Key Points
FDG-PET–negative status achieved with salvage therapy is the most important determinant of favorable outcome after HSCT, for patients with R/R cHL.
Our modified Bv+Bs salvage regimen led to deep metabolic responses in the majority of patients, establishing a bridge to transplant.
Abstract
We evaluated the impact on progression-free survival (PFS) of achieving a deep metabolic response at 2-deoxy-2[18F] fluoro-d-glucose positron emission tomography (FDG-PET) in patients with refractory or relapsed (R/R) classic Hodgkin lymphoma (cHL) following a new salvage regimen named Bv+Bs (brentuximab vedotin + bendamustine supercharge), from 2013 to 2017. In this real-life study, 20 consecutive patients (aged <60 years) with R/R cHL after failure of ≥1 salvage treatments received Bv+Bs regimen consisting of 3-days outpatient IV infusions of 1.8 mg/kg of Bv on day 1 of each 3-week cycle combined in sequence to bendamustine on days 2 and 3 of the treatment cycle at a fixed dose of 120 mg/m2 per day, for a total of 4 courses. A robust primary prophylaxis approach, including premedication, antimicrobials, stimulating factors, and cytomegalovirus monitoring, was systematically performed. The 20 patients (all evaluable) underwent 4 courses of Bv+Bs with a median dose intensity of 100% for both Bv and Bs. Ten patients (50%) experienced grade ≥3 treatment-related adverse events, without requiring hospitalization. At post-Bv+Bs reevaluation, 80% of patients had deep metabolic responses with Deauville 5-point scale scores ≤2. Thereafter, 14 patients (70%) received autologous hematopoietic stem cell transplantation (HSCT; peripheral blood stem cells previously harvested in 12 cases), and 4 patients (10%) received allogeneic HSCT. At a median follow-up of 27 months from Bv+Bs regimen initiation, the 2-year PFS of the entire population was 93.7% (95% confidence interval, 62.7% to 99.6%). Our data suggest that Bv+Bs regimen-driven strategy may be a promising salvage option to improve long-term control of high-risk Hodgkin lymphoma.
Introduction
Brentuximab vedotin (Bv) and bendamustine show encouraging results in the most challenging subset of patients with classic Hodgkin lymphoma (cHL).1 Phase 1/2 studies, including cohorts with refractory and/or relapsed (R/R) cHL, have investigated the optimal treatment schedule with combined Bv and bendamustine.2-4 The recommended dose by IV infusion was deemed to be1.8 mg/kg of Bv on day 1 of a 21-day cycle, plus 1 dose of bendamustine 90 mg/m2 IV on days 1 and 2 of the treatment cycle.2-5 This schedule had a manageable toxicity profile with grade 3 to 4 neutropenia and lung infection in 25% and 14% of patients, respectively, and infusion-related reactions (fever, chills, dyspnea, flushing, rash, and/or pruritis) in ∼35% of patients in the absence of systematic premedication with corticosteroids and antihistamines.2,4 After a median of 4 courses, the reported overall response rates were ≥80% with an average of complete remissions (CRs) of ∼70%.2-5 This therapy’s favorable expectations should be tempered by the follow-up data: the pooled 2-year progression-free survival (PFS) rate was ∼50%.2-5 Thus, the proportion of patients with R/R cHL achieving long-term CR after salvage therapy with conventional Bv and bendamustine regimen remains low, and attempts to improve it are welcome.6
Clinical trials conducted in this setting present convincing evidence that increasing dosage of bendamustine had good anticancer activity with no dose-limiting toxicity.7-9 Improvements in antilymphomatous potency occurred especially when increasing doses of bendamustine followed Bv infusion, more likely due to an enhanced synergistic effect which was perceived as a great advantage in this subset.10 Emerging in vitro data allowed the speculation that high-dose bendamustine, administered right after Bv, facilitated intracellular trafficking, internalization, and metabolism of anti-CD30–auristatin conjugates and thus targeted delivery of anticancer therapeutics.11-14
We report a real-life experience on the efficacy and safety of a salvage program based on sequential combination of Bv standard dose and bendamustine supercharge (Bs) for a total of 4 courses, named “Bv+Bs” regimen, in a series of R/R cHL patients.
