Key Points
In children undergoing tonsillectomy, low VWF levels did not predict surgical bleeding in the absence of major bleeding history.
Children have lower VWF levels than adults and these lower levels do not appear to be associated with bleeding.
Abstract
von Willebrand disease is a common bleeding disorder, but diagnosis can be difficult in young children who have not had bleeding challenges. We sought to evaluate the correlation between bleeding and von Willebrand factor (VWF) levels in children undergoing surgical challenge with tonsillectomy. Children ages 0 to 18 undergoing tonsillectomy without a personal or family history of bleeding were enrolled prospectively following informed consent and institutional review board approval. VWF levels were obtained at the time of surgery. VWF antigen (VWF:Ag) and VWF activity (VWF:GPIbM) were tested via enzyme-linked immunosorbent assay. Bleeding score was calculated using the International Society of Hematology bleeding assessment tool (BAT). Surgical and postoperative bleeding were determined using questionnaires filled out by the surgeon and patient/family. A total of 1399 subjects were enrolled with evaluable data, with a median age of 5 years. The median VWF:Ag was 85 IU/dL and the median VWF:GPIbM was 100 U/dL. Median BAT for the entire population was 0, including those with postoperative bleeding. There was no difference in VWF level between those who experienced postoperative bleeding and those who did not, with median VWF:Ag 85 vs 85 (P = .89) and mean VWF:GPIbM 98 vs 100 (P = .5). Interestingly, there was a difference in VWF levels with age, with median VWF:Ag 81 for those younger than 3 years, 82 for those 3 to 6 years, 90 for those 7 to 10 years, and 100 for those 11 to 18 years. A similar trend was noted for VWF:GPIbM. Of the 2 to 6 year olds, 5% had VWF:Ag <50, which would meet criteria for low VWF, but only 1.8% had an abnormal BAT at study entry and only 2.5% bled after surgery. Only 1 subject with low VWF had an elevated postoperative BAT >2. These data suggest that low VWF levels do not correlate with bleeding in children undergoing tonsillectomy. In addition, VWF levels outside the adult normal range in young children may be more common than previously thought and do not necessarily predict surgical bleeding.
Introduction
von Willebrand disease (VWD) is a common bleeding disorder. Although previous studies have suggested that 1 in 100 people have low von Willebrand factor (VWF) and possibly, VWD, more recent studies of primary care patients show VWD potentially affects 1 in about 1000 individuals.1,2 Diagnosis typically requires the combination of personal history of bleeding, family history of bleeding or diagnosed VWD, and abnormal laboratory testing for VWD.3 However, VWD may be more difficult to diagnose in children for several reasons. First, bleeding history may or may not be helpful in young children who have not had time to experience significant challenges. Many clinicians remain concerned that a negative bleeding history in a young child may not exclude the diagnosis of VWD. Second, some bleeding symptoms such as bruising or nosebleeds4 are often normal symptoms of childhood. Therefore, presence of bleeding in and of itself may not necessarily be indicative of a bleeding disorder. Third, low VWF levels of 30 to 50 IU/dL do not necessarily suggest that a person has VWD.5
These challenges are particularly evident when considering a common childhood surgery, tonsillectomy. This surgery is associated with potential postoperative bleeding, with reported frequencies of around 2% to 5% along with the possibility of life-threatening hemorrhage and pneumonia.6,7 Some groups advocate for coagulation testing before surgery to identify patients at increased risk of bleeding.8 Conversely, others have suggested that coagulation screening does not reliably identify those at risk, but adds unnecessary cost.9-11 There are also guidelines, including the British Committee for Standards in Haematology, that recommend against routine coagulation testing.12 A recent meta-analysis confirmed the lack of sensitivity with current screening tests.13
We sought to investigate the relationship between VWF levels and bleeding history in the preoperative and in the immediate postoperative tonsillectomy period. This study has also provided the opportunity to evaluate VWF levels in children of different ages.
