TO THE EDITOR:
Treatment of myelodysplastic syndrome (MDS) is suboptimal. Responses to hematopoietic growth factors, hypomethylating agents, and lenalidomide in 5q− syndromes are not sustained, and not all patients benefit. Allogeneic stem cell transplantation is potentially curative but only used in a minority of MDS patients.1 Evidence for immune-mediated myelosuppression in ∼1 quarter of MDS patients2 is the rationale for the use of immunosuppressive treatment (IST) to restore bone marrow function. In selected patients, response rates of 30% to 68% are reported, with improved hematopoiesis and reduced transfusion requirements.3-11 Younger age, lower transfusion burden, and HLA-DR15+ status were predictors of response to IST with antithymocyte globulin (ATG) alone or in combination with cyclosporine (CsA).3 However, because of the toxicity profile of ATG and CsA, especially in older patients, hospitalization and prolonged CsA administration and monitoring may be required. The immunosuppressant alemtuzumab is a humanized monoclonal antibody against CD52, capable of inducing profound lymphopenia. In 2010, we reported a 68% overall response rate in 31 MDS patients treated with alemtuzumab.4 We report here the long-term follow-up of this cohort, expanded to 39 patients, which includes 6 exceptional responders who remain alive and in complete hematologic remission ≥5 years from enrollment.
Between 2005 and 2013, 142 patients were screened, 40 of whom were enrolled, after providing informed consent in accordance with the Declaration of Helsinki, onto this institutional review board–approved phase 1/2 pilot study of alemtuzumab in MDS patients (NCT00217594). Patients with de novo MDS (refractory anemia [RA], RA with ring sideroblasts, refractory cytopenia with multilineage dysplasia with ring sideroblasts, refractory cytopenia with multilineage dysplasia, or RA with excessive blasts 1 according to World Health Organization classification) age 18 to 72 years were eligible if they had ≥1 of the following: transfusion dependence (≥2 units of packed red blood cells or 5 units of platelets per month for a period of 2 months), thrombocytopenia (platelet count ≤50 × 109/L), neutropenia (neutrophil count ≤0.5 × 109/L), and anemia (hemoglobin ≤9 g/dL or absolute reticulocyte count [ARC] ≤60 × 109/L). Enrollment was based on a prediction model12 for patients likely to respond to IST that included age, months of red cell transfusion dependence (RCTD), and HLA-DR15 status. In HLA-DR15− patients, the sum of age plus months of RCTD had to be <58; in HLA-DR15+ patients, age plus months of RCTD had to be <72. Patients received a test dose of 3 mg of alemtuzumab subcutaneously followed by a 10-mg dose either IV (n = 37 patients) or subcutaneously daily (n = 3 patients) for 10 days. Patients who relapsed were eligible to receive CsA. All patients received Pneumocystis carinii, antiviral, and antibacterial prophylaxis with 300 mg of inhaled pentamidine every 4 weeks, 500 mg of valacyclovir daily, and 500 mg of ciprofloxacin twice daily, respectively. Cytomegalovirus and Epstein-Barr virus polymerase chain reaction was assessed before treatment, weekly for the first month, every 2 weeks for the second month, monthly until month 8, and then at 12 months. Response assessments were performed at 3, 6, and 12 months and then yearly until year 5.13 Annual follow-up after completion of the 5-year study was optional. The previously reported median follow-up was 13 months4 ; we now present data with a median follow-up of 5 years.
Treatment was generally well tolerated. Infusion reactions were common, typically occurring with the first dose and decreasing during subsequent doses. These did not limit treatment, with the exception of 1 patient who developed severe hypotension after the test dose and was excluded from the study. Two patients died on study from causes other than disease progression: 1 patient with severe thrombocytopenia before treatment (4 × 109/L) died as a result of an intracranial hemorrhage during the first month of treatment, and another died as a result of pneumonia 3 years after alemtuzumab. Adverse events were recorded for 6 months after last treatment done. Excluding hematologic laboratory abnormalities, no grade 4 toxicity was reported. Transient grade 3 elevated liver function tests were noted in 10 patients and thought to be possibly related to drug. Syncope was reported twice and was the only clinical grade 3 toxicity observed in >1 patient. Cytomegalovirus and Epstein-Barr virus reactivations were common, transient, and self limiting and did not result in disease.
