Key Points
The majority of older adults or unfit acute leukemia patients are not offered intensive therapy, resulting in dismal long-term survival.
A novel cytarabine prodrug BST-236 enables delivery of high-dose cytarabine and appears to be safe and efficacious in these patients.
Abstract
High-dose cytarabine is the backbone of acute myeloid leukemia (AML) treatment. Nevertheless, its use in older patients is considerably limited due to increased toxicity. BST-236 (INN aspacytarabine) is a novel cytarabine prodrug designed to deliver high-dose cytarabine to target cells with reduced systemic exposure to free cytarabine. This phase 1/2a dose-escalation study was designed to evaluate BST-236 safety, pharmacokinetics, and efficacy in older or unfit-for-intensive-therapy patients with acute leukemia. Twenty-six patients, unfit for standard therapy, who were either relapsed/refractory or newly diagnosed, received BST-236 in 6 dose-escalating cohorts (range 0.3 to 6 g/m2 per day). BST-236 was administered intravenously once daily over 60 minutes for 6 consecutive days. The median age was 76.5 (26 to 90), with 84.6% of patients ≥70 years. BST-236 was safe and well tolerated. The maximal tolerated dose was 6 g/m2 per day. Overall response rate was 29.6%. A subgroup analysis of newly diagnosed patients with AML, de novo or secondary to myelodysplastic syndrome, unfit for standard induction (median age 78), demonstrated overall response of 45.5%. The median overall survival was 6.5 months and was not reached in patients achieving complete remission. The findings of this phase 1/2 study suggest that BST-236 safely delivers high and efficacious cytarabine doses to older patients who are unfit for standard induction and lays the foundation for further studies of BST-236 in AML. This trial was registered at www.clinicaltrials.gov as #NCT02544438.
Introduction
The prevalence of acute myeloid leukemia (AML), recognized as a disease of older adults with a median age at presentation of 67 years, is increasing in recent years due to the overall aging population, toxic exposures, and prior use of chemotherapy or radiation for the treatment of other malignancies.1,2 Intensified treatment improves remission rate, leukemia-free survival, and overall survival (OS), but is associated with increased treatment-related mortality. Decisions regarding treatment intensity are based on disease-associated prognostic factors such as cytogenetic and molecular risk score and presence of antecedent hematologic malignancy, on the one hand, and patient-related factors including age, performance status, and comorbidities, on the other hand. The backbone of intensive AML therapy for the last 45 years has been cytarabine (cytosine arabinoside), which is included in virtually all standard induction regimens, generally in combination with anthracyclines, and in consolidation protocols. It is also commonly used as front-line and relapse treatment in acute lymphoblastic leukemia (ALL). The cytarabine toxicity profile depends on the dose and duration of its administration. Age is identified as the most important risk factor for toxicity development, specifically when using high-dose cytarabine (HiDAC). The fact that older age is associated with adverse cytogenetics, a higher likelihood of chemoresistance, and inability to deliver high doses of chemotherapy2-4 results in poor disease-free survival.
Cytarabine pharmacokinetics (PK) is characterized by rapid disappearance from plasma owing to its deamination by the liver enzyme cytidine deaminase (CDA)5 to uracil arabinoside (Ara-U), which is excreted by the kidneys. Hence, the dose should be adjusted for liver and kidney function. In addition, a highly polymorphic gene codes CDA, and patients with a poor metabolizer phenotype are prone to increased cytarabine toxicity, whereas rapid metabolizers may be prone to cytarabine resistance.6,7 Therefore, balancing between effective antileukemic cytarabine doses, the administration mode, and its toxicity is challenging, particularly in older and frail patients, which leaves a significant number of patients with AML with suboptimal therapy and with no consensus regarding a standard-of-care protocol.
BST-236 (INN aspacytarabine) is a novel cytarabine prodrug, composed of cytarabine covalently bound to asparagine via its cytosine residue (supplemental Figure 1) designed to deliver high cytarabine doses with reduced systemic toxicity. BST-236 is inactive in its intact prodrug form, and as long as it remains intact, its asparagine residue protects cytarabine from deamination into its inactive metabolite Ara-U. In vitro studies demonstrate that BST-236 is cytotoxic to a variety of acute and chronic leukemia cell lines which is mediated by cytarabine release. BST-236 is shown to enter leukemic cells intact, followed by cellular accumulation of its metabolite cytarabine, resulting in cell death via apoptosis. In vivostudies show that BST-236 is highly effective in eliminating leukemia tumors, while enabling better recovery of normal white blood cells compared with free cytarabine and with no apparent clinical toxicity. Overall, preclinical pharmacology, PK, and toxicology studies have determined that BST-236 enables delivery of high cytarabine doses with significantly reduced exposure to free cytarabine, resulting in reduced systemic toxicity and relative sparing of normal tissues.8,9 These findings have led to the design and conduction of the phase 1/2a clinical study presented herein.
