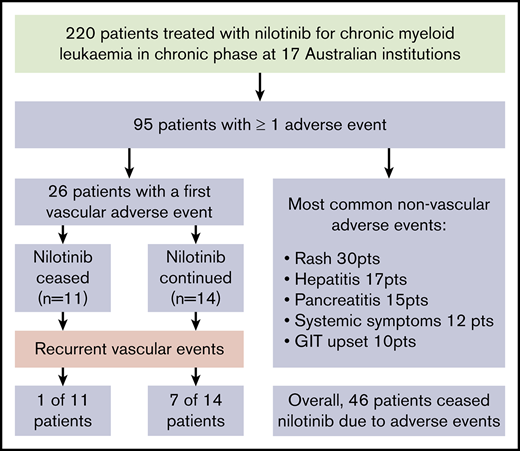
Key Points
In contrast to other AEs, there is a high risk of recurrent vascular AEs with continuing nilotinib therapy for CML.
Abstract
Although second-generation tyrosine kinase inhibitors (TKIs) show superiority in achieving deep molecular responses in chronic myeloid leukemia in chronic phase (CML-CP) compared with imatinib, the differing adverse effect (AE) profiles need consideration when deciding the best drug for individual patients. Long-term data from randomized trials of nilotinib demonstrate an increased risk of vascular AEs (VAEs) compared with other TKIs, although the natural history of these events in response to dose modifications or cessation has not been fully characterized. We retrospectively reviewed the incidence of nilotinib-associated AEs in 220 patients with CML-CP at 17 Australian institutions. Overall, AEs of any grade were reported in 95 patients (43%) and prompted nilotinib cessation in 46 (21%). VAEs occurred in 26 patients (12%), with an incidence of 4.1 events per 100 patient-years. Multivariate analysis identified age (P = .022) and dyslipidemia (P = .007) as independent variables for their development. There was 1 fatal first VAE, whereas the remaining patients either continued nilotinib (14 patients) or stopped it immediately (11 patients). Recurrent VAEs were associated with ongoing therapy in 7 of 14 who continued (with 2 fatal VAEs) vs 1 of 11 who discontinued (P = .04). Nineteen of the 23 evaluable patients surviving a VAE ultimately stopped nilotinib, of whom 14 received an alternative TKI. Dose reduction or cessation because of VAEs did not adversely affect maintenance of major molecular response. These findings demonstrate that in contrast to other AEs, VAEs are ideally managed with nilotinib cessation because of the increased risk of additional events with its ongoing use.
Introduction
Nilotinib is a second-generation tyrosine kinase inhibitor (TKI) that shows deeper molecular responses and superior kinetics of response compared with the first-generation TKI imatinib in the treatment of newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CML-CP).1 It is approved for use as frontline therapy and as second-line treatment after failure or intolerance of an alternative TKI.
Nilotinib has a unique adverse effect (AE) profile, including both hematological and nonhematological AEs.2 Five-year follow-up of phase 3 data documented grade 3 to 4 AEs of any kind in 61% and 72% of patients receiving 300 mg of nilotinib twice daily and 400 mg twice daily, respectively.1 Other nonhematological AEs associated with nilotinib use include pancreatitis,3 hyperlipidemia,4 and elevations of blood glucose.3,5 Of particular concern is the risk of vascular AEs (VAEs), which seem to be almost exclusively arterial in nature.6,7 The incidence of nilotinib-associated VAEs ranges from 0.5% to 35% of patients in published literature.2,8-11 Factors contributing to the variability include inconsistent duration of follow-up, lack of a standardized definition of vascular events,8 and exclusion of patients with a history of impaired cardiac function in some randomized controlled trials.3,10
Despite the increasing understanding of the incidence and predisposing risk factors for nilotinib-associated AEs, there is a paucity of data to assist clinicians in their management. Although strategies such as dose reduction, treatment breaks, and nilotinib cessation are commonly used in practice and form part of the recommendations by expert groups,12 the effect of these interventions on the subsequent risk of AEs and CML disease response remains unclear. This study describes the incidence and natural history of the most common nilotinib-associated AEs, with a particular focus on VAEs, in a real-world cohort of CML-CP patients treated in multiple institutions with the intention of recommending strategies for their management.
