Key Points
BEGEV/ASCT salvage therapy: 49 of 59 patients responded to BEGEV, 43 were autografted, and 33 remained in CR at 5 years and may be cured.
At 5-year follow-up, the BEGEV/ASCT program shows similar long-term safety and efficacy in both relapsed and primary refractory patients.
Abstract
The complete remission (CR) rate achieved with induction chemotherapy prior to autologous stem cell transplantation (ASCT) represents the strongest prognostic factor in relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL). By inducing a CR rate of 75%, the bendamustine, gemcitabine, vinorelbine (BEGEV) regimen represents an optimal chemotherapy regimen prior to ASCT. Presented here are the 5-year results of BEGEV followed by ASCT in R/R cHL. With a median follow-up of 5 years, progression-free survival (PFS) and overall survival (OS) for the whole series (n = 59) were 59% and 78%, respectively. ASCT was performed in 43 of 49 responding patients (73% by intention to treat [ITT]; 88% by response to BEGEV) and resulted in 33 with continuous CR (56% by ITT; 77% of transplanted patients), 7 with disease relapse, and 3 with nonrelapse mortality. For patients who received transplants, the 5-year PFS and OS were 77% and 91%, respectively, with no significant difference between relapsed and refractory patients. No patient experienced secondary leukemia or myelodysplasia. In summary, the long-term efficacy data, the benefits for both relapsed and refractory patients, and the excellent safety profile provide a strong rationale for further development of the BEGEV regimen. This trial was registered at EudraCT as #2010-022169-91 and at www.clinicaltrials.gov as #NCT01884441.
Introduction
The consensus emerging from phase 3 studies is that a substantially higher percentage of classical Hodgkin lymphoma (cHL) patients who are refractory to or relapse after first-line therapy may be cured with second-line salvage chemotherapy followed by autologous stem cell transplantation (ASCT) than with standard-dose chemotherapy.1,2 Thus, achieving complete remission (CR) prior to ASCT represents the strongest prognostic factor in refractory/relapsed (R/R) cHL.3 Over the past decades, a variety of combination chemotherapy regimens have been proposed as induction regimens prior to ASCT for R/R cHL.4-9 These regimens resulted in pre-ASCT overall response rates (ORRs) and CR rates that ranged from 60% to 80% and from 20% to 54%, respectively.10 Notwithstanding the heterogeneity of these results, which likely resulted from differences in risk stratification and improvements in disease assessment, combination chemotherapy may still represent a reliable backbone for the optimization of second-line salvage therapy in R/R cHL. We previously reported a prospective multicenter single-arm phase 2 study showing that the combination of bendamustine, gemcitabine, and vinorelbine (BEGEV) as second-line chemotherapy before ASCT, in 59 patients with R/R cHL, resulted in an ORR of 83% and a CR of 73%.11 Here, we present the 5-year efficacy, durability, and long-term safety results of the BEGEV regimen followed by ASCT.
Study design
The complete study design and statistical analysis have been previously reported.11 Briefly, patients who were primary refractory (n = 27) to or had relapsed (n = 32) after receiving 1 previous line of chemotherapy were enrolled in a prospective multicenter single-arm phase 2 study using BEGEV followed by ASCT as second-line salvage therapy in R/R cHL (supplemental Appendix). Refractory disease was defined as disease progression during or within 3 months after doxorubicin-based chemotherapy, whereas relapsed disease was defined as reappearance of disease after a complete response lasting ≥3 months.12 The BEGEV regimen consisted of 800 mg/m2 gemcitabine on days 1 and 4, 20 mg/m2 vinorelbine on day 1, and 90 mg/m2 bendamustine on days 2 and 3.11 Prednisolone (100 mg) was administered on days 1 to 4. Four cycles of the BEGEV regimen were administered every 21 days. Patients who achieved CR or partial response (PR) after completion of the planned 4 cycles received myeloablative therapy with carmustine, etoposide, cytarabine, and melphalan (BEAM; n = 20) or fotemustine, etoposide, cytarabine, and melphalan (FEAM; n = 23), followed by the reinfusion of CD34+ cells. Disease responses were assessed before and after the fourth BEGEV cycle by computed tomography and [18F]fluorodeoxyglucose–positron emission tomography scans according to the International Working Group response criteria.13 Progression-free survival (PFS) was determined from first BEGEV administration to disease progression/relapse or death, whichever occurred first, or until the last disease assessment for patients who were alive and did not have progressive disease, and overall survival (OS) was calculated from first BEGEV administration to death or last contact. The study was conducted in accordance with the Declaration of Helsinki, and the ethical committees of the participating centers approved the study protocol and the informed consent form. All patients signed an informed consent form before inclusion.