Patients and methods
Study design, participants, and procedures
This study was conducted in the Hematology Unit of the Federico II University of Naples (Italy). All necessary approvals were obtained from our ethics committee, and a specific consent form dedicated to immunochemotherapy treatment was obtained from each patient according to the Declaration of Helsinki. From September 2013 to November 2017, consecutive biopsy-proven CD30-positive R/R cHL15 patients scheduled to receive salvage treatment with Bv+Bs regimen were included. The Bv+Bs schedule consisted of 3-day outpatient IV infusions of 1.8 mg/kg of Bv on day 1 of each 3-week cycle (as established by Younes and colleagues)16 combined in sequence with bendamustine (at least 24 hours after Bv, precisely on days 2 and 3 of the treatment cycle; this timing is a personal extrapolation from published in vitro and in vivo data, which was not previously established)7,11,13,14 at a fixed dose of 120 mg/m2 per day. This dosage was used on the basis of 2 studies, Corazzelli and colleagues and Moskowitz and colleagues, on 25 and 34 patients, respectively, treated with high-dose bendamustine, showing no discontinued treatment due to drug-related adverse events.8,9
The complete scheme of the Bv+Bs regimen is shown in Figure 1. For study purposes, particular attention was given to the primary prophylactic strategy. For every 3-week cycle, patients routinely received methylprednisolone at 200 mg IV and diphenhydramine at 50 mg IV on days 1 to 3, febuxostat at 80 mg orally on days 1 to 5 (plus hyperhydration), and peg-filgrastim 6 mg subcutaneously on day 6. In addition, antimicrobial drugs were administered for each patient as follows: trimethoprim-sulfamethoxazole at 960 (160 + 800) mg orally every 12 hours 2 times a week and acyclovir at 800 mg orally daily from the start of Bv+Bs regimen until 1 month after the last cycle. Moreover, serum cytomegalovirus (CMV)-DNA monitoring was always performed on day 1 of every 3-week cycle, for each patient.
Bv+Bs regimen schedule. Bv, at 1.8 mg/kg IV on day 1 of the 3-week cycle; Bs, at 120 mg/m2 IV on days 2 and 3 of the 3-week cycle; P, peg-filgrastim 6 mg subcutaneous on day 6 of the 3-week cycle; FDG-PET, at baseline and 5 weeks after salvage treatment completion; C, serum CMV-DNA monitoring on day 1 of the 3-week cycle. Moreover, as support prophylactic treatment: methylprednisolone at 200 mg IV on days 1 to 3 of the 3-week cycle, diphenhydramine at 50 mg IV on days 1 to 3 of the 3-week cycle, febuxostat at 80 mg orally on days 1 to 5 of the 3-week cycle (plus hyperhydration); trimethropin-sulfamethoxazole at 960 (160 + 800) mg orally every 12 hours 2 times a week and acyclovir at 800 mg orally daily from the start of Bv+Bs regimen until 1 month after the last cycle. Clinical visits were performed on days 1 to 3 of the 3-week cycle. Routine laboratory tests were performed every 2 weeks.