Methods
Patients
Seventeen institutions agreed to participate in a survey of CML patients treated with nilotinib and dasatinib; the dasatinib component of this survey has been published.13
All eligible patients at each site were included in the study if they received nilotinib for CML-CP at any time between 1 February 2005 and 30 June 2015 and had sufficient clinical details available. A comprehensive medical record review was undertaken, with key data collected including sex, date of birth, starting dose and duration of nilotinib therapy, sequential BCR-ABL1 transcript levels, prior CML-CP therapy, reasons for and details of any dose modifications, including temporary or permanent cessation, and AEs of all grades (graded as per Common Terminology Criteria for Adverse Events version 4.0).
Specific details of the management of VAEs were collected, including established vascular risk factors (defined as a clinician-reported diagnosis of diabetes mellitus, hypertension, or dyslipidemia requiring therapy, current or past cigarette smoking, or history of ischemic heart disease, peripheral vascular disease, or cerebrovascular disease), pharmacological treatment (antiplatelet agents, antihypertensives, and lipid-lowering therapy), and surgical interventions. VAEs were defined as acute coronary syndrome, ischemic cerebrovascular events, or peripheral vascular disease.1 Deidentified patient information was collected in an Access database (Microsoft Access 2007). CML response assessment was defined using standard published criteria.12 Deep molecular response was defined as a response of MR4.0 or greater. Ethical approval was obtained at each site.
Statistics
Data were analyzed using SPSS statistics software (version 25.0; SPSS Inc, Chicago, IL). Continuous data were compared using the Student t test, ordinal data using the Mann-Whitney U test, and categorical data using the Fisher’s exact test. Multiple regression was performed to evaluate the association between age and cardiovascular risk factors with VAEs; P < .05 was considered significant. Incidence rates of VAEs were determined at the time of first VAE; subsequent VAEs were not included in the calculation. Time-to-event analyses used the Kaplan-Meier method and are presented as cumulative incidence. Hazard ratios were calculated using Cox regression.
Results
Background demographic and treatment data are summarized in Table 1. Nearly half of all patients commenced 800 mg of nilotinib daily; for more than half of patients, this was second-line therapy. At some point during the follow-up period, 132 (60%) of the 220 patients were treated as part of a clinical trial; the most frequent trials were ENESTxtnd14 (n = 55), TIDEL-II15 (n = 38), and ENESTcmr16 (n = 29); individual treatment protocols for these studies have been published.
Patient demographics and treatment summary
| Characteristic . | n (%) . |
|---|---|
| No. of patients | 220 |
| Female sex | 90 (41) |
| Age at nilotinib commencement, y | |
| Median | 55 |
| Range | 20-91 |
| Starting daily dose, mg | |
| 800 | 106 (48) |
| 600 | 90 (41) |
| 400 | 15 (7) |
| 300 | 6 (3) |
| 150 | 2 (1) |
| Not available | 1 (1) |
| Line of therapy | |
| First | 76 (35) |
| Second | 112 (51) |
| Third | 32 (14) |
| Follow-up from commencement, mo | |
| Median | 42 |
| Range | 6-117 |
| Characteristic . | n (%) . |
|---|---|
| No. of patients | 220 |
| Female sex | 90 (41) |
| Age at nilotinib commencement, y | |
| Median | 55 |
| Range | 20-91 |
| Starting daily dose, mg | |
| 800 | 106 (48) |
| 600 | 90 (41) |
| 400 | 15 (7) |
| 300 | 6 (3) |
| 150 | 2 (1) |
| Not available | 1 (1) |
| Line of therapy | |
| First | 76 (35) |
| Second | 112 (51) |
| Third | 32 (14) |
| Follow-up from commencement, mo | |
| Median | 42 |
| Range | 6-117 |
VAEs
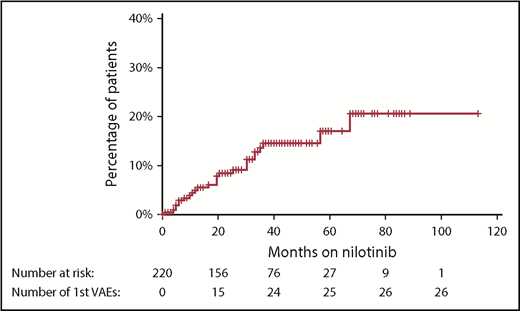
With a median follow-up of 42 months from the commencement of therapy, an initial VAE was experienced by 26 patients (12% of patients), equating to 4.1 events per 100 patient-years of exposure to nilotinib. The median time to first VAE was 19 months, with >90% of events occurring within 3 years of nilotinib commencement (Figure 1). All events occurred in patients actively taking nilotinib, with no first VAE recorded during the follow-up period in patients who had stopped nilotinib. Acute coronary syndrome was the most common first VAE (13 patients), followed by peripheral vascular disease (10 patients) and ischemic cerebrovascular events (3 patients). There were no venous thrombotic events.