Results and discussion
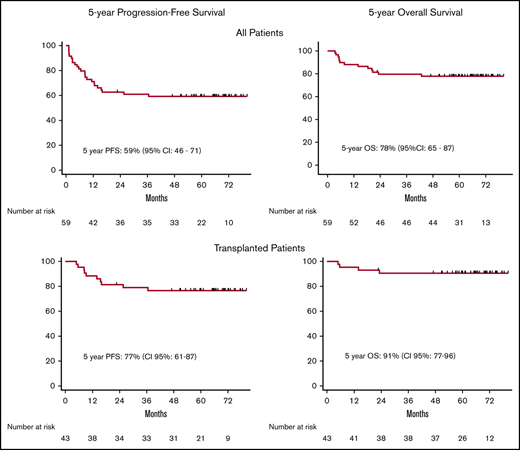
As previously reported, 59 patients with relapsed (46%) or primary refractory (54%) cHL received the BEGEV/ASCT program as second-line salvage therapy.11 BEGEV induced CR in 44 patients (75%) and PR in 5 (8%), for an ORR of 83% (Figure 1). The CR rate achieved with BEGEV was substantially higher than that reported for the ifosfamide, gemcitabine, vinorelbine, and prednisolone (IGEV) regimen (75% vs 54%)9 as well as a variety of second-line chemotherapy-based salvage regimens (eg, ifosfamide, carboplatin, and etoposide [ICE]; dexamethasone, high-dose cytarabine (ara-C), and cisplatin [DHAP]; gemcitabine, vinorelbine, and pegylated liposomal doxorubicin [GVD]; gemcitabine, dexamethasone, and cisplatin [GDP]; and etoposide, methylprednisolone (solumedrol), high-dose cytarabine (ara-C), and cisplatin [ESHAP]).4-8 By design, all BEGEV responders (n = 49) were eligible for ASCT, and 43 of 49 patients (73% by intention to treat; 88% by response to BEGEV) were indeed autografted (Figure 1). With a median follow-up of 5 years (range, 3-89 months) following BEGEV initiation, the 5-year PFS for all series was 59% (95% confidence interval, 46% to 71%), and OS was 78% (95% confidence interval, 65% to 87%) (Figure 2A-B).
CONSORT diagram showing the flow of participants. *One patient considered to be in PR at the time of the initial report11 was classified as being in CR when disease response was reassessed for the current analysis. CCR, continuous complete remission; NRM, nonrelapse mortality; PD, progressive disease; SD; stable disease.
CONSORT diagram showing the flow of participants. *One patient considered to be in PR at the time of the initial report11 was classified as being in CR when disease response was reassessed for the current analysis. CCR, continuous complete remission; NRM, nonrelapse mortality; PD, progressive disease; SD; stable disease.
Kaplan-Meier curves. PFS (A) and OS (B) of the overall population. PFS (C) and OS (D) of patients with relapsed (blue line) or refractory (red line) disease prior to BEGEV. PFS (E) and OS (F) of patients who achieved CR or PR and underwent ASCT.
Kaplan-Meier curves. PFS (A) and OS (B) of the overall population. PFS (C) and OS (D) of patients with relapsed (blue line) or refractory (red line) disease prior to BEGEV. PFS (E) and OS (F) of patients who achieved CR or PR and underwent ASCT.
According to the risk factors (ie, primary refractory disease, relapse within 12 months, or extranodal disease at relapse) recently described by the AETHERA study, 91% of patients had ≥1 risk factor and would have met eligibility criteria to be considered at high risk for relapse after ASCT.14 With a median 5-year follow-up, 33 autografted patients (56% by intention to treat; 77% by ASCT) experienced continuous CR, 7 relapsed, and 3 died due to nonrelapse mortality (pneumonia, n = 1; infection, n = 1; multiorgan failure, n = 1). Disease relapse after transplant occurred at a median of 6.2 months (range, 3-28 months) from ASCT, with only 2 patients relapsing beyond 12 months. Notwithstanding the high relapse risk expected after ASCT for our patient population,14 the therapeutic BEGEV/ASCT program allowed autograft recipients to experience long-term disease control with a 5-year OS and PFS of 91% and 77%, respectively (Figure 2E-F). Despite the reduced activity of BEGEV in inducing CR in primary refractory patients,11 disease status at study entry did not impact on 5-year PFS and OS (Figure 2C-D).