Bv+Bs regimen schedule. Bv, at 1.8 mg/kg IV on day 1 of the 3-week cycle; Bs, at 120 mg/m2 IV on days 2 and 3 of the 3-week cycle; P, peg-filgrastim 6 mg subcutaneous on day 6 of the 3-week cycle; FDG-PET, at baseline and 5 weeks after salvage treatment completion; C, serum CMV-DNA monitoring on day 1 of the 3-week cycle. Moreover, as support prophylactic treatment: methylprednisolone at 200 mg IV on days 1 to 3 of the 3-week cycle, diphenhydramine at 50 mg IV on days 1 to 3 of the 3-week cycle, febuxostat at 80 mg orally on days 1 to 5 of the 3-week cycle (plus hyperhydration); trimethropin-sulfamethoxazole at 960 (160 + 800) mg orally every 12 hours 2 times a week and acyclovir at 800 mg orally daily from the start of Bv+Bs regimen until 1 month after the last cycle. Clinical visits were performed on days 1 to 3 of the 3-week cycle. Routine laboratory tests were performed every 2 weeks.
The results of 2-deoxy-2[18F] fluoro-d-glucose positron emission tomography (FDG-PET) scans, conducted at the end of the fourth cycle of Bv+Bs, defined the responses to the salvage therapy. The key to interpreting imaging reporting was the Deauville 5-point scale (DS), as already described.17 PET-negative results (DS score ≤3) led to a switch to hematopoietic stem cell transplantation (HSCT) or other 2 consolidation courses of Bv+Bs. Thereafter, FDG-PET scans were performed every 3 to 6 months.
Outcomes
Efficacy analysis focused primarily on PFS (defined as the time from Bv+Bs first dose to disease progression/relapse or to death from any cause) and secondarily on PET-negative results and bridge to transplant after the fourth cycle of Bv+Bs. Safety analysis focused on grade ≥3 hematological and/or nonhematological toxicity (National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03).
Results
Patient characteristics and treatment
During the 4-year study period, 20 patients (median age, 40 years; range, 23-54) with R/R cHL who received the Bv+Bs regimen for salvage therapy constituted the entire population included in the final assessment (Table 1). The median number of prior therapies was 3 (range, 2-6). The Bv+Bs treatment was the second or third salvage regimen used in most cases. In fact, 16 patients had received Ifosfamide, Gemcitabine, Vinorelbine and Prednisolone (IGEV) as first-salvage regimen, followed by autologous HSCT in 5 cases. Five patients underwent prior Bv treatment as a single agent at a dosage of 1.8 mg/kg IV for a median of 3 courses (range, 2-4); of these, after Bv, 3 patients achieved a partial response, whereas the remaining 2 had stable disease.
Baseline characteristics of analyzed patients
| Characteristics . | n (%) . |
|---|---|
| Number of patients | 20 |
| Age | |
| Median (range), y | 40 (23-54) |
| Sex | |
| Female | 12 (60) |
| Male | 8 (40) |
| Histological subtypes | |
| Nodular sclerosis | 16 (80) |
| Mixed cellularity | 3 (15) |
| Lymphocyte rich | 1 (5) |
| Frontline therapy received | |
| ABVD, 4 courses | 5 (25) |
| ABVD, 6 courses | 15 (75) |
| Radiotherapy after ABVD | 6 (30) |
| Response to frontline therapy | |
| Primary refractory | 11 (55) |
| Relapsed | |
| CR ≤1 y | 5 (25) |
| CR >1 y | 4 (20) |
| Number of previous treatments | |
| 2 | 5 (25) |
| 3-4 | 14 (70) |
| >4 | 1 (5) |
| Median (range) | 3 (2-6) |
| Previous salvage therapy* | |
| IGEV | 16 (80) |
