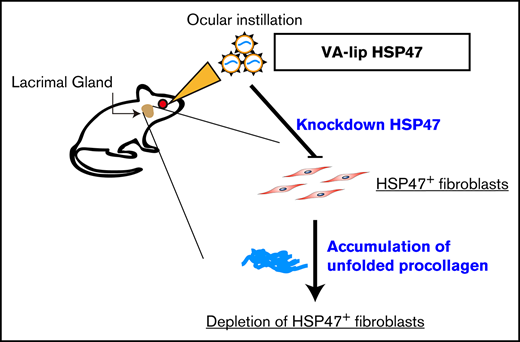
Key Points
HSP47+ fibroblasts play a critical role in lacrimal gland fibrosis in chronic GVHD.
Ocular instillation of VA-lip HSP47 ameliorates dry eye syndrome in chronic GVHD.
Abstract
Chronic graft-versus-host disease (GVHD) profoundly affects the quality of life of long-term survivors of allogeneic hematopoietic stem cell transplantation (SCT). The eyes are frequently involved, and dry eye syndrome is the most common manifestation of ocular chronic GVHD. We explored the role of heat shock protein 47 (HSP47) in ocular GVHD and developed a novel antifibrotic topical therapy using vitamin A–coupled liposomes containing HSP47 small interfering RNA (siRNA) against HSP47 (VA-lip HSP47). In a mouse model of chronic GVHD, infiltration of HSP47+ fibroblasts and massive fibrosis surrounding the lacrimal ducts were observed after allogeneic SCT, leading to impaired tear secretion. After ocular instillation, VA-lip HSP47 was distributed to the lacrimal glands, knocked down HSP47 expression in fibroblasts, reduced collagen deposition, and restored tear secretion after allogeneic SCT. Ocular instillation of VA-lip HSP47 also ameliorated established lacrimal gland fibrosis and dry eye syndrome. VA-lip HSP47 eye drops are a promising prophylactic and therapeutic option against dry eye syndrome in chronic GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is a curative therapy for various hematologic malignant and nonmalignant diseases. Recent progress in allogeneic SCT results in increased long-term survivors of SCT1 ; however, the incidence of chronic graft-versus-host disease (GVHD) has increased in recent years.2 Chronic GVHD involves various organs, such as the skin, liver, lung, mouth, eye, and hematopoietic system, and it profoundly affects the quality of life of long-term survivors of SCT.3 Prolonged inflammatory responses after SCT initiate the fibrotic cascade; fibrosis of epithelial and mucosal tissues is a cardinal feature of chronic GVHD.4 On the other hand, chronic GVHD is associated with antileukemia effects.5 Corticosteroids are the mainstay of treatment for chronic GVHD but the higher doses and the longer duration of corticosteroid use are associated with poor prognosis because of the increased risk for nonrelapse mortality and leukemia relapse.6,7 Development of novel treatment strategies for chronic GVHD that can spare systemic corticosteroids is an urgent unmet medical need.
Ocular GVHD affects 40% to 60% of allogeneic SCT recipients and is characterized by dysfunction of lacrimal glands and Meibomian glands, resulting in dry eye syndrome due to reduced tear production and excessive evaporation of tears.8-10 Dry eye syndrome in chronic GVHD significantly decreases patients’ quality of life by causing symptoms such as photophobia, foreign body sensation, blurring of vision, and pain.3 The histopathological features of ocular chronic GVHD include fibrosis and inflammatory cellular infiltration in the glandular interstitium of the lacrimal glands and fibrosis and atrophy of the Meibomian glands, indicating that a novel antifibrotic therapy could be beneficial for patients suffering from ocular GVHD.11-13
Heat shock protein 47 (HSP47) is a stress protein with a unique character as a molecular chaperone that specifically binds to procollagen in endoplasmic reticulum. HSP47+ fibroblasts represent activated fibroblasts, which secrete excessive amounts of collagen and are responsible for fibrosis in various disorders, such as liver cirrhosis, systemic sclerosis, and chronic GVHD.14-16 In chronic GVHD, HSP47 is overexpressed in the skin and salivary glands in mice and in the lacrimal glands in humans.16,17 We recently reported that vitamin A–coupled liposomes containing short interfering RNA (siRNA) against HSP47 (VA-lip HSP47) specifically delivered HSP47 siRNA to pathogenic fibroblasts and ameliorated skin fibrosis in chronic GVHD.16 In the current study, we developed a novel topical antifibrotic treatment against ocular chronic GVHD using VA-lip HSP47 eye drops.