Cumulative incidence of first VAE using the Kaplan-Meier method. Hatches represent censoring because of cessation of nilotinib or end of follow-up period. There were no first VAEs recorded after nilotinib was ceased.
Cumulative incidence of first VAE using the Kaplan-Meier method. Hatches represent censoring because of cessation of nilotinib or end of follow-up period. There were no first VAEs recorded after nilotinib was ceased.
The characteristics of patients who experienced VAEs are summarized in Table 2. Patients experiencing a VAE were more likely to be older and to have a higher number of cardiovascular risk factors. Smoking history and dyslipidemia were individually associated with VAEs, with a trend toward hypertension. Multivariate analysis demonstrated age (P = .022) and dyslipidemia (P = .007) as independent risk factors for developing VAEs. VAEs occurred in 15% of patients who commenced taking 800 mg compared with 9% receiving ≤600 mg daily (odds ratio, 1.8; 95% confidence interval, 0.8-4.2; P = .29). Nilotinib dose at time of first VAE was the same as commencement dose in all but 1 patient, in whom the first VAE occurred while receiving 600 mg daily after commencing 800 mg.
Characteristics of patients who developed VAEs vs those without VAEs
| Characteristic . | n (%) . | P . | OR (95% CI) . | |
|---|---|---|---|---|
| Patients with VAEs (n = 26) . | Patients without VAEs (n = 194) . | |||
| Male/female | 15/11 | 115/79 | .99 | 0.94 (0.4-2) |
| Age at nilotinib commencement, y | .014* | |||
| Median | 61 | 53 | ||
| Range | 37-80 | 21-91 | ||
| Duration of nilotinib therapy to time of first VAE, mo | NA | |||
| Median | 19 | |||
| Range | 1-68 | |||
| Mean no. of cardiovascular risk factors per patient | 1.7 | 0.9 | <.001 | |
| Individual cardiovascular risk factors | ||||
| Diabetes | 3 (12) | 19 (10) | .73 | 1.2 (0.3-4.3) |
| Hypertension | 12 (46) | 52 (26) | .06 | 2.3 (0.9-5.3) |
| Smoking history (former or current) | 14 (54) | 59 (30) | .025 | 2.7 (1.2-6.4) |
| History of VAEs | 5 (20) | 20 (10) | .19 | 2.1 (0.8-5.8) |
| Dyslipidemia | 8 (31) | 13 (7) | <.001* | 6.1 (2.2-17.2) |
| Line of therapy† | ||||
| First | 9 (35) | 67 (35) | .99 | |
| Second | 17 (65) | 95 (49) | .15 | |
| First-line therapy, imatinib | 17 | 90 | ||
| First-line therapy, dasatinib | 0 | 5 | ||
| Third | 0 (0) | 32 (16) | .018 | |
| Nilotinib dose at commencement, mg† | ||||
| 800 | 16 (62) | 90 (46) | .21 | |
| 600 | 10 (38) | 80 (41) | .84 | |
| 400 | 0 (0) | 15 (8) | .39 | |
| 300 | 0 (0) | 6 (3) | .99 | |
| 150 | 0 (0) | 2 (1) | .99 | |
| Data not available | 0 (0) | 1 (1) | .99 | |
| Characteristic . | n (%) . | P . | OR (95% CI) . | |
|---|---|---|---|---|
| Patients with VAEs (n = 26) . | Patients without VAEs (n = 194) . | |||
| Male/female | 15/11 | 115/79 | .99 | 0.94 (0.4-2) |
| Age at nilotinib commencement, y | .014* | |||
| Median | 61 | 53 | ||