| DHAP | 7 (35) |
| Bv | 5 (25) |
| BEACOPP | 1 (5) |
| Radiotherapy | 1 (5) |
| Transplant | |
| Autologous HSCT | 5 (25) |
| At the time of Bv+Bs start | |
| Characteristics of disease | |
| ≥3 nodal sites involved | 12 (60) |
| Extranodal involvement | 10 (50) |
| Mediastinal bulky | 6 (30) |
| Bone marrow involvement | 1 (5) |
| LDH > normal limits | 7 (35) |
| ESR >50 | 6 (30) |
| Ann Arbor/Cotswold staging† | |
| I-II | 6 (30) |
| III-IV | 14 (70) |
| B symptoms | 11 (55) |
| ECOG-PS | |
| 0-1 | 14 (70) |
| ≥2 | 6 (30) |
| Characteristics . | n (%) . |
|---|---|
| Number of patients | 20 |
| Age | |
| Median (range), y | 40 (23-54) |
| Sex | |
| Female | 12 (60) |
| Male | 8 (40) |
| Histological subtypes | |
| Nodular sclerosis | 16 (80) |
| Mixed cellularity | 3 (15) |
| Lymphocyte rich | 1 (5) |
| Frontline therapy received | |
| ABVD, 4 courses | 5 (25) |
| ABVD, 6 courses | 15 (75) |
| Radiotherapy after ABVD | 6 (30) |
| Response to frontline therapy | |
| Primary refractory | 11 (55) |
| Relapsed | |
| CR ≤1 y | 5 (25) |
| CR >1 y | 4 (20) |
| Number of previous treatments | |
| 2 | 5 (25) |
| 3-4 | 14 (70) |
| >4 | 1 (5) |
| Median (range) | 3 (2-6) |
| Previous salvage therapy* | |
| IGEV | 16 (80) |
| DHAP | 7 (35) |
| Bv | 5 (25) |
| BEACOPP | 1 (5) |
| Radiotherapy | 1 (5) |
| Transplant | |
| Autologous HSCT | 5 (25) |
| At the time of Bv+Bs start | |
| Characteristics of disease | |
| ≥3 nodal sites involved | 12 (60) |
| Extranodal involvement | 10 (50) |
| Mediastinal bulky | 6 (30) |
| Bone marrow involvement | 1 (5) |
| LDH > normal limits | 7 (35) |
| ESR >50 | 6 (30) |
| Ann Arbor/Cotswold staging† | |
| I-II | 6 (30) |
| III-IV | 14 (70) |
| B symptoms | 11 (55) |
| ECOG-PS | |
| 0-1 | 14 (70) |
| ≥2 | 6 (30) |
Values are n (%) unless otherwise noted.
ABVD, doxorubicin 25 mg/m2 IV on days 1 and 15, bleomycin 10 IU/m2 IV on days 1 and 15, vinblastine 6 mg/m2 IV on days 1 and 15, dacarbazine 375 mg/m2 IV on days 1 and 15; IGEV, ifosfamide 2000 mg/m2 IV on days 1 to 4, gemcitabine 800 mg/m2 IV on days 1 and 4, vinorelbine 20 mg/m2 IV on day 1, and prednisolone 100 mg orally on days 1 to 4; BEACOPP (escalated), bleomycin 10 UI/m2 IV on day 8, etoposide 200 mg/m2 IV on days 1 to 3, doxorubicin 35 mg/m2 IV on day 1, cyclophosphamide 1250 mg/m2 IV on day 1, vincristine 1.4 mg/m2 IV on day 8, procarbazine 100 mg/m2 orally on days 1 to 7, prednisone 40 mg/m2 orally on days 1 to 14; DHAP, cisplatin 100 mg/m2 by continuous IV infusion over 24 hours, followed by cytosine arabinoside IV in 2 pulses each at a dose of 2 g/m2 given 12 hours apart. Dexamethasone, 40 mg IV, was given on days 1 through 4; Bv, at a dosage of 1.8 mg/kg IV every 3 weeks for a median of 3 courses (range, 2-4); ECOG-PS, Eastern Cooperative Oncology Group–Performance Status; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase.
Four patients received several lines of salvage therapy, before Bv+Bs regimen.
Stage I is defined as involvement of 1 nodal group or lymphoid organ; stage II is defined as involvement of 2 or more nodal groups on the same side of diaphragm; stage III is defined as involvement of nodal groups on both sides of the diaphragm; and stage IV is defined as disseminated involvement of 1 or more extralymphatic organs (eg, lung, bone) with or without any nodal involvement.