Methods
Study design
This 2-center, open-label, dose-escalating phase 1/2a first-in-human study aimed to assess safety, tolerability, PK, and efficacy of BST-236 as a single agent in relapsed/refractory or newly diagnosed patients with AML or ALL, unfit for standard induction therapy.
Patient demographics and medical history were recorded at baseline. Physical examination, vital signs, and Eastern Cooperative Oncology Group (ECOG) performance status (PS) were documented at baseline and throughout the study. Safety, based on adverse event (AE) assessment, was evaluated and graded according to the National Cancer Institute Common Terminology Criteria for AEs, version 4.0.
The treatment included an induction course of BST-236, administered intravenously once daily over 60 minutes for 6 consecutive days. The patients received dexamethasone eye drops for prophylaxis for 10 days. A second course was optional, at the discretion of the treating physician. Patients were followed for 90 days. Poststudy follow-up of OS was conducted until data lock.
The study had a 3+3 design. In the first 4 dose-escalation cohorts, patients were treated with 0.5, 1.5, 3, or 4.5 g/m2 per day of BST-236. In each of these cohorts, a 50% dose reduction was applied for patients >50 years, in accordance with the requirement by the Israel Ministry of Health. Upon completion of the treatment of cohort 4 with the maximal tolerated dose (MTD) not reached, the protocol was amended to enroll 2 additional cohorts, 5 and 6, with 3 and 6 patients at a minimal age of 70 years, treated with 4.5 or 6 g/m2 per day BST-236, respectively, and no dose adjustment.
If a patient in any cohort was withdrawn from the study for a reason other than a dose-limiting toxicity (DLT) event during the first 6-day treatment course, the patient was replaced. A DLT event was determined as any grade 3 or higher nonhematologic toxicity (excluding tumor lysis syndrome and myelosuppression-related complications, such as fever, infections, and bleeding), observed during the first 30 days of each treatment course.
The safety committee assessed all safety data following treatment completion in each cohort and provided recommendations regarding the study continuation.
Patients
Patients with AML or ALL, either relapsed/refractory or newly diagnosed unfit for standard induction, with ECOG PS ≤ 2 were considered eligible. Patients ≥18 years old were included in cohorts 1 to 4 and those ≥70 years were included in cohorts 5 to 6. Exclusion criteria were central nervous system involvement, serum creatinine >1.5 (the upper limit of normal [ULN]), aspartate aminotransferase, alanine aminotransferase >2.5 × ULN, total bilirubin level >1.5 × ULN, compromised pulmonary function requiring oxygen therapy, and a known infection with HIV and/or active viral hepatitis B or C. The study was approved by the institutional review boards of the participating centers. All participants gave written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Study endpoints
The primary study endpoint was to establish the MTD by identifying DLTs of BST-236 as a single agent from treatment start until day 30 in the study population. Secondary endpoints were BST-236 PK and efficacy.
PK
Samples for PK analysis were collected from all patients during their first treatment course. Blood was collected at the following time points in cohorts 1 to 5: on days 1 and 6, samples were collected at predose and 15, 30, 60, 90, 120, 240, 360, and 600 minutes after completion of BST-236 administration; on days 2 to 5, samples were collected at predose and 30 minutes after completion of BST-236 administration; and 24 and 48 hours after the last BST-236 dose on day 6. In cohort 6, the PK schedule included collection at 2 additional time points during infusion on day 1, and only predose samples were collected on days 2 to 6. Samples were centrifuged within 30 minutes of collection at 3000 rpm for 5 minutes at 4°C, and plasma was collected, added to ice-cold methanol, and frozen at −80°C. Plasma concentrations of BST-236 and cytarabine were determined by liquid chromatography with tandem mass spectrometry under Good Laboratory Practice compliance.
Outcome definitions
Hematological responses, including complete remission (CR), morphologic CR with incomplete blood count recovery (CRi), and morphologic CR with incomplete blood platelet recovery (CRp), were assessed according to the 2017 European LeukemiaNet (ELN) AML Recommendations.2 CR was defined as morphologic CR of < 5% blasts in a bone marrow (BM) aspirate sample with marrow spicules and with a count of ≥200 nucleated cells (no blasts with Auer rods or persistent extramedullary disease), with blood count recovery (absolute neutrophil count [ANC] >1 × 109/L and platelets ≥100 × 109/L). CRi was defined as CR with either ANC ≤1 × 109/L or a platelet count ≤100 × 109/L. CRp was defined as CR with ANC ≥1 × 109/L, a platelet count <100 × 109/L, and platelet transfusion independence. Partial response (PR) was defined as a decrease of at least 50% in the percentage of BM blasts to 5% to 25%, with no need for hematopoietic recovery. Overall response rate (ORR) was defined as CR + Cri + CRp + PR.
