can deduce the reason for early therapy discontinuation for some patients, we lack information on patient preferences or clinician recommendation regarding persistence in treatment. The study sample was population based yet limited to older AML patients (median age, 77 years) with continuous Medicare fee-for-service coverage. Our results may not be generalizable to younger AML patients or to Medicare beneficiaries enrolled in Medicare Advantage (health maintenance organization or preferred provider organization) plans.1 Despite these potential limitations, the study sample was large, included data on patient sociodemographic characteristics and comorbidities, and enabled measurement of HMA-agent dosing relative to schedule and number of cycles, with longitudinal follow-up of RBC TI status and OS. The rich data and sophisticated measures of treatment and outcomes implemented in this analysis allowed for a comprehensive examination in this older HMA-treated population.
In conclusion, we report one of the largest studies examining the outcomes of older AML patients receiving HMA monotherapy in a real-world setting. The population-level median OS with HMAs were 7 to 8 months. Differences in efficacy between azacitidine and decitabine are often questioned and remain relevant given their increasing use in combination with investigational agents. However, a large, randomized trial is unlikely to be undertaken, so our data provide the closest answer to how these agents compare in the real-world setting. We observed no clinically meaningful differences in OS or RBC TI between azacitidine- and decitabine-treated patients, but a substantial proportion of patients did not receive therapy on the standard and studied dosing scheduled. Further, the majority of patients did not complete the minimum recommended 4 cycles of HMA, which likely limits achievement of response and survival benefit for these patients. Therefore, combination HMA-based therapies (like those incorporating venetoclax) with shorter time to response are urgently needed for older AML patients.
Requests for data sharing should be sent to the corresponding author, Amer M. Zeidan (amer.zeidan@yale.edu).
Presented in part in a platform session at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
Acknowledgments
This work was supported by an investigator-initiated grant from Celgene Corporation (principal investigator: X.M.). A.M.Z. is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was in part supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA016359. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute’s SEER Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute, and the Centers for Disease Control and Prevention’s National Program of Cancer Registries under agreement #U55/CCR921930-02 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health; the National Cancer Institute; and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services, Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Authorship
Contribution: A.M.Z., R.W., A.J.D., and X.M. contributed to conception and design; R.W. and X.M. provided study materials or patients and collected and assembled data; A.M.Z., R.W., R.M.S., A.J.D., and X.M. wrote the manuscript; and all authors contributed to data analysis and interpretation and critical revision and final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: A.M.Z. received research funding (institutional) from Celgene, Acceleron, AbbVie, Novartis, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics; had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Ionis, Epizyme, and Takeda; and received travel support for meetings from Pfizer, Novartis, and Trovagene. R.W. received research funding from Celgene Corp. S.D.G. received research support from Celgene and Boehringer-Ingelheim and consulted for AbbVie. A.J.D. received research funding from Celgene Corp. X.M. received research funding (institutional) from Celgene Corporation for the work included in this article. The remaining authors declare no competing financial interests.
Correspondence: Amer M. Zeidan, Section of Hematology, Department of Internal Medicine, Yale School of Medicine, 333 Cedar St, New Haven, CT 06510-3222; e-mail: amer.zeidan@yale.edu.
References
Author notes
A.M.Z. and R.W. are joint first authors.
A.J.D. and X.M. are joint senior authors.
The full-text version of this article contains a data supplement.