At the start of the Bv+Bs regimen, the majority of patients had very extensive disease with Ann Arbor stage III to IV in 70% of cases, extranodal involvements in 50%, and bulky disease in 30%.
All patients underwent 4 courses of Bv+Bs: median-dose intensity during sequential therapy was 100% (range, 88.6% to 102.4%) for Bv and 100% (range, 88.7% to 102.4%) for Bs. The vigorous support drug treatment and clinical and laboratory monitoring as shown in Figure 1 were systematically performed in all patients.
Adverse events
A total of 10 patients (50%) experienced treatment-related adverse events of grade 3 or 4. The most common grade ≥3 hematologic toxicity was neutropenia (3 cases), which required supplemental administrations of granulocyte-colony stimulating factors (G-CSF), whereas the most common grade ≥3 extrahematologic toxicity was CMV reactivation with viremia (median CMV-DNA, 1810 IU/mL; range, 620-170 000 IU/mL) with fever (5 patients), which was successfully treated with preemptive therapy with valganciclovir. Notably, by virtue of the specific premedication against acute toxicity during the IV administration of Bv+Bs, serious infusion-related reactions (both during cycle 2 of combination therapy) with fever, chills, and dyspnea occurred in only 2 patients (10%).
Overall, a total of 8 patients (40%) temporarily discontinued therapy due to treatment-related adverse events. In particular, these cases received at least 1 Bv and/or Bs dose modification in terms of delays (n = 4) or reductions (n = 4). However, all of them were efficacy-evaluable patients having completed 4 courses of Bv plus bendamustine.
No patient required hospitalization to manage treatment-related adverse events.
Treatment response
All patients were assessable for the final responses after Bv+Bs regimen. FDG-PET scans were performed between 5 and 6 weeks (median, 5) after the fourth immune-chemotherapy cycle. All cases had FDG-PET–negative results, thus achieving complete metabolic response. The analysis of post-Bv+Bs imaging scans assigned a DS as follows: DS, 1 to 8 patients; DS 2 to 8 patients, and DS 3 to 4 patients. The response data based on the percentage change in tumor volume from baseline as evaluated by morphologic imaging (ie, computed tomography scans) showed a 100% decrease in lymph node size for each patient, except for 2 cases with residual tissue (ie, nodes ≥ 2 cm long axis) in the initial mediastinal bulky site.
Post-Bv+Bs stem cell mobilization and transplant
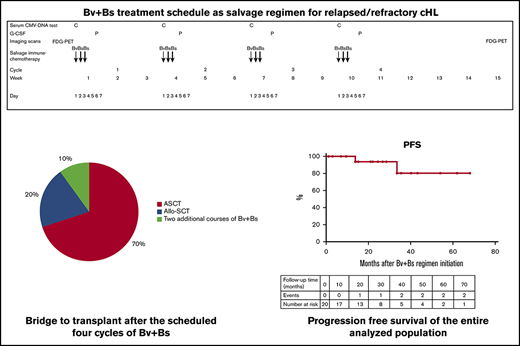
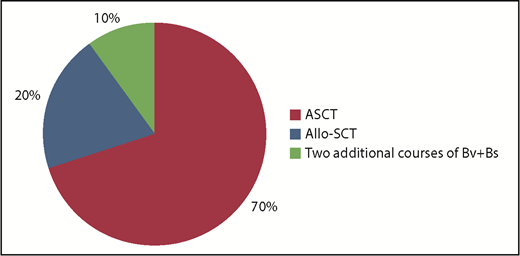
Overall, after the 4 courses of Bv+Bs, the bridge to autologous HSCT occurred in 14 patients (70%) and to allogeneic HSCT in 4 patients (10%). The remaining 2 patients received 2 additional courses of Bv+Bs (for a total of 6; Figure 2).