Materials and methods
Mice
Female BALB/c (H-2d) mice were purchased from CLEA Japan (Tokyo, Japan), and B10.D2 (H-2d) mice were purchased from Japan SLC (Shizuoka, Japan). All animal experiments were performed under the auspices of the Institutional Animal Care and Research Advisory Committee of Hokkaido University (approval number 17-0026).
Bone marrow transplantation
Bone marrow transplantation (BMT) was performed as previously described.16 Briefly, BALB/c mice received a single dose of 6 Gy total body irradiation (TBI), followed by IV injection of 8 × 106 bone marrow (BM) cells plus 2 × 107 splenocytes from minor histocompatibility antigen–mismatched B10.D2 or syngeneic BALB/c donors on day 0. Mice were maintained in specific pathogen–free conditions and received normal chow and autoclaved hyperchlorinated water.
Evaluation of ocular chronic GVHD
The lacrimal glands were harvested at the indicated time points after BMT, fixed in 4% paraformaldehyde, and embedded in paraffin; 7-μm-thick paraffin sections of the tissues were stained with hematoxylin and eosin (H&E) or Masson’s trichrome (MT). Images were taken at room temperature using a digital camera (DP20) mounted on a microscope (BX50; both from Olympus, Tokyo, Japan). The area staining positive with MT was calculated as a ratio of the blue area/total area of a low-power field (20×/0.50 NA objective lens) using ImageJ software (National Institutes of Health; Bethesda, MD; http://imagej.nih.gov/ij/).
VA-lip HSP47
VA-lip HSP47, VA-lip HSP47 labeled with immunofluorescent Dy647 (VA-lip Dy647), and VA-lip containing scrambled siRNA with the same nucleotide composition as HSP47 siRNA (VA-lip Scr) were prepared as previously described and provided by Nitto Denko (Tokyo, Japan).16 VA-lip HSP47 or VA-lip Scr eye drops were instilled once or twice daily into both eyes of allogeneic recipients (2 μL per mouse) from days +2 to +34 or from days +21 to +34 after BMT. In the indicated experiments, VA-lip Scr eye drops were instilled daily into the left eyes of allogeneic recipients and VA-lip HSP47 eye drops were instilled into the right eyes of the same mice from days +2 to +34 after BMT. To track the in vivo distribution of VA-lip HSP47 after eye instillation, allogeneic recipient mice were unilaterally administered 2 μL of VA-lip Dy647 eye drops on days +29 to +33 after BMT, and the lacrimal glands on the treated side and the control untreated side were harvested 1 hour after the last treatment.
Collagen assay
Lacrimal glands were digested in 0.5 M acetic acid with 0.1 mg/mL pepsin solution for 72 hours. The amount of collagen deposition was measured using a Sircol Collagen Assay Kit (Biocolor, Carrickfergus, United Kingdom) and a GloMax-Multi Luminescence System (Promega, Tokyo, Japan), according to the manufacturers’ instructions. Collagen deposition was evaluated as the weight-normalized amount of collagen in the lacrimal glands.
Tear-secretion analysis
Exocrine function of the lacrimal glands was determined with the cotton thread test using standardized phenol red–impregnated cotton threads (Zone-Quick; Menicon, Nagoya, Japan). The cotton threads were inserted under the lower eyelids for 15 seconds, and the average of the bilateral length of tear-absorbing color-changed thread was calculated.
Immunofluorescent studies
The extraorbital lacrimal glands were harvested and fixed in 4% paraformaldehyde for 24 hours. Fixed tissues were embedded in paraffin for histological studies. After antigen retrieval with target retrieval solution (HistoVT One; nacalai tesque, Kyoto, Japan), paraffin-embedded sections were blocked with goat serum (Nichirei Biosciences, Tokyo, Japan), followed by incubation with primary antibodies (Abs), such as anti-HSP47 (ab77609, ab109117) and biotinylated anti–α-smooth muscle actin (α-SMA; ab125057; all from Abcam, Cambridge, United Kingdom) Abs diluted to 1:800 or 1:200, respectively. After incubation overnight at 4°C, primary Abs were visualized with anti-rabbit immunoglobulin G conjugated to Alexa Fluor 488 or Alexa Fluor 555 or streptavidin conjugated to Alexa Fluor 555 (Thermo Fisher Scientific, Waltham, MA). To detect Dy647-labeled VA-lip HSP47, frozen lacrimal gland sections were prepared after fixation with 4% paraformaldehyde overnight and 30% sucrose for the following 24 hours. Nuclear staining was done with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Dojindo Laboratories, Kumamoto, Japan). Images of tissue sections were acquired at room temperature using an FV-1000 microscope (Olympus) with a 20×/0.75 NA, 40×/0.95 NA, or 60×/1.35 NA objective lens. Data were analyzed using FluoView software (Olympus).