The 39 evaluable patients had a median age of 56 years (range, 23-71 years); 67% were men, 59% had had no previous treatments, 74% were transfusion dependent, and 46% had abnormal cytogenetics at baseline. According to International Prognostic Scoring System (IPSS) score,14 74% were intermediate 1, 18% were intermediate 2, and 8% were low. By revised IPSS,15 46% were intermediate, 26% were low, 23% were high, and 3% were very low or very high risk. Most (85%) had <5% blasts on bone marrow at baseline, with a median hemoglobin level of 8.8 g/dL (range, 5.7-13 g/dL), platelet count of 30 × 109/L (range, 5 × 109/L to 439 × 109/L), absolute neutrophil count (ANC) of 0.9 × 109/L (range, 0.08 × 109/L to 8.02 × 109/L), and ARC of 46.3 × 109/L (range, 1.8 × 109/L to 202.4 × 109/L).
Hematologic improvement (HI)13 was seen in 20 (51%) of 39 evaluable patients and, by IPSS, in 17 (53%) of 32 (53%) intermediate-1–risk patients, 2 (50%) of 4 intermediate-2–risk patients, and 1 (33%) of 3 low-risk patients. The median time to HI was 3 months, and all 15 patients who were transfusion dependent became transfusion independent. The median duration of HI after alemtuzumab in these patients was 17 months (range, 3-70 months).
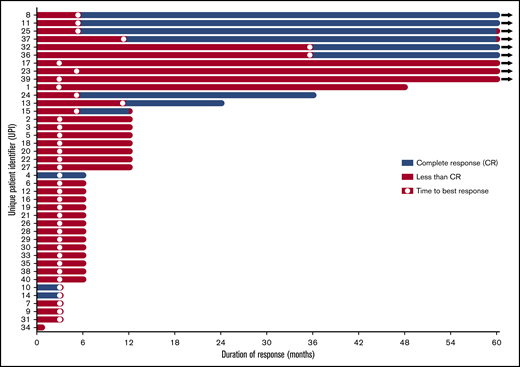
Twelve (31%) of 39 patients had a complete response (CR), which we define here as recovery of peripheral blood counts (hemoglobin >11 g/dL, ANC >1.5 × 109/L, platelets >100 × 109/L) with no peripheral blasts, together with a bone marrow examination showing <5% myeloblasts and normal maturation of all cell lines with no evidence of dysplasia. The median time to CR was 6 months (range, 3-36 months), with a median response duration of 30 months (Figure 1). Median overall survival in complete responders was 72.4 months (range, 19.3-123.1 months), compared with 37 months (range, 1-110 months) in nonresponders. Eight (62%) of 13 women treated achieved a CR, compared with only 4 (15%) of 26 men (P = .01). Other characteristics were not significantly different between CR and non-CR patients, including HLA-DR, number of prior treatments, transfusion dependence, cytogenetics, IPSS, revised IPSS, baseline blast percentage, and blood counts. Most CR patients (83%) had <5% blasts on baseline bone marrow biopsy. Baseline blood counts in those achieving a CR included a mean hemoglobin level of 8.9 g/dL, platelet count of 59 × 109/L, ANC of 0.89 × 109/L, and ARC of 52.1 × 109/L. CR patients were often untreated (58%) and transfusion dependent (67%) before treatment.
Duration of response and time to best response in all patients treated with alemtuzumab.
Duration of response and time to best response in all patients treated with alemtuzumab.