| Range | 37-80 | 21-91 | ||
| Duration of nilotinib therapy to time of first VAE, mo | NA | |||
| Median | 19 | |||
| Range | 1-68 | |||
| Mean no. of cardiovascular risk factors per patient | 1.7 | 0.9 | <.001 | |
| Individual cardiovascular risk factors | ||||
| Diabetes | 3 (12) | 19 (10) | .73 | 1.2 (0.3-4.3) |
| Hypertension | 12 (46) | 52 (26) | .06 | 2.3 (0.9-5.3) |
| Smoking history (former or current) | 14 (54) | 59 (30) | .025 | 2.7 (1.2-6.4) |
| History of VAEs | 5 (20) | 20 (10) | .19 | 2.1 (0.8-5.8) |
| Dyslipidemia | 8 (31) | 13 (7) | <.001* | 6.1 (2.2-17.2) |
| Line of therapy† | ||||
| First | 9 (35) | 67 (35) | .99 | |
| Second | 17 (65) | 95 (49) | .15 | |
| First-line therapy, imatinib | 17 | 90 | ||
| First-line therapy, dasatinib | 0 | 5 | ||
| Third | 0 (0) | 32 (16) | .018 | |
| Nilotinib dose at commencement, mg† | ||||
| 800 | 16 (62) | 90 (46) | .21 | |
| 600 | 10 (38) | 80 (41) | .84 | |
| 400 | 0 (0) | 15 (8) | .39 | |
| 300 | 0 (0) | 6 (3) | .99 | |
| 150 | 0 (0) | 2 (1) | .99 | |
| Data not available | 0 (0) | 1 (1) | .99 | |
NA, not applicable.
Significant (P < .05) on multivariate analysis.
Percentage indicates percentage of patients receiving that line or dose.
Management and subsequent history of VAEs
The first VAE was managed with surgical intervention in 6 patients, consisting of coronary artery bypass (n = 2) grafting or femoral bypass surgery (n = 4; supplemental Materials). An additional 9 patients underwent endovascular intervention comprising balloon angioplasty and/or stent insertion. New pharmacological interventions after first VAE included commencement of an antiplatelet agent (n = 19), antihypertensive agent (n = 3), and therapy for dyslipidemia (n = 6). There was 1 death from the first VAE as a result of mesenteric ischemia and gut infarction.
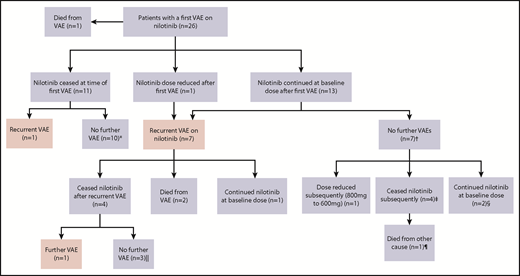
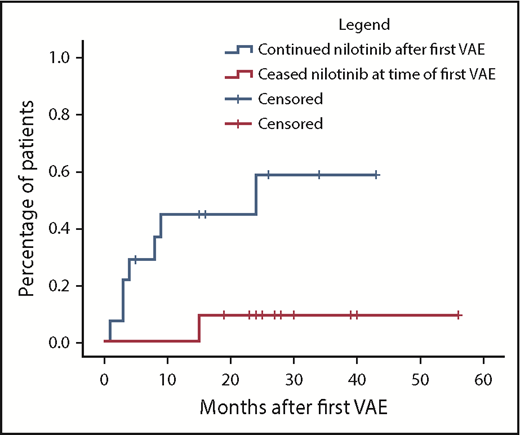
Nilotinib was immediately stopped in 11 of 25 evaluable patients and continued in 14, with recurrent events occurring in 1 of 11 vs 7 of 14, respectively (hazard ratio, 8.3; 95% confidence interval, 1.01-68; P = .019). There was a longer duration of prior nilotinib therapy in the group who stopped nilotinib after a first VAE (mean, 31.3 vs 14.5 months; P = .009), with no other significant differences in baseline characteristics between the groups (supplemental Materials). Twenty recurrent VAEs occurred overall in these 8 patients. Recurrent events did not necessarily occur at the same site. Two of the 8 patients had a subsequent event at an alternative arterial site in the same system (contralateral leg arteries); another 5 patients experienced an event in a different arterial system altogether (supplemental Materials). There was no difference in the risk of recurrence if the first event was managed with pharmacological therapy alone compared with surgical or endovascular intervention (P = .40).