Statistical analysis
The Mann-Whitney U test or 1-way analysis of variance, followed by the Tukey post test, was used to compare data. P < .05 was considered statistically significant, and all data represent the mean ± standard error of the mean (SEM). Analyses were performed using Prism software version 6 (GraphPad, La Jolla, CA).
Results
Accumulation of HSP47+ fibroblasts in the lacrimal glands in chronic GVHD
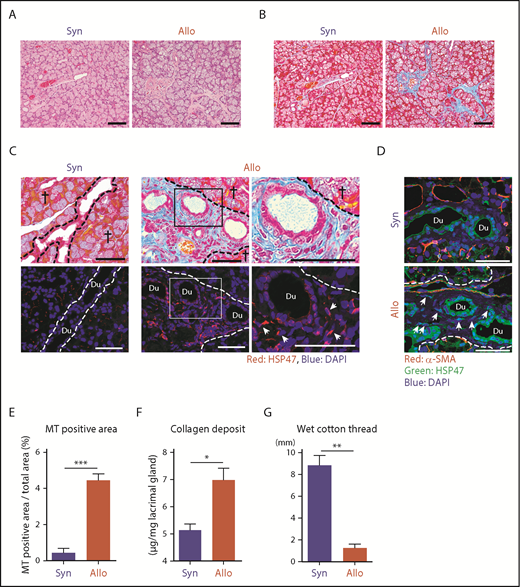
HSP47+ activated fibroblasts secrete excessive amounts of collagen and are responsible for pathologic fibrosis, including liver cirrhosis, systemic sclerosis, and chronic GVHD.14-16 We evaluated whether HSP47+ fibroblasts could be accumulated in the fibrotic lacrimal glands in ocular chronic GVHD using a well-established murine model of chronic GVHD. BALB/c mice were conditioned with 6 Gy TBI and injected IV with 8 × 106 BM cells plus 2 × 107 splenocytes from allogeneic B10.D2 or syngeneic BALB/c donors. H&E and MT staining of the lacrimal glands harvested on day +35 after allogeneic BMT demonstrated classical features of ocular chronic GVHD, with massive fibrosis in the glandular interstitium and periductal area (Figure 1A-B).13,18 Immunofluorescent studies showed an accumulation of HSP47+ fibroblasts in the fibrotic interstitium identified in the consecutive section stained with MT in allogeneic recipients (Figure 1C). In contrast to the other organs, such as liver and skin, in which α-SMA+ HSP47+ myofibroblasts are responsible for fibrosis,14,16 HSP47+ fibroblasts accumulated in the lacrimal glands did not express α-SMA (Figure 1D).17 These pathological findings were not observed in syngeneic controls, whereas physiological expression of HSP47 in the acinar basement membrane and α-SMA in acinar myoepithelial cells were observed equally in syngeneic and allogeneic recipients (Figure 1A-D). The ratio of MT-stained area/total area of the lacrimal gland section was also significantly increased in allogeneic animals compared with syngeneic controls (Figure 1E). A Sircol collagen assay showed that collagen deposition was significantly increased in the lacrimal glands of allogeneic recipients compared with that in syngeneic controls (Figure 1F). Next, lacrimal excretory function was evaluated on day +35 after BMT using a cotton thread test, an alternative for the clinical Schirmer's tear test. Tear production was significantly reduced in allogeneic mice but not in syngeneic controls (Figure 1G).