Bridge to transplant following Bv+Bs regimen. Autologous hematopoietic stem cell transplantation (ASCT; n = 14) was performed with PBSC previously harvested in 12 cases, or with post-Bv+Bs PBSC successfully collected (by G-CSF and cyclophosphamide) in 2 cases. Allogeneic hematopoietic stem cell transplantation (Allo-SCT; n = 4): haploidentical in 3 cases, and sibling in 1 case. Two patients received 2 additional courses of Bv+Bs (for a total of 6).
Bridge to transplant following Bv+Bs regimen. Autologous hematopoietic stem cell transplantation (ASCT; n = 14) was performed with PBSC previously harvested in 12 cases, or with post-Bv+Bs PBSC successfully collected (by G-CSF and cyclophosphamide) in 2 cases. Allogeneic hematopoietic stem cell transplantation (Allo-SCT; n = 4): haploidentical in 3 cases, and sibling in 1 case. Two patients received 2 additional courses of Bv+Bs (for a total of 6).
Autologous HSCT was performed with peripheral blood stem cells (PBSC) previously harvested (after 2 courses of IGEV, which was the first salvage regimen) in 12 cases, or with post-Bv+Bs PBSC successful collection, after mobilization with G-CSF and cyclophosphamide (average of CD34+ cells collected: 3.5 × 106 per kilogram of body weight) in 2 cases. Regarding allogeneic HSCT, 3 cases underwent haploidentical transplant and 1 case sibling transplant. Among the patients who proceeded to HSCT, the most used conditioning regimen was BEAM (Carmustine, Etoposide, Cytarabine, and Melphalan) or FEAM (Fotemustine, Etoposide, Cytarabine, and Melphalan). All patients successfully engrafted: median times to neutrophil and platelet engraftment were 10 and 15 days, respectively.
Long-term follow-up
With a median follow-up of 27 months (range, 4-68) from Bv+Bs regimen initiation, the estimated 2-year PFS of the entire analyzed population was 93.7% (95% confidence interval, 62.7% to 99.6%), as shown in Figure 3.
PFS. Kaplan-Meier curve of 2-year PFS of 20 patients with R/R cHL who received the Bv+Bs regimen (see “Patients and methods”). Table shows number of events and number at risk during follow-up.
PFS. Kaplan-Meier curve of 2-year PFS of 20 patients with R/R cHL who received the Bv+Bs regimen (see “Patients and methods”). Table shows number of events and number at risk during follow-up.
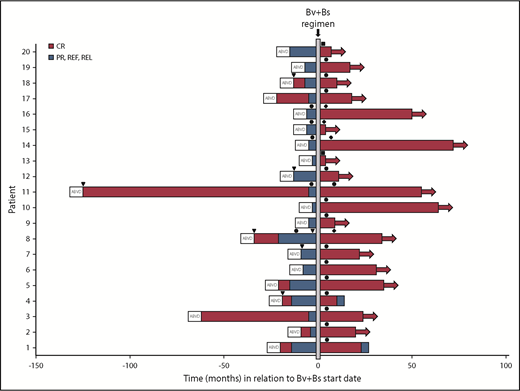
Two patients relapsed after 13 and 33 months, respectively (Figure 4). Both had received autologous HSCT after Bv+Bs regimen. One of them was refractory to prior treatment with Bv as single agent. The 2 patients had residual tissue in the initial mediastinal bulky site with a DS score of 3 at FDG-PET scans following Bv+Bs regimen.
Swimmers plot. Duration of patients’ response following Bv and Bs salvage treatment. Horizontal arrows denote ongoing response. Arrowheads (▾) denote radiation therapy. Circles (●) denote autologous HSCT. Rhombuses (♦) denote allogeneic HSCT. Squares (▪) denote 2 additional Bv+Bs courses. PR, partial remission; REF, refractory; REL, relapse.
Swimmers plot. Duration of patients’ response following Bv and Bs salvage treatment. Horizontal arrows denote ongoing response. Arrowheads (▾) denote radiation therapy. Circles (●) denote autologous HSCT. Rhombuses (♦) denote allogeneic HSCT. Squares (▪) denote 2 additional Bv+Bs courses. PR, partial remission; REF, refractory; REL, relapse.