Methods
Subjects were recruited from the Children’s Hospital of Wisconsin Ear, Nose, and Throat (ENT) Clinic. Informed consent was obtained from all subjects and their families. Eligibility included planned tonsillectomy, with or without accompanying adenoidectomy, and age ≤18. Exclusion criteria included a known bleeding disorder, known abnormal coagulation testing, or significant personal history of bleeding given to the ENT provider before scheduling. Those subjects and/or their families who disclosed a significant bleeding history or a family history of bleeding or known bleeding disorder were referred to hematology to perform clinical and laboratory evaluation. The study was approved by the Children’s Hospital of Wisconsin institutional review board. Informed consent was obtained from all parents and assent obtained from children aged 7 to 17 years old. Enrollment occurred over a 4-year period between 2014 and 2018.
Bleeding history was obtained and scored using the International Society of Hematology (ISTH) Bleeding Assessment Tool (BAT).14 Because the majority of subjects were children were younger than 18, a score of 2 or less was considered normal. For those subjects who were 18 years of age at the time of taking the bleeding history, a normal score for males was 3 or less and a normal score for females was 5 or less.15 Sex, race, and ethnicity were self-reported and subjects and their families had the option to not respond to any questions if desired.
All subjects had blood drawn at the time of anesthesia for plasma VWF levels. The majority of samples were obtained following induction of anesthesia. Blood was collected into sodium citrate and plasma obtained for analysis. VWF antigen (VWF:Ag) was performed as previously described.16 VWF binding to platelet GPIb was assessed using the VWF:GPIbM assay as previously described.17 VWF:Ag values <50 IU/dL and VWF:GPIbM values <50 U/dL were considered to be abnormal. The reference range for the VWF:Ag in the research laboratory for adult healthy control subjects is 48 to 224 IU/dL and the reference range for the VWF:GPIbM is 49 to 243 U/dL.
Bleeding during surgery was identified via the surgeon’s operative note in the electronic medical record and a form filled out by the surgeon following surgery (supplemental Table 1). Bleeding during the postoperative period was identified by a diary filled out either by the patient and/or family or by the research coordinator over the phone if the family failed to send its diary back to the research coordinators (supplemental Figure 1). Virtually all bleeding that resulted in a return to the hospital was to our emergency room and therefore captured. In some cases, data were supplemented by information from the patient’s medical chart if the family did not return complete information. Bleeding was monitored for 14 days following surgery.
Continuous variables were summarized as median (range) and categorical variables as frequency (%). For comparison of different groups, the χ2 test or Fisher’s exact test was used for categorical variables, and the Mann-Whitney U test was used for continuous variables. Linear regression was used to compare VWF levels and age at enrollment. Statistical analyses were implemented using SAS (Cary, NC).
Results
A total of 1447 subjects were enrolled, with 1399 subjects having fully evaluable data. Most of the subjects excluded from analysis were missing the postoperative bleeding diary. The median age of enrolled subjects was 5 years and mean age was 6.0 years. The largest segment of children undergoing tonsillectomy fell into the 3- to 6-year-old age range. Subject demographics and VWF levels are given in Table 1.
Demographics of subjects enrolled in tonsillectomy study
| No. of subjects . | 1399 . |
|---|---|
| Age, median (IQR), y | 5 (4-7) |
| Female, n (%) | 650 (46) |
| Male, n (%) | 748 (54) |
| White, n (%) | 928 (70) |
| African American, n (%) | 343 (26) |
| Race: other, n (%) | 49 (4) |
| Hispanic, n (%) | 246 (18) |
| Non-Hispanic, n (%) | 1141 (82) |
| VWF:Ag, median (IQR), IU/dL | 85 (67-109) |
| VWF:GPIbM, median (IQR), U/dL | 100 (77-131) |
| ISTH BAT, median (IQR) | 0 (0-0) |
| Postoperative bleeding score, median (IQR) | 0 (0-0) |
| No. of subjects . | 1399 . |
|---|---|
| Age, median (IQR), y | 5 (4-7) |
| Female, n (%) | 650 (46) |
| Male, n (%) | 748 (54) |
| White, n (%) | 928 (70) |
| African American, n (%) | 343 (26) |
| Race: other, n (%) | 49 (4) |
| Hispanic, n (%) | 246 (18) |
| Non-Hispanic, n (%) | 1141 (82) |
| VWF:Ag, median (IQR), IU/dL | 85 (67-109) |
| VWF:GPIbM, median (IQR), U/dL | 100 (77-131) |
| ISTH BAT, median (IQR) | 0 (0-0) |
| Postoperative bleeding score, median (IQR) | 0 (0-0) |
IQR, interquartile range.