HSP47+myofibroblasts accumulated in fibrotic lesions of the lacrimal gland after allogeneic BMT. BALB/c mice were transplanted with 8 × 106 BM cells plus 2 × 107 splenocytes from allogeneic (Allo) B10.D2 or syngeneic (Syn) BALB/c donors on day 0, following 6 Gy TBI. Lacrimal glands were harvested on day +35 after BMT. H&E-stained (A) and MT-stained (B) images of the lacrimal glands. Scale bars, 100 μm. (C) MT staining (upper panels) and immunofluorescent images (lower panels) for HSP47 (red), with DAPI nuclear staining (blue) on the pairs of consecutive serial sections. Areas in the rectangles (middle panels) were magnified (right panels). Scale bars, 50 μm. Crosses indicate glandular parenchyma. Arrows indicate HSP47+ fibroblasts. (D) Immunofluorescent staining of HSP47 (green) and α-SMA (red) with DAPI nuclear staining (blue). Arrows indicate HSP47+ myofibroblasts. Scale bars, 50 μm. (E) Proportion of fibrotic area stained with MT compared with the total area of the sections of lacrimal glands from Syn (n = 6) and Allo (n = 9) mice. (F) The amount of collagen deposition in the lacrimal glands from Syn (n = 7) and Allo (n = 7) mice was normalized with tissue weight. Data from 2 independent experiments were combined and are shown as mean ± SEM. (G) The volume of tear secretion was measured as the length of wet cotton threads from Syn (n = 8) and Allo (n = 9) mice on day +35 posttransplant. Data from 2 independent experiments were combined and are shown as mean ± SEM. Dashed lines indicate the borders between the glandular parenchyma and interstitium. *P < .05, **P < .01, ***P < .005. Du, ductal lumen.
HSP47+myofibroblasts accumulated in fibrotic lesions of the lacrimal gland after allogeneic BMT. BALB/c mice were transplanted with 8 × 106 BM cells plus 2 × 107 splenocytes from allogeneic (Allo) B10.D2 or syngeneic (Syn) BALB/c donors on day 0, following 6 Gy TBI. Lacrimal glands were harvested on day +35 after BMT. H&E-stained (A) and MT-stained (B) images of the lacrimal glands. Scale bars, 100 μm. (C) MT staining (upper panels) and immunofluorescent images (lower panels) for HSP47 (red), with DAPI nuclear staining (blue) on the pairs of consecutive serial sections. Areas in the rectangles (middle panels) were magnified (right panels). Scale bars, 50 μm. Crosses indicate glandular parenchyma. Arrows indicate HSP47+ fibroblasts. (D) Immunofluorescent staining of HSP47 (green) and α-SMA (red) with DAPI nuclear staining (blue). Arrows indicate HSP47+ myofibroblasts. Scale bars, 50 μm. (E) Proportion of fibrotic area stained with MT compared with the total area of the sections of lacrimal glands from Syn (n = 6) and Allo (n = 9) mice. (F) The amount of collagen deposition in the lacrimal glands from Syn (n = 7) and Allo (n = 7) mice was normalized with tissue weight. Data from 2 independent experiments were combined and are shown as mean ± SEM. (G) The volume of tear secretion was measured as the length of wet cotton threads from Syn (n = 8) and Allo (n = 9) mice on day +35 posttransplant. Data from 2 independent experiments were combined and are shown as mean ± SEM. Dashed lines indicate the borders between the glandular parenchyma and interstitium. *P < .05, **P < .01, ***P < .005. Du, ductal lumen.
VA-lip HSP47 is distributed to the lacrimal glands and ameliorates fibrosis of the lacrimal glands in chronic GVHD
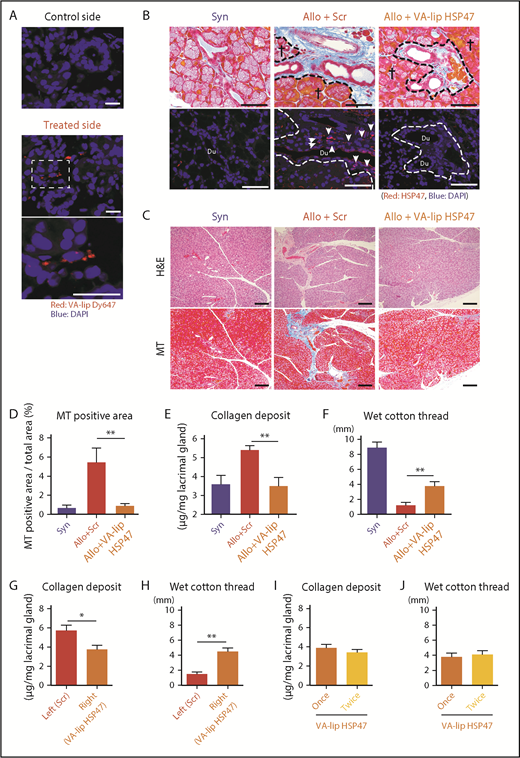
To establish a novel antifibrotic therapy against lacrimal gland fibrosis in chronic GVHD, we tested whether HSP47 could be a therapeutic target of fibrosis in chronic GVHD by using VA-lip HSP47 eye drops. When fluorescent-labeled VA-lip Dy647 was instilled into 1 eye from day +29 to day +33 after allogeneic BMT, VA-lip Dy647 particles were detected in the lacrimal glands on the treated side but not on the nontreated control side (Figure 2A).