The BEGEV regimen has excellent stem cell mobilization activity, which allowed successful harvest in 55 of 57 evaluable patients (96.5%), with all patients experiencing full hematopoietic engraftment with a favorable long-term toxicity profile, with no patients developing secondary leukemia or myelodysplasia. These results further confirm that BEGEV is a second-line salvage regimen with an excellent safety profile.
The patients who did not receive transplants (n = 16) showed a median survival of 32 months with a 5-year OS of 43%. At the last follow-up, 7 patients were alive, whereas 9 patients died due to disease progression (n = 7), infection (n = 1), and unknown cause (n = 1). Overall, 18 of 23 patients who failed the BEGEV/ASCT program (16 who did not receive transplants and 7 who relapsed after ASCT) received further treatments, including radiation therapy (n = 3), brentuximab vedotin (n = 13), nivolumab (n = 2), standard-dose chemotherapy (n = 7), autologous (n = 4), allogeneic transplants (n = 4), tandem autologous/allogeneic transplants (n = 2). Response to these heterogeneous treatments was CR (n = 9), PR (n = 1), and progressive disease (n = 6) whereas 2 patients were responding but still on therapy at the time of the present analysis.
Several studies are evaluating brentuximab vedotin or programmed cell death 1 inhibitors in combination with conventional chemotherapy for the treatment of R/R cHL. The addition of brentuximab vedotin to chemotherapy combination significantly improved the efficacy of salvage treatments.15-20 However, although therapy with brentuximab vedotin–based regimens reached ORR and CR rates comparable to those achieved with the BEGEV regimen, the combination of brentuximab vedotin and bendamustine is associated with significant toxicity, including 56% G3/4 hematological and nonhematological toxicities and 36% treatment discontinuation.17 More recently, a second-line chemotherapy-free approach combining brentuximab vedotin with nivolumab has been proposed.21 This combination is an effective and well-tolerated second-line salvage regimen with a CR rate of 62% and an ORR rate of 82%. However, 20% of these patients did require salvage chemotherapy before ASCT, and an adequate follow-up is needed to assess the efficacy on a long-term basis and determine whether the brentuximab vedotin/nivolumab combination may eventually replace the need for cytotoxic chemotherapy before ASCT.10
In conclusion, further validation of the efficacy and safety of the BEGEV regimen has recently been provided by a real-world analysis including 70 patients who received BEGEV as second-line salvage treatment (C.C.-S., manuscript in preparation), resulting in efficacy and toxicity data comparable to those obtained in the present study. Combination with programmed cell death 1 inhibitors will be the next step of BEGEV development to improve the outcome of primary refractory patients who fail to respond to BEGEV.
Acknowledgments
The authors thank the patients who participated in this study, the families of the participants, and the investigators and staff at the clinical sites.
This work was partially supported by an unrestricted grant provided by Mundipharma Pharmaceuticals srl, which also provided bendamustine.
The funder was not involved in the interpretation of the data or the writing of the report.
Authorship
Contribution: A.S., L.G., and C.C.-S. conceived of and designed the study, contributed to the analysis and interpretation of the data, and wrote the manuscript; and all authors provided study materials or enrolled patients in the study, collected and assembled data, reviewed and commented on the analysis, contributed to the writing of the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.S. reports receiving honoraria for speaker engagements and scientific advisory fees from Bristol-Myers Squibb, Servier, Gilead, Pfizer, Eisai, Bayer, Merck Sharp & Dohme, ArQule, Takeda, Roche, AbbVie, Amgen, Celgene, AstraZeneca, Pfizer, Lilly, Sandoz, and Novartis. A. Pulsoni reports receiving personal fees from Roche, Merck Sharp & Dohme, Pfizer, Sandoz, Takeda, Gilead, and Bristol-Myers Squibb. F.M. reports receiving nonfinancial support from Roche, Takeda, and Celgene and personal fees from Janssen and Gilead. S.L. reports personal fees from Roche, Celgene, Teva Pharmaceuticals, Gilead Sciences, and Takeda Pharmaceuticals. C.C.-S. reports receiving honoraria for speaker engagements from Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen, Janssen Oncology, and AstraZeneca, and scientific advisory fees from Sanofi, ADC Therapeutics, Servier, Boehringer Ingelheim, Novartis, Roche, Genenta Science srl, and Rhizen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Armando Santoro, Humanitas Cancer Center, Via Manzoni 56, 20089 Rozzano (MI), Italy; e-mail: armando.santoro@cancercenter.humanitas.it.
References
Author notes
Deidentified clinical data will be provided to the Protocol Registration and Results System of clinicaltrials.gov within 1 year of publication.
The full-text version of this article contains a data supplement.