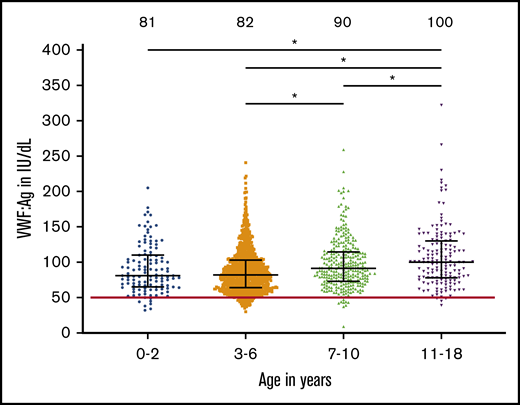
The mean VWF:Ag was 85 IU/dL (range, 9-322) and mean VWF:GPIbM was 100 U/dL (range, 10-411) for all enrolled subjects, but levels did differ by age. Figure 1 shows VWF levels by age, with lowest average levels seen in the youngest subjects and highest average levels seen in the oldest subjects. The median VWF:Ag was 81 for those younger than 3 years, 82 for those 3 to 6 years, 90 for those 7 to 10 years, and 100 for those 11 to 18 years. A similar trend was noted for VWF:GPIbM. Of the 3- to 6-year-old subjects, 5% (44 subjects) had VWF:Ag <50 IU/dL, which would meet the criteria for low VWF. Linear regression analysis demonstrated a significant association of VWF:Ag with age (P < .001).
VWF levels are lower in young children. This graph shows the VWF:Ag on the y-axis for the indicated age groups. Bars indicate the median interquartile range. The median for each group is given at the top of the graph. *Groups with a significant difference (P < .001) are noted by lines.
VWF levels are lower in young children. This graph shows the VWF:Ag on the y-axis for the indicated age groups. Bars indicate the median interquartile range. The median for each group is given at the top of the graph. *Groups with a significant difference (P < .001) are noted by lines.
Previous bleeding history was assessed using the ISTH BAT score. The median BAT for the entire population was 0 (range, 0-8). The median BAT was also 0 for those subjects who went on to experience a postoperative bleed. Only 2% of subjects had an abnormal bleeding score for their age. Of all subjects enrolled, 84% had a bleeding score of 0. There was no difference between males and females (both with median of 0). Median bleeding scores were still 0 for children <10 years of age and for those ages 10 to 18, but there was a statistically significant difference (P < .001) in ISTH BAT, with older children having the highest bleeding scores. Abnormal bleeding scores were due to bruising, epistaxis, surgical bleeding, gastrointestinal bleeding, tooth extraction, minor wounds, intracranial bleeding, and heavy menstrual bleeding. Other than the presence of heavy menstrual bleeding exclusively in adolescent females, there was no apparent age association between positive bleeding score and specific symptoms.
Postoperative bleeding (supplemental Table 1), as reported by patient/parent-completed diaries, occurred in 248 subjects (18%). Only 46 subjects (3%) had postoperative bleeding scores of >2 and 27 subjects (2%) had postoperative bleeding scores >3. Only 8 subjects had a postoperative bleeding score >5 (0.6%). Postoperative bleeding occurred across the 14-day period of observation without any particular pattern.
There was no difference in VWF level between those who experienced postoperative bleeding and those who did not, as shown in Table 2. For those who did not experience a postoperative bleed, mean VWF:Ag was 85 IU/dL (interquartile range, 9-322) and mean VWF:GPIbM was 100 U/dL (interquartile range, 10-411). For those who did experience a postoperative bleed, mean VWF:Ag was 85 IU/dL (range, 37-190) and mean VWF:GPIbM was 98 U/dL (range, 36-290). One subject had a VWF:Ag of 9 and VWF:GPIbM of 10, with an ISTH BAT of 0 and a postoperative bleeding score of 0. Table 3 shows mean VWF level for each age group studied.