Figure 2 represents the management of nilotinib therapy and outcome after the first and subsequent VAEs. Of the 14 patients who initially continued nilotinib after a VAE, the first recurrent events in the 7 affected patients occurred early, a majority within 12 months of the initial event (Figure 3). Of the surviving 12 patients within this group, only 3 continued with nilotinib at the original dose. Of these, 1 had a second VAE and 2 patients did not experience another VAE. Eight of the 12 patients stopped the drug because of recurrent VAEs (n = 4) and for alternative reasons (Figure 2 footnote). The remaining patient continued with a dose reduction for pancreatitis.
Outcomes in patients with VAEs depending upon management strategy of first VAE. *Median follow-up, 27.5 months (range, 19-56 months) after first VAE. †Median follow-up, 35 months (range, 16-45 months) after first VAE. ‡Stopped because of resistance (at 6 and 12 months), metabolic syndrome (at 36 months), and interaction with melanoma treatment (at 15 months). §Follow-up of 16 and 35 months after first VAE. Both patients experienced ACS as the first VAE. Patient 1 was treated with aspirin alone, and patient 2 commenced aspirin, clopidogrel, and rosuvastatin. ‖Median follow-up, 26 months (range, 20-73 months) after first VAE. ¶Died as a result of metastatic melanoma.
Outcomes in patients with VAEs depending upon management strategy of first VAE. *Median follow-up, 27.5 months (range, 19-56 months) after first VAE. †Median follow-up, 35 months (range, 16-45 months) after first VAE. ‡Stopped because of resistance (at 6 and 12 months), metabolic syndrome (at 36 months), and interaction with melanoma treatment (at 15 months). §Follow-up of 16 and 35 months after first VAE. Both patients experienced ACS as the first VAE. Patient 1 was treated with aspirin alone, and patient 2 commenced aspirin, clopidogrel, and rosuvastatin. ‖Median follow-up, 26 months (range, 20-73 months) after first VAE. ¶Died as a result of metastatic melanoma.
Cumulative incidence of second VAEs in patients continuing vs ceasing nilotinib after a first VAE using the Kaplan-Meier method. In the group initially continuing nilotinib (n = 14), hatches represent censoring for end of follow-up or nilotinib cessation. In the group initially stopping nilotinib (n = 11), hatches represent end of follow-up. Hazard ratio, 8.3; 95% confidence interval, 1.01-68; P = .019.
Cumulative incidence of second VAEs in patients continuing vs ceasing nilotinib after a first VAE using the Kaplan-Meier method. In the group initially continuing nilotinib (n = 14), hatches represent censoring for end of follow-up or nilotinib cessation. In the group initially stopping nilotinib (n = 11), hatches represent end of follow-up. Hazard ratio, 8.3; 95% confidence interval, 1.01-68; P = .019.
In the VAE group as a whole, nilotinib was eventually stopped in 19 of 26 patients, of whom 14 commenced an alternative TKI (Table 3). Recurrent VAEs occurred in only 2 of these patients (at 8 and 27 months), with a median follow-up of 24 months (range, 5-56 months) after cessation.
Subsequent line of therapy after nilotinib cessation
| Therapy . | No. of patients . | Median total time on nilotinib, mo . | Molecular response at time of nilotinib cessation . | Progress at 6 mo after cessation of nilotinib . |
|---|---|---|---|---|
| Imatinib | 3 | 16 | MR3.0 (n = 2) | Maintained MMR (n = 3) |
| MR4.5 (n = 1) | ||||
| Dasatinib | 11 | 30 | MR2.0 (n = 2) | Maintained or attained MMR (n = 10) |
| MR3.0 (n = 1) | ||||
| MR4.0 (n = 2) | Resistance to second TKI (n = 1) | |||
| MR4.5 (n = 6) | ||||
| No TKI | 5 | 34 | MR3.0 (n = 1) | Died of other cause (n = 1) |
| MR4.5 (n = 4) | Maintained MR4.5 (n = 4) |
| Therapy . | No. of patients . | Median total time on nilotinib, mo . | Molecular response at time of nilotinib cessation . | Progress at 6 mo after cessation of nilotinib . |
|---|---|---|---|---|
| Imatinib | 3 | 16 | MR3.0 (n = 2) | Maintained MMR (n = 3) |
| MR4.5 (n = 1) | ||||
| Dasatinib | 11 | 30 | MR2.0 (n = 2) | Maintained or attained MMR (n = 10) |
| MR3.0 (n = 1) | ||||
| MR4.0 (n = 2) | Resistance to second TKI (n = 1) | |||
| MR4.5 (n = 6) | ||||
| No TKI | 5 | 34 | MR3.0 (n = 1) | Died of other cause (n = 1) |
| MR4.5 (n = 4) | Maintained MR4.5 (n = 4) |
MMR, major molecular response.