VA-lip HSP47 ameliorates fibrosis of the lacrimal glands in chronic GVHD. Mice were transplanted as in Figure 1. (A) VA-lip Dy647 eye drops were administered daily in 1 eye from day +29 to day +33 after allogeneic BMT. One hour later, the lacrimal glands were harvested from the treated and control sides. Images of VA-lip Dy647 particles (red) with DAPI (blue) counterstaining. The area in the dashed rectangle (middle panel) is magnified (lower panel). Scale bars, 20 μm. (B-F) A group of allogeneic recipients (Allo) was treated with VA-lip HSP47 eye drops from day +2 to day +34, and the lacrimal glands were harvested on day +35 after BMT. VA-lip Scr was administered to Allo control mice. (B) MT staining (upper panels) and immunofluorescent (lower panels) images for HSP47 (red) with DAPI nuclear staining (blue) on pairs of consecutive serial sections. Scale bars, 50 μm. Dashed lines indicate the borders between the glandular parenchyma and interstitium, crosses represent glandular parenchyma; and arrows point to HSP47+ fibroblasts. (C) H&E staining (upper panels) and MT staining (lower panels) of pairs of consecutive serial sections. Scale bars, 100 µm. (D) Proportion of fibrotic area to whole area of the sections of lacrimal glands stained with MT. Data from syngeneic mice (Syn; n = 3), allogeneic controls treated VA-lip Scr (Allo+Scr; n = 6), and allogeneic recipients treated with VA-lip HSP47 (Allo+VA-lip HSP47, n = 9) are shown as mean ± SEM. (E) The amount of collagen deposition in the lacrimal glands from Syn (n = 8), Allo+Scr (n = 9), and Allo+VA-lip HSP47 (n = 8) mice was normalized with the weight of tissues. (F) The volume of tear secretion was measured as the length of wet cotton thread inserted under the lower eyelids on day +35 posttransplant. Data from Syn (n = 3), Allo+Scr (n = 6), and Allo+VA-lip HSP47 (n = 9) mice are shown as mean ± SEM. (G-H) Allogeneic recipient mice (n = 13) were treated with VA-lip HSP47 eye drops in the right eye and VA-lip Scr eye drops in the left eye from day +2 to day +34 after BMT. (I-J) Allogeneic recipient mice were treated with VA-lip HSP47 eye drops once daily (n = 8) or twice daily (n = 8) from day +2 to day +34 after BMT. The amount of collagen deposition in the lacrimal glands (G,I) and the volume of tear secretion (H,J) on day +35 are shown as mean ± SEM. (D-J) Data from 2 independent experiments were combined. *P < .05; **P < .01.