Comparison of demographics, bleeding, and VWF levels for subjects who experienced postoperative bleeding as compared with subjects who did not experience bleeding
| . | Bleed . | No bleed . | . |
|---|---|---|---|
| Number of subjects | 248 | 1151 | |
| Age, median (IQR), y | 6 (0-18) | 5 (0-18) | P < .005 |
| 0-2, n (%) | 19 (16) | 97 (84) | |
| 3-6, n (%) | 123 (14) | 740 (86) | |
| 7-10, n (%) | 75 (27) | 199 (73) | |
| 11-18, n (%) | 31 (21) | 115 (79) | |
| Female, n (%) | 102 (16) | 548 (84) | P = NS |
| Male, n (%) | 145 (19) | 603 (81) | |
| White, n (%) | 149 (16) | 779 (84) | P = NS |
| African American, n (%) | 74 (21) | 269 (78) | |
| Hispanic, n (%) | 49 (20) | 197 (80) | P = NS |
| Non-Hispanic, n (%) | 197 (17) | 944 (83) | |
| VWF:Ag, median (IQR), IU/dL | 85 (37-190) | 85 (9-322) | P = NS |
| VWF:GPIbM, median (IQR), U/dL | 98 (36-290) | 100 (10-411) | P = NS |
| ISTH BAT: normal (≤2), n (%) | 236 (17) | 1125 (83) | P < .005 |
| . | Bleed . | No bleed . | . |
|---|---|---|---|
| Number of subjects | 248 | 1151 | |
| Age, median (IQR), y | 6 (0-18) | 5 (0-18) | P < .005 |
| 0-2, n (%) | 19 (16) | 97 (84) | |
| 3-6, n (%) | 123 (14) | 740 (86) | |
| 7-10, n (%) | 75 (27) | 199 (73) | |
| 11-18, n (%) | 31 (21) | 115 (79) | |
| Female, n (%) | 102 (16) | 548 (84) | P = NS |
| Male, n (%) | 145 (19) | 603 (81) | |
| White, n (%) | 149 (16) | 779 (84) | P = NS |
| African American, n (%) | 74 (21) | 269 (78) | |
| Hispanic, n (%) | 49 (20) | 197 (80) | P = NS |
| Non-Hispanic, n (%) | 197 (17) | 944 (83) | |
| VWF:Ag, median (IQR), IU/dL | 85 (37-190) | 85 (9-322) | P = NS |
| VWF:GPIbM, median (IQR), U/dL | 98 (36-290) | 100 (10-411) | P = NS |
| ISTH BAT: normal (≤2), n (%) | 236 (17) | 1125 (83) | P < .005 |
NS, not significant.
Range of VWF antigen values by age
| Age, y . | Mean ± 2 SD . |
|---|---|
| 0-2 | 91 ± 68 |
| 3-6 | 88 ± 63 |
| 7-10 | 98 ± 71 |
| 11-18 | 107 ± 88 |
| Age, y . | Mean ± 2 SD . |
|---|---|
| 0-2 | 91 ± 68 |
| 3-6 | 88 ± 63 |
| 7-10 | 98 ± 71 |
| 11-18 | 107 ± 88 |
SD, standard deviation.
There was minimal overlap between low VWF levels and postoperative bleeding. Although 59 subjects (4%) had low VWF levels as defined by VWF:Ag or VWF:GPIbM <50 IU/dL and 46 subjects (3%) had significant postoperative bleeding as defined by a postoperative bleeding score >2, only 2 subjects fell into both categories (0.1% of enrolled subjects). The overlap is shown graphically in Figure 2. Only 1 subject with a postoperative bleeding score >2 had both VWF:Ag and VWF:GPIbM <50 IU/dL. One additional subject had VWF levels <50 IU/dL and a postoperative bleeding score of 1. Overall, 84% of subjects had no reported postoperative bleeding. Of those who reported bleeding, no association with timing of bleeding symptoms was seen.
Minimal overlap between low VWF levels and postoperative bleeding. This Venn diagram shows the number of subjects with decreased VWF:Ag, the number of subjects with increased postoperative bleeding, and the minimal overlap between the 2 groups.
Minimal overlap between low VWF levels and postoperative bleeding. This Venn diagram shows the number of subjects with decreased VWF:Ag, the number of subjects with increased postoperative bleeding, and the minimal overlap between the 2 groups.