Deaths resulting from VAEs
In addition to the single death with a first VAE, 2 deaths occurred as a result of subsequent VAEs: fatal myocardial infarction and intraoperative myocardial ischemia during coronary artery bypass grafting. Both patients had continued nilotinib at 800 mg after the first VAE without dose reduction.
Molecular responses and VAEs
Patients who did and did not experience a VAE had a similar rate of deep molecular response at 6 months from nilotinib commencement (62% vs 45%, respectively). The subsequent requirement for therapy and CML outcomes of the 19 patients who stopped nilotinib at some time after VAEs is summarized in Table 3. All but 1 evaluable patient maintained a major molecular response, including the 4 evaluable patients who did not resume TKI therapy.
Non-VAEs
Table 4 indicates other AEs reported in ≥5% of patients. The most common of these was rash (30 patients) and was described as erythematous reaction or folliculitis in most cases. There was a single case of erythema nodosum and 2 cases of psoriatic-like plaque formations. Four patients permanently stopped nilotinib therapy because of rash; the remaining 26 continued at the same or lowered doses with recurrence in only 1, who was able to continue nilotinib after a period of temporary cessation.
Non-VAEs in ≥5% of patients
| AE . | No. of patients experiencing AE (% all patients) . | No. of patients stopping nilotinib because of AE (% of all patients) . | |
|---|---|---|---|
| Any grade . | Grade 3-4 . | ||
| Rash | 30 (14) | 8 (4) | 4 (2) |
| Hepatitis | 17 (8) | 4 (2) | 7 (3) |
| Pancreatitis | 15 (7) | 5 (2) | 7 (3) |
| Systemic | 12 (5) | 2 (1) | 3 (1) |
| Other gastrointestinal (nausea, diarrhea) | 10 (5) | 0 (0) | 1 (<1) |
| AE . | No. of patients experiencing AE (% all patients) . | No. of patients stopping nilotinib because of AE (% of all patients) . | |
|---|---|---|---|
| Any grade . | Grade 3-4 . | ||
| Rash | 30 (14) | 8 (4) | 4 (2) |
| Hepatitis | 17 (8) | 4 (2) | 7 (3) |
| Pancreatitis | 15 (7) | 5 (2) | 7 (3) |
| Systemic | 12 (5) | 2 (1) | 3 (1) |
| Other gastrointestinal (nausea, diarrhea) | 10 (5) | 0 (0) | 1 (<1) |
In the 17 patients with hepatitis, there were variable biochemical patterns, including cholestasis, transaminitis, and mixed derangement. These patients were managed with nilotinib adjustments in all but 1 case, with permanent cessation in 4, temporary cessation in 11, and dose reduction in 1. Six of 13 evaluable patients experienced recurrent hepatitis despite these adjustments after a median of 4.5 months (range, 1-34 months); 3 then stopped nilotinib and switched to an alternative TKI. In a majority of the other 7 cases, reescalation to baseline dose was possible without additional events.
Pancreatitis was reported in 15 patients; 10 with biochemical changes alone and 5 with clinical symptoms, 4 of whom required hospitalization and permanent nilotinib cessation. The asymptomatic patients did not require admission and were managed with monitoring alone or temporary cessation; in most cases (7 of 10), full-dose therapy was resumed without symptomatic or biochemical recurrence.
Other gastrointestinal AEs included nausea and diarrhea in 7 and 3 patients, respectively. One patient stopped nilotinib because of severe nausea. Of the remainder, only 3 required a dose reduction or temporary cessation, all with subsequent symptom resolution.
Systemic AEs, including lethargy, fatigue, sweats, and muscle aches and pains, occurred in 12 patients (5%) and were considered severe enough for dose reductions in 6 and cessation in 3 patients.