VA-lip HSP47 ameliorates fibrosis of the lacrimal glands in chronic GVHD. Mice were transplanted as in Figure 1. (A) VA-lip Dy647 eye drops were administered daily in 1 eye from day +29 to day +33 after allogeneic BMT. One hour later, the lacrimal glands were harvested from the treated and control sides. Images of VA-lip Dy647 particles (red) with DAPI (blue) counterstaining. The area in the dashed rectangle (middle panel) is magnified (lower panel). Scale bars, 20 μm. (B-F) A group of allogeneic recipients (Allo) was treated with VA-lip HSP47 eye drops from day +2 to day +34, and the lacrimal glands were harvested on day +35 after BMT. VA-lip Scr was administered to Allo control mice. (B) MT staining (upper panels) and immunofluorescent (lower panels) images for HSP47 (red) with DAPI nuclear staining (blue) on pairs of consecutive serial sections. Scale bars, 50 μm. Dashed lines indicate the borders between the glandular parenchyma and interstitium, crosses represent glandular parenchyma; and arrows point to HSP47+ fibroblasts. (C) H&E staining (upper panels) and MT staining (lower panels) of pairs of consecutive serial sections. Scale bars, 100 µm. (D) Proportion of fibrotic area to whole area of the sections of lacrimal glands stained with MT. Data from syngeneic mice (Syn; n = 3), allogeneic controls treated VA-lip Scr (Allo+Scr; n = 6), and allogeneic recipients treated with VA-lip HSP47 (Allo+VA-lip HSP47, n = 9) are shown as mean ± SEM. (E) The amount of collagen deposition in the lacrimal glands from Syn (n = 8), Allo+Scr (n = 9), and Allo+VA-lip HSP47 (n = 8) mice was normalized with the weight of tissues. (F) The volume of tear secretion was measured as the length of wet cotton thread inserted under the lower eyelids on day +35 posttransplant. Data from Syn (n = 3), Allo+Scr (n = 6), and Allo+VA-lip HSP47 (n = 9) mice are shown as mean ± SEM. (G-H) Allogeneic recipient mice (n = 13) were treated with VA-lip HSP47 eye drops in the right eye and VA-lip Scr eye drops in the left eye from day +2 to day +34 after BMT. (I-J) Allogeneic recipient mice were treated with VA-lip HSP47 eye drops once daily (n = 8) or twice daily (n = 8) from day +2 to day +34 after BMT. The amount of collagen deposition in the lacrimal glands (G,I) and the volume of tear secretion (H,J) on day +35 are shown as mean ± SEM. (D-J) Data from 2 independent experiments were combined. *P < .05; **P < .01.
VA-lip HSP47 or VA-lip Scr was instilled into eyes daily from day +2 after BMT (2 μL per mouse). An immunofluorescent study on day +35 demonstrated that VA-lip HSP47 knocked down HSP47 expression in the lacrimal glands after allogeneic BMT (Figure 2B). Histological studies with H&E and MT staining on day +35 showed that VA-lip HSP47 eye drops, but not VA-lip Scr eye drops, significantly ameliorated fibrosis in the lacrimal glands (Figure 2C-D). VA-lip HSP47 significantly reduced collagen deposition in the lacrimal glands in allogeneic recipients, in association with preserved tear-secreting function (Figure 2E-F). When 1 eye was treated with VA-lip HSP47 eye drops and the other eye of the same mouse was treated with control VA-lip Scr, collagen deposition was again significantly reduced and tear secretion was significantly restored in the eyes treated with VA-lip HSP47 eye drops compared with the eyes treated with control VA-lip Scr (Figure 2G-H). To test whether more frequent eye instillation of VA-lip HSP47 could be more effective against lacrimal gland fibrosis, VA-lip HSP47 eye drops were given once or twice daily from day +2 to day +34 after BMT; however, there was no difference in collagen deposition or tear secretion between these 2 treatment schedules (Figure 2I-J). Prolonged treatment with VA-lip HSP47 eye drops could be more effective against lacrimal gland fibrosis; however, wasting syndrome due to systemic chronic GVHD in allogeneic recipients hampered prolonged observation of ocular GVHD.
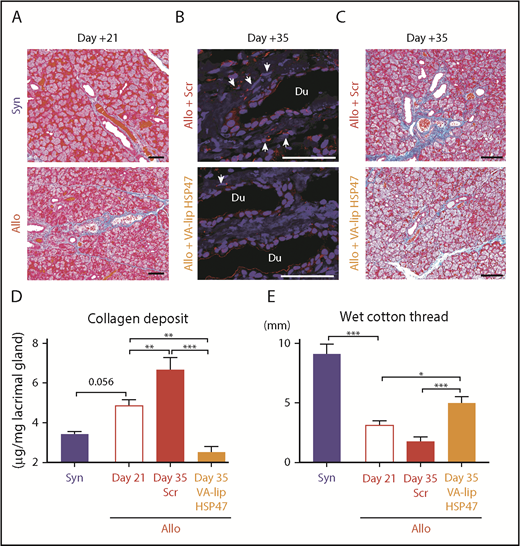
Finally, we tested whether VA-lip HSP47 eye drops could ameliorate established lacrimal gland fibrosis in chronic GVHD. In this model, lacrimal gland fibrosis developed by day +21 (Figure 3A). VA-lip HSP47 eye drops were administered daily to allogeneic recipient mice from day +21 to day +34 after BMT, and fibrosis was evaluated on day +35. VA-lip HSP47 knocked down HSP47 expression in the lacrimal glands and ameliorated fibrosis (Figure 3B-C). Importantly, VA-lip HSP47 significantly reduced collagen volume in the lacrimal glands on day +35 compared with the baseline level on day +21, whereas collagen volume was significantly increased on day +35 compared with that on day +21 in VA-lip Scr–treated controls. Ocular instillation of VA-lip HSP47 also significantly increased tear secretion on day +35 compared with that on day +21 (Figure 3D-E). Altogether, VA-lip HSP47 eye drops are promising local therapy for prophylaxis and treatment of lacrimal gland fibrosis and dry eye syndrome in chronic GVHD.