Similarly, there was minimal overlap between abnormal bleeding score (ISTH BAT) obtained preoperatively and postoperative bleeding. Of those subjects with significant postoperative bleeding, only 2 subjects had an abnormal ISTH BAT (4%). When any amount of postoperative bleeding was considered, only 12 subjects had an abnormal ISTH BAT (5%). The vast majority of subjects had a score of 0 for both. For those with positive scores, the subjects with elevated preoperative bleeding scores were not necessarily those with elevated postoperative bleeding scores.
Discussion
The results from this study showed no significant overlap between posttonsillectomy bleeding and VWF levels <50 IU/dL. The vast majority of subjects, as would be expected in a pediatric population, had no history of bleeding, with a median bleeding score of 0. The postoperative bleeding was in line with previous reports of bleeding complications following tonsillectomy.6,7 As shown in Figure 2, there were some subjects with low VWF levels and some subjects with documented posttonsillectomy bleeding, but only 2 (or 0.1%) with both a history of bleeding as well as low VWF levels. One subject with VWF:Ag of 9 IU/dL had both preoperative and postoperative bleeding scores of 0. This is of interest given previous population studies suggesting that clinically significant low VWF with bleeding occurs in about 1 in 1000 individuals.2
Bleeding histories are difficult in young children because they may not have had sufficient challenges. The largest group of subjects in our study included children ages 3 to 6 years, and very few subjects in this group had an abnormal ISTH BAT. Even in older children, the median bleeding score was 0. Given the lack of postoperative bleeding seen in our study subjects, this may simply support the notion that a prior history of bleeding is the most critical factor in assessing future medical or surgical bleeding. In adult patients with VWD, there is evidence that history of bleeding is one of the key factors in future bleeding.18 However, even the subjects with high bleeding scores in our study did not overlap with the subjects who experienced postoperative bleeding. We also cannot exclude the possibility that young children with normal bleeding scores and low VWF levels would have higher levels with age, and therefore never experience significant bleeding, but longitudinal data will be required to assess this possibility.
In our study, 5% of children younger than age 7 had a VWF level below 50 IU/dL, yet this did not correlate with postoperative bleeding score. Previous reports have suggested VWF levels increase with age.19,20 The data from this study suggest that most normal ranges may be predicated on adult populations, and that “normal” VWF levels for young children may be lower than a typical adult. If the normal range is defined as mean ± 2 standard deviations, it would be expected that 2.5% of any population would have levels below the lower limit of normal. Our observation here suggests that an even higher percentage of younger children have “low VWF.”
This raises the question of what to do with subjects with low VWF levels (30-49 IU/dL) whose levels and symptoms might meet criteria for a diagnosis of VWD or low VWF. There is significant debate at present about the correct cutoff for a diagnosis of VWD with some sources considering only VWF levels <30 IU/dL to be pathogenic, whereas others argue that patients with VWF levels between 30 and 50 IU/dL can have significant bleeding and thus deserve a diagnosis of VWD.3,21 Without wishing to specify age-specific normal ranges, it should be noted that in patients without any history of bleeding, an isolated low VWF level does not necessarily merit a diagnosis of VWD. There are risks in assigning a diagnosis of VWD, including increased cost of care, exposure to medications, insurance concerns, and changes in lifestyle such as avoidance of contact sports. Therefore, we suggest that assigning a diagnosis of VWD solely on the basis of VWF levels lower than the normal range may not be appropriate.
Previous reports have suggested that preoperative laboratory screening for bleeding disorders, including VWD, is not cost effective.22 Our results would support such a conclusion, given the lack of correlation between VWF levels and surgical bleeding. One study has suggested use of educational tools to decrease inappropriate testing.23 Current published guidelines from the British Committee on Standards in Haematology confirm that routine coagulation screening is unnecessary.12 There may be other reasons for bleeding beyond coagulation factor abnormalities, such as platelet function defects or collagen vascular disease, that would not be captured by coagulation factor bleeding, and there may also be a baseline risk of bleeding to surgical procedures such as tonsillectomy apart from congenital hemostatic abnormalities.
One limitation of this study is that children with a history of major bleeding were excluded. Although there were subjects with abnormal bleeding scores, anyone who disclosed significant bleeding or a family history of a bleeding disorder at their ENT visit was excluded from the study. This may limit the generalizability of our data because many clinicians would elect to perform laboratory testing on patients presenting with bleeding. However, these results do also support the contention that screening laboratory testing on asymptomatic patients does not reduce postoperative bleeding complications.