BM analysis was performed for aspiration and biopsy, analyzed by microscopic examination, immunohistochemistry, and flow cytometry. In the case of discrepancy between the methods, the higher blast number was considered for response evaluation. Response to treatment was evaluated locally at the trial site according to the above standard criteria.
OS was defined as time from treatment initiation to death or to the end of study follow-up.
Statistical analysis
Safety and efficacy analyses were performed in the intent-to-treat (ITT) population, defined as all subjects who received at least 1 BST-236 dose. Efficacy measurements were summarized using descriptive statistics.
Survival analysis using Kaplan-Meier survival function curves was applied for analyzing OS from the first treatment day. Subjects were censored on the last day of follow-up or early termination not due to death. Median time to event was calculated. A log-rank test or Cox models (as appropriate) were applied to compare the times across treatment groups.
Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
A subgroup analysis was performed for newly diagnosed patients with AML, either de novo or secondary to myelodysplastic syndrome (MDS), who were unfit for standard induction.
PK variables were tabulated, and descriptive statistics were calculated for each group. Geometric means and coefficients of variation were presented for Cmax and area under the curve (AUC) for each group.
Results
Patients
Twenty-six patients (median age 76.5 years, range 26 to 90) were enrolled to the study between April 2014 and August 2017 at 2 Israeli sites and were treated with at least 1 BST-236 dose. These patients formed the ITT population (Table 1). Table 2 presents patient demographics and baseline characteristics. Most patients (n = 24, 92.3%) had AML and 2 patients (7.7%) had ALL. Of the 24 patients with AML, 10 patients (42%) had relapsed/refractory AML and 14 patients (58.3%) had newly diagnosed AML, including 3 de novo AML and 11 secondary AML, of whom 8 patients had AML secondary to MDS and 3 had AML secondary to myeloproliferative disorders (MPDs). The 2 patients with ALL were newly diagnosed.
Treatment cohorts
| Cohort no. . | BST-236 dose . | No. of patients per protocol . |
|---|---|---|
| 1 | Age <50 y: 0.5 g/m2 per d | 1 patient |
| Age ≥50 y: 0.3 g/m2 per d | 2 patients | |
| 2 | Age <50 y: 1.5 g/m2 per d | 0 patients |
| Age ≥50 y: 0.8 g/m2 per d | 3 patients (1 patient received 2 induction courses) | |
| 3 | Age <50 y: 3 g/m2 per d | 0 patients |
| Age ≥50 y: 1.5 g/m2 per d | 4 patients (1 patient discontinued on day 2) | |
| 4 | Age <50 y: 4.5 g/m2 per d | 1 patient |
| Age ≥50 y: 2.3 g/m2 per d | 5 patients | |
| 5 | 4.5 g/m2 per d (no age limit) | 3 patients |
| 6 | 6 g/m2 per d (no age limit) | 7 patients (1 patient discontinued on day 2; 1 patient discontinued on day 3; 1 patient received 2 induction courses) |
| Total | 26 |
| Cohort no. . | BST-236 dose . | No. of patients per protocol . |
|---|---|---|
| 1 | Age <50 y: 0.5 g/m2 per d | 1 patient |
| Age ≥50 y: 0.3 g/m2 per d | 2 patients | |
| 2 | Age <50 y: 1.5 g/m2 per d | 0 patients |
| Age ≥50 y: 0.8 g/m2 per d | 3 patients (1 patient received 2 induction courses) | |
| 3 | Age <50 y: 3 g/m2 per d | 0 patients |
| Age ≥50 y: 1.5 g/m2 per d | 4 patients (1 patient discontinued on day 2) | |
| 4 | Age <50 y: 4.5 g/m2 per d | 1 patient |
| Age ≥50 y: 2.3 g/m2 per d | 5 patients | |
| 5 | 4.5 g/m2 per d (no age limit) | 3 patients |
| 6 | 6 g/m2 per d (no age limit) | 7 patients (1 patient discontinued on day 2; 1 patient discontinued on day 3; 1 patient received 2 induction courses) |
| Total | 26 |
Patient demographics and baseline characteristics
| . | All patients, N = 26 . | AML, n = 24 . | ALL, n = 2 . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 76.5 (26-90) | 76.0 (26-88) | 84.5 (79-90) |
| Age ≥75, n (%) | 17 (65.4) | 15 (62.5) | 2 (100.0) |
| Age ≥80, n (%) | 9 (34.6) | 8 (33.3) | 1 (50.0) |
| Sex, n (%) | |||
| M | 14 (53.8) | 12 (50.0) | 2 (100.0) |
| F | 12 (46.2) | 12 (50.0) | 0 (0.0) |
| Leukemia type, n (%) | |||
| AML | 24 (92.3) | ||
| ALL | 2 (7.7) | ||
| AML status, n (%) | |||
| Refractory | 6 (25.0) | ||
| Relapse | 4 (16.7) | ||
| Newly diagnosed, unfit for standard therapy | 14 (58.3) | ||
| De novo