VA-lip HSP47 eye drops resolve established lacrimal gland fibrosis in chronic GVHD. Mice were transplanted as in Figure 1. A group of allogeneic recipients (Allo) was treated with VA-lip HSP47 eye drops or VA-lip Scr eye drops daily from day +21 to day +34, and the lacrimal glands were harvested on day +35 post-BMT. (A) Representative images of MT staining of lacrimal glands harvested from syngeneic (Syn; upper panel) or Allo (lower panel) recipients on day +21 posttransplant. Scale bars, 50 µm. (B) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining on day +35. Arrows point to HSP47+ fibroblasts. Scale bars, 50 µm. (C) MT staining of lacrimal glands harvested from Allo mice given VA-lip Scr (Allo + Scr; upper panel) and Allo mice given VA-lip HSP47 (Allo + VA-lip HSP47; lower panel) on day +35 post-BMT. Scale bars, 50 µm. (D) The amount of collagen deposition in the lacrimal glands was evaluated before (Allo Day 21, n = 8) and after ocular instillation with VA-lip Scr (Allo + Scr Day 35, n = 8) or VA-lip HSP47 (Allo + VA-lip HSP47 Day 35, n = 7). Syn controls were evaluated on day +21 (n = 8). (E) Tear secretion was assessed before initiation of ocular instillation (Allo Day 21, n = 18) and after ocular instillation with VA-lip Scr (Allo + Scr Day 35, n = 8) or VA-lip HSP47 (Allo + VA-lip HSP47 Day 35, n = 10). Syn controls were evaluated on day +21 (n = 8). (D-E) Data from 2 similar experiments were combined. *P < .05; **P < .01; ***P < .005.
VA-lip HSP47 eye drops resolve established lacrimal gland fibrosis in chronic GVHD. Mice were transplanted as in Figure 1. A group of allogeneic recipients (Allo) was treated with VA-lip HSP47 eye drops or VA-lip Scr eye drops daily from day +21 to day +34, and the lacrimal glands were harvested on day +35 post-BMT. (A) Representative images of MT staining of lacrimal glands harvested from syngeneic (Syn; upper panel) or Allo (lower panel) recipients on day +21 posttransplant. Scale bars, 50 µm. (B) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining on day +35. Arrows point to HSP47+ fibroblasts. Scale bars, 50 µm. (C) MT staining of lacrimal glands harvested from Allo mice given VA-lip Scr (Allo + Scr; upper panel) and Allo mice given VA-lip HSP47 (Allo + VA-lip HSP47; lower panel) on day +35 post-BMT. Scale bars, 50 µm. (D) The amount of collagen deposition in the lacrimal glands was evaluated before (Allo Day 21, n = 8) and after ocular instillation with VA-lip Scr (Allo + Scr Day 35, n = 8) or VA-lip HSP47 (Allo + VA-lip HSP47 Day 35, n = 7). Syn controls were evaluated on day +21 (n = 8). (E) Tear secretion was assessed before initiation of ocular instillation (Allo Day 21, n = 18) and after ocular instillation with VA-lip Scr (Allo + Scr Day 35, n = 8) or VA-lip HSP47 (Allo + VA-lip HSP47 Day 35, n = 10). Syn controls were evaluated on day +21 (n = 8). (D-E) Data from 2 similar experiments were combined. *P < .05; **P < .01; ***P < .005.
Discussion
HSP47 plays a critical role in the maturation of procollagen to collagen; thus, inhibition of HSP47 results in accumulation of unfolded collagen bundles and apoptosis of fibroblasts resulting from endoplasmic reticulum stress. These findings suggest that HSP47 is a promising therapeutic target for various fibrotic disorders.19 In the current study, we demonstrated that HSP47 plays a critical role in lacrimal gland fibrosis in chronic GVHD, and HSP47 is a promising therapeutic target of ocular GVHD.