Another potential limitation is that not all children undergoing surgery were enrolled. However, observations by study staff as well as the varied demographics of the study population (which is similar to those seen in the referral area for our hospital), both suggest that no particular subgroup was excluded. The vast majority of subjects were English speaking, but there is no reason to suspect that the prevalence of low VWF or bleeding is higher in non-English–speaking patients.
It is also possible that there was a difference in recall of bleeding for those families that filled out diaries and those who required a phone call from the research coordinator. However, any bleeding requiring return to the hospital or surgery would have been captured in our system. In addition, the majority of the subjects reported no bleeding and for those families who reported postoperative bleeding, it appears to have been memorable enough to have been recalled clearly regardless of the form used to quantify it.
A final concern is that many subjects had their blood drawn following induction of anesthesia. It is possible that this resulted in lower VWF levels than would be expected had they been drawn while awake. Of note, the majority of the blood samples were obtained after masking the patient asleep with anesthesia gases and during placement of the peripheral intravenous line, which occurs within the first 5 minutes of the anesthetic. There remains a concern in the pediatric population that VWF levels are increased when drawn in a clinic laboratory setting resulting from stress. Similarly, there is concern that young children can also be stressed in the preoperative setting when separated from their parents.
In conclusion, this is the largest prospective study investigating VWF levels among children undergoing a common surgical setting and it suggests 3 important take-home messages. First, assessment of VWF levels in healthy children undergoing tonsillectomy appears to be insufficient to predict bleeding at surgery, with the important caveat that should a patient report a history of significant bleeding, it would be prudent to investigate this subject for VWD or another bleeding disorder. Second, bleeding score in a relatively healthy population did not predict postoperative bleeding, although the majority of subjects in this study were quite young with relatively scant time to develop bleeding history. Third, VWF levels appear to be, on average, lower in younger children, without evidence of an increased bleeding risk associated with these lower levels. Five percent of our study population had low VWF levels (<50 IU/dL) and this percentage is much higher than initial studies suggesting that 1% of the population might have VWD.1 The 5% of subjects in our study with low VWF also contrasts to the 2.5% that would be expected to have levels below the normal range. Therefore, low VWF in young children may require more cautious interpretation.
Acknowledgments
The authors thank the patients who graciously participated in this study, the research coordinators and anesthesiologists who collected the data, and all the Ear, Nose, and Throat Clinic staff for their enthusiasm and assistance.
This work was funded in part by the National Institutes of Health, National Heart, Lung, and Blood Institute grants HL081588 and HL144457 (R.R.M.), HL126810 (V.H.F.), and HL112614 (R.R.M. and T.C.A.). Additional funding was provided by the MACC Fund Center for Cancer and Blood Disorders, the Versiti Blood Center Research Fund, and grant H30MC24052 from the US Department of Health and Human Services, Health Resources and Services Administration.
The authors acknowledge their coauthor, Joan Cox Gill, who was an instrumental part of the study’s initiation and eagerly analyzed data as they became available. Although she unfortunately passed away before the final enrollment, she will always be remembered as a leader and mentor for many of us in the field.
Authorship
Contribution: J.C.G., S.F.C., T.C.A., R.R.M., and V.H.F. conceived the study and analyzed data; V.P.J. supervised enrollment of subjects and data collection; P.A.C., S.L.H., C.D.D., and T.C.S. collected and analyzed data; J.Z. and P.S. performed the statistical analysis; V.H.F. wrote the manuscript; and all authors edited and approved the manuscript.
Conflict-of-interest disclosure: R.R.M. has a patent assigned to the Versiti BloodCenter of Wisconsin on a von Willebrand factor platelet-binding assay. The remaining authors declare no competing financial interests.
Joan Cox Gill died on 9 May 2019.
Correspondence: Veronica H. Flood, Comprehensive Center for Bleeding Disorders, 8739 Watertown Plank Rd, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: vflood@mcw.edu.
References
Author notes
Send data sharing requests via e-mail to the corresponding author, Veronica H. Flood (vflood@mcw.edu).
The full-text version of this article contains a data supplement.