Discussion
Herein, we present a cohort of patients with high-risk Hodgkin lymphoma treated with a modified Bv plus bendamustine regimen. In particular, we have increased the bendamustine dose and made modifications to the timing of administration of bendamustine based on preclinical rationale that bendamustine may best synergize with Bv when administered afterward.11-14
In this series of patients with cHL at very poor prognosis,1,6 which included 55% with primary refractory disease, 25% with early relapse, and 20% with heavy pretreatments for multiple relapses, the immune-chemotherapy sequential combination of Bv followed by Bs (actually delivered dose-intensity median: 80 mg/m2 per week) obtained a truly powerful tumoricidal effect against Reed-Sternberg cells, leading to an impressive improvement of PFS. In fact, the PFS was ∼94% at 2 years, indicating that our approach is clinically very promising in terms of efficacy. On the other hand, the reported rates of PFS in patients rescued with standard platinum-based chemotherapy (such as ICE [Ifosfamide, Carboplatin and Etoposide] or DHAP [Cisplatin, Cytosine arabinoside, and Dexamethasone])1 and, more recently, with BeGEV (Bendamustine, Gemcitabine, Etoposide, and Vinblastine)6,18 were ∼45% and 62% at 2 years, respectively. Foremost, particular homogeneity of post-Bv+Bs reevaluation findings in terms of achieving a best result of CR was noticed in the study population. At FDG-PET scans, 80% of the treated patients had deep metabolic responses with DS scores ≤2 (DS scores 3 in the remaining 20%). According to the Lugano classification,19 PET-negative status achieved with salvage therapy is the most important determinant of favorable outcome after HSCT.2,4,6 Second, the number of patients proceeding to HSCT after Bv+Bs regimen was very high (18/20, 90%), and the main reason for this was a lasting response without progression while waiting for transplant procedure. Thus, the activity of our salvage regimen may serve not only as an initial debulking therapy but also to maintain response duration. However, we do not have enough data regarding the impact of the Bv+Bs regimen on CD34+ cells mobilization and collection (especially in terms of impairments).6 Finally, Bv+Bs treatment-emergent serious adverse events included ≤25% grade 3 to 4 neutropenia or infection or infusion-related reaction, substantiating that the combination of Bv+Bs was slightly more toxic than the single agents taken alone.8,9,16 However, a robust primary prophylaxis with broad-spectrum support drug treatment, and strict clinical and laboratory (in particular, serum CMV-DNA) monitoring, is strongly recommended.
Our study has limitations that must be pointed out. First, it is a single-center study with a small number of patients and events, which limits statistical results. Second, the study was not performed in the context of a clinical trial. Hence, our findings need to be validated in a prospective multicenter large trial. Finally, other studies are needed to demonstrate leverage from therapy with Bv+Bs in patients with R/R cHL and aged 60 years and over.20
To the best of our knowledge, this is the first report in a clinical background of a very effective synergistic effect between Bv and high-dose bendamustine when administered thereafter, in patients with R/R cHL. Altogether, our data suggest that Bv+Bs regimen-driven strategy may be a new salvage option to increase the proportion of patients, aged <60 years, achieving long-term disease control of high-risk Hodgkin lymphoma.
Authorship
Contribution: M.P. designed the research; M.P., R.D.P., C.G., and N.P. performed the research and wrote the paper; C.M., F.T., M.G.R., I.C., M.R., M. Memoli, M. Monteverde, and M. Mascolo collected data; N.P. analyzed data; and F.P. and M.P. performed the final revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roberta Della Pepa, Department of Clinical Medicine and Surgery, Federico II University Medical School, Via S. Pansini 5, 80131 Naples, Italy; e-mail: roberta.dellapepa@unina.it.