HSP47+ fibroblasts accumulate in the fibrotic interstitium of the lacrimal glands in human and experimental chronic GVHD.17,20 Although α-SMA+ myofibroblasts play an important role in various fibrotic disorders, including skin GVHD,16,21-23 HSP47+ fibroblasts in the lacrimal glands did not express α-SMA in chronic GVHD. Our results are consistent with previous reports showing that HSP47+ fibroblasts in GVHD-induced lacrimal gland fibrosis are negative for α-SMA in humans and mice.17,24 The phenotype of pathogenic fibroblasts may be context and tissue dependent.17
Dry eye syndrome is caused by chronic GVHD, as well as by various factors, such as inflammatory disorders and aging, or it could be idiopathic.25 Lacrimal gland fibrosis is responsible for various types of dry eye syndrome, including chronic GVHD, suggesting that VA-lip HSP47 could be effective for dry eye syndrome in general. Although the major lacrimal glands in mice are located outside of the orbital cavity, unlike in humans, we found VA-lip Dy647 distributed in the lacrimal glands after eye instillation, suggesting that particles could move against tear excretion and reach the extraorbital lacrimal glands. In humans, VA-lip HSP47 eye drops may have better access to the lacrimal glands within the orbital cavity than in mice, although this needs to be investigated in clinical studies.
Although siRNA can specifically inhibit the transcription of target genes and is a potentially useful agent to treat various diseases, siRNA is rapidly degraded after in vivo administration, hampering its clinical application. Vitamin A–coupled liposomes protect HSP47 siRNA from degradation and preferentially deliver the siRNA to activated fibroblasts or myofibroblasts, in which vitamin A is stored or degraded.14,26,27 Systemic administration of VA-lip HSP47 has been shown to be effective against liver, pulmonary, and pancreatic fibrosis in animal models,14,28,29 and its antifibrotic effects are now being tested in patients suffering from liver cirrhosis (NCT02227459). We previously demonstrated that systemic administration of VA-lip HSP47 ameliorates cutaneous and salivary gland fibrosis in mouse chronic GVHD.16 The current study demonstrated that VA-lip HSP47 eye drops ameliorated lacrimal gland fibrosis in experimental BMT. Topical therapy has an advantage over systemic therapy by sparing systemic toxicity.30 Several topical treatments have been developed to treat ocular chronic GVHD, such as ocular instillation of corticosteroids, cyclosporine, pharmaceutical lubricants, and autologous serum.31,32 Liposomal agents are now being developed for the treatment of ophthalmic disorders, and clinical trials demonstrated their feasibility and safety for eye instillation.33 We found that systemic administration of VA-lip HSP47 did not affect engraftment or immune reconstitution in the current BMT model16 ; however, the safety of VA-lip HSP47 needs to be carefully assessed in preclinical and clinical studies. In conclusion, topical administration of VA-lip HSP47 is a novel strategy against lacrimal gland fibrosis in chronic GVHD, and it may have broad therapeutic implications in various dry eye diseases.
Acknowledgments
This work was supported by Japan Society for the Promotion of Science KAKENHI (21390295 and 17H04206 [T.T.], 17K09945 [D.H.]), the Japan Society of Hematology Research Fund (T.T.), and the Center of Innovation Program from Japan Science and Technology Agency (T.T.), as well as by research grants from the Mochida Memorial Foundation for Medical and Pharmaceutical Research (D.H.).
Authorship
Contribution: T.T. and D.H. developed the conceptual framework of the study, designed the experiments, conducted studies, analyzed data, and wrote the manuscript; H.O. designed the experiments, conducted studies, analyzed data, and wrote the manuscript; and E.H., S.T., T.A., T.Y., J.S., M.O., and M.N. conducted studies and analyzed data.
Conflicts-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takanori Teshima, Hokkaido University Faculty of Medicine, Graduate School of Medicine, N15 W7, Kita-ku, Sapporo 060-8638, Japan; e-mail: teshima@med.hokudai.ac.jp; and Daigo Hashimoto, Hokkaido University Faculty of Medicine, Graduate School of Medicine, N15 W7, Kita-ku, Sapporo 060-8638, Japan; e-mail: d5hash@pop.med.hokudai.ac.jp.