Uncommon AEs included infection (2 patients: pneumonia and otitis media) and peripheral edema (2 patients). One patient developed a pleural effusion, which resolved after prednisolone therapy and did not recur on rechallenge. Grade 3 to 4 neutropenia occurred in 4 patients, with grade 3 in 3 patients and grade 4 in 1. All patients required dose reduction, 2 with temporary cessation beforehand; nilotinib was stopped in 1 patient for persistent neutropenia.
Drug cessation because of AEs
Eighty-two patients (37% of the study population) stopped nilotinib after a median duration of 19 months of treatment, with 46 patients (21%) stopping because of AEs. Other causes for cessation included treatment failure (n = 23; 10%), death (n = 8; 4%), poor compliance (n = 1; <1%), and attempted treatment-free remission (n = 3; 1%). For the cohort stopping because of AEs, the median age was 68 years (range, 29-94 years), with a median duration of nilotinib of 13 months (range, 0.1-73 months). A majority of patients stopped at the time of first AE (27 [59%] of 46), with the remainder doing so after a period of temporary cessation or dose reduction. The most common AE leading to cessation was a VAE (n = 15), followed by hepatitis (n = 7), pancreatitis (n = 7), and rash (n = 4). Other reasons included systemic symptoms (n = 3), worsening diabetes (n = 2), prolonged QTc (n = 1), severe nausea (n = 1), and grade 4 neutropenia (n = 1). Cessation because of an AE occurred in 18%, 24%, and 16% of patients receiving nilotinib as first-, second-, and third-line therapy, respectively.
Summary of deaths
There were 8 deaths during the follow-up period, with a median follow-up of 24 months (range, 10-79 months). Three resulted from VAEs, and 2 resulted from another malignancy. One patient died as a result of blast transformation of CML, and the remaining 2 patients died as a result of other causes.
Discussion
With a mixture of patients treated on CML trials and not on trials, our data demonstrated AEs of any grade in 43% of all patients, with 21% stopping nilotinib because of AEs. The ENESTnd trial found higher rates of AEs, with grade 3 to 4 AEs in 65% of patients and substantially more grade 1 to 2 AEs, but a lower rate of AE-related cessation (16%). This may reflect the real-world nature of our data, including possible underreporting of less severe AEs and a greater willingness to change TKI therapy outside of a trial setting.
A major focus of our study was to document not only the incidence of VAEs but also the relevant risk factors and particularly the natural history of VAEs according to nilotinib cessation or not. With a median follow-up of 42 months, VAEs occurred in 12% of patients at a rate of 4.1 events per 100 patient-years of exposure, broadly consistent with findings from large randomized trials and observational studies. At 5-year follow-up, VAEs were experienced by 7.5% of patients receiving 300 mg of nilotinib twice daily and 13.4% of patients receiving 400 mg twice daily in ENESTnd.1 In contrast, our findings are considerably higher than those of a Swedish registry study, which demonstrated a rate of 2.9 VAEs per 100 exposure-years10 ; this may reflect the longer nilotinib exposure time in our study (average, 2.9 vs 0.9 exposure-years, respectively) and reiterates that the risk of VAEs continues beyond the early treatment period.
Numerous studies have sought to define risk factors for VAE development during nilotinib treatment, with a key commonality being the high incidence of at least 1 preexisting cardiovascular risk factor in patients who experience VAEs.9 Strategies to predict the overall likelihood of VAEs have been proposed1,17,18 and have formed the basis of recommendations for pre-TKI risk assessment and the avoidance of nilotinib where possible in those at higher risk.2,9 In addition, regular monitoring for risk factors throughout treatment is recommended, with some authors suggesting a role for angiologic testing such as ankle-brachial index assessment2,19 and computed tomography coronary calcium scores.9 In line with previous findings, we demonstrated that VAEs were more common in older patients20 and in those with preexisting cardiovascular risk factors.17,18 However, unlike early studies where risk factors were assessed together,1,17 our study specifically identified smoking history and dyslipidemia as independent risk factors for VAEs, with dyslipidemia remaining significant on multivariate analysis. Although higher doses of nilotinib (800 mg daily vs ≤600 mg daily) have been associated with an increased risk of VAEs,1 this was not statistically significant in our population and may be reflective of insufficient power to demonstrate this difference.