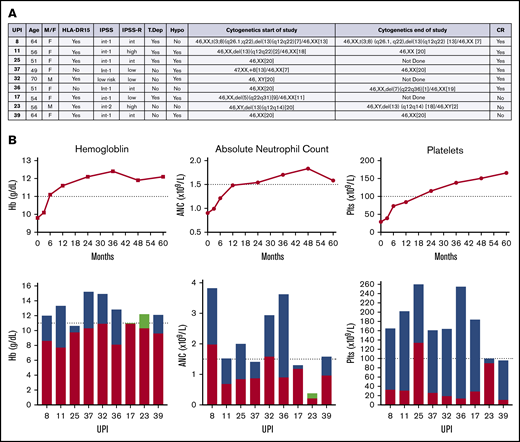
Nine of the 39 patients completed the 5-year study and were alive without additional treatment (Figure 2). The median age of these patients was 56 years (range, 49-70 years). A majority (78%) had no prior treatment, were HLA-DR15+, and were women. Six of these 9 long-term responders had baseline pretreatment bone marrow examination interpreted as hypocellular for age. All but 1 patient had <5% bone marrow blasts at baseline. Two of these 9 patients converted from an abnormal karyotype to normal over the course of 5 years, 3 did not change, 1 converted from normal to abnormal, and 3 did not have end-of-study cytogenetics performed. Peripheral blood counts before treatment showed a median hemoglobin level of 9.8 g/dL (range, 7.7-12.2 g/dL), platelet count of 29 × 109/L (range, 11 × 109/L to 134 × 109/L), ANC of 0.9 × 109/L (range, 0.38 × 109/L to 1.98 × 109/L), and ARC of 69.3 × 109/L (range, 17.1 × 109/L to 89.3 × 109/L). Three were treated with CsA on protocol. Six were complete responders, for whom time to best response ranged from 6 to 36 months. Two long-term CR patients lost their response at the time of year-5 assessment because of decreasing ANC (unique patient identifier 37; 1.41 × 109/L; requirement for CR, 1.5 × 109/L) in 1 and decreasing hemoglobin level (unique patient identifier 25; 10.6 g/dL; requirement for CR, 11 g/dL) in the other. All of these 9 patients remain alive at a median of 8 years (range, 5-10.25 years) from initial study enrollment.
Characteristics of patients completing the study. (A) Characteristics of 9 patients who completed the study and were alive at last follow-up. (B) Trend of median hemoglobin (Hb), ANC, and platelets over time (months) in patients who completed the study. Individual patient changes in hemoglobin, ANC, and platelets from baseline compared with end of study. Red + green, baseline; blue, increase from baseline; green, decrease from baseline. Hypo, hypocellular MDS; Int, intermediate; IPSS-R, revised IPSS; plts, platelets; T dep, T-cell depletion; UPI, unique patient identifier.
Characteristics of patients completing the study. (A) Characteristics of 9 patients who completed the study and were alive at last follow-up. (B) Trend of median hemoglobin (Hb), ANC, and platelets over time (months) in patients who completed the study. Individual patient changes in hemoglobin, ANC, and platelets from baseline compared with end of study. Red + green, baseline; blue, increase from baseline; green, decrease from baseline. Hypo, hypocellular MDS; Int, intermediate; IPSS-R, revised IPSS; plts, platelets; T dep, T-cell depletion; UPI, unique patient identifier.
In summary, in a cohort of MDS patients selected for likelihood of response to IST on the basis of age, HLA-DR15 status, and transfusion duration, we observed durable objective responses to alemtuzumab in a subset of patients. A recent multicenter retrospective study of IST in MDS reported an overall response rate of 49% and a median overall survival of 47.4 months.7 The authors noted that despite its apparent efficacy, IST is used infrequently for MDS in clinical practice, perhaps at least in part because of toxicity; ∼30% of patients discontinued IST because of adverse effects. In contrast to ATG, alemtuzumab therapy was relatively well tolerated, requires only a single cycle, and represents an attractive option for selected patients with MDS. The inability to precisely identify those patients most likely to benefit is likely responsible for the limited use of IST in MDS. We show here that although long-term survivors were typically previously untreated HLA-DR15+ middle-aged women with hypocellular MDS, exceptions to this general clinical profile do exist. Achievement of CR to IST was not required for long-term transfusion-free survival. Future studies may integrate assessment of those genetic and immunologic factors associated with a meaningful clinical response to IST in MDS.
Acknowledgment:
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health through grant 1-ZIA-HL006163.
Contribution: C.L. performed research, analyzed data, and wrote the paper; V.R. analyzed data and wrote the paper; C.W. analyzed data; M.J.O., A.R.P., A.S., J.T., B.W., and P.S. performed research; A.J.B. performed research and wrote the paper; R.D. performed research; N.S.Y. designed and performed research and wrote the paper; and C.S.H. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: C.L. has served on an advisory board for Agios and as a speaker for Jazz Pharma and Astellas. A.R.P. has served as a consultant for Foundation Medicine. C.S.H. has received laboratory research funding from Merck and Sellas. The remaining authors declare no competing financial interests.
Correspondence: Catherine Lai, Georgetown University Medical Center, 3800 Reservoir Rd NW, Washington, DC 20007; e-mail: catherine.lai@gunet.georgetown.edu.
References
Author notes
The full-text version of this article contains a data supplement.