Nilotinib itself can exacerbate existing vascular risk factors (by increasing low-density lipoprotein cholesterol4 and inducing hyperglycemia3 ) and has been shown to induce platelet activation21 and inhibit endothelial repair mechanisms.22 The overall effect seems to result from direct prothrombotic effects and longer-term promotion of atherosclerosis. Despite this increasing understanding of the incidence of, risk factors for, and pathogenesis of VAEs, data to guide subsequent management after a VAE are scarce. It has been suggested that overall survival and ongoing molecular responses are not adversely affected by the occurrence and management of a VAE23 ; however, it remains unclear whether continuation or dose reduction alters the incidence of subsequent VAEs. In our findings, there was a clear and statistically significant increase in the risk of recurrent and often multiple VAEs if nilotinib was continued after a first event. This risk was present despite surgical and/or pharmacological management; all patients who experienced a subsequent VAE were receiving an antiplatelet agent, suggesting that this alone is insufficient to protect against additional VAEs. Recurrence was not associated with a longer overall exposure time to nilotinib and was fatal in 2 patients. Conversely, recurrent VAEs were rare once nilotinib was stopped. Importantly, in those patients who stopped nilotinib because of VAEs, ongoing responsiveness to other TKI therapies was preserved, consistent with other findings in this context.23 A vast majority of patients either did not require further TKI therapy or maintained their preexisting molecular response on an alternative TKI.
Published guidelines for the management of TKI-associated AEs have adopted a practical approach and aim to balance the morbidity of AEs against the need to provide effective CML therapy with minimal treatment interruptions and generally recommend a graded approach based upon AE severity.24 In contrast to VAEs, our findings regarding the natural history of the most frequent non-VAEs support such an approach. In the cases of rash, gastrointestinal upset, and systemic symptoms, symptomatic treatment and temporary nilotinib withdrawal were generally successful in a majority of patients able to continue nilotinib without recurrent events. There were higher rates of recurrence of hepatitis and pancreatitis, but 70% of these patients remained on effective nilotinib therapy after temporary cessation and/or dose reduction.
Limitat"ions of this study include its retrospective nature and paucity of specific information relating to the management and status of comorbid conditions such as hypertension and diabetes. In particular, there was insufficient granularity of data regarding lipid-lowering therapy to detect an association with VAE incidence or recurrence; this may be of particular interest given the independent association demonstrated between a history of dyslipidemia and risk of VAEs. Despite these limitations, considerable effort was made to ensure the data were as complete and accurate as possible.
In conclusion, the main finding of this study was the high rate of recurrence of VAEs in patients continuing nilotinib after a first event despite standard management approaches. Whether targeted pharmacological therapy can influence this natural history remains an area for further research. Nevertheless, our findings strongly suggest that the optimal approach to a first VAE is to stop nilotinib and use another TKI or attempted TFR if indicated rather than continue nilotinib therapy and attempt to mitigate various cardiovascular risk factors.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the following people who participated in data collection at their institutions: Lisa Carne (Royal Adelaide Hospital), Michele Gambrill (Calvary Mater Hospital), Jennifer Devos (Concord Hospital, Sydney, Australia), Zar Nwe (Royal Hobart Hospital), and Kimberley Bury (Townsville Hospital, Townsville, Australia).
Authorship
Contribution: A.G. conceptualized the project, interpreted data, and edited the manuscript; L.F., K.C., D.Y., K.B., J.S., S.F., A.S., R.C., C.F., M.T., O.M., A.M., R.H., A.H., S.R., K.-L.C., W.-H.H., A.A., and F.P. performed data collection; A.G.M. collated, analyzed, and interpreted the data and wrote the manuscript; B.C. performed statistical analysis and manuscript review; F.P. managed the database; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.S. and D.Y. have received honoraria from Novartis and Bristol-Myers Squibb (BMS) and research funding from BMS and participated in advisory boards for Novartis. S.F. has received honoraria from BMS and research funding from BMS and participated in advisory boards for Ariad. C.F. has received a travel grant from BMS. A.G. has participated in advisory boards for Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Adrian G. Minson, Austin Hospital, 145 Studley Rd, Heidelberg, VIC 3084, Australia; e-mail: agminson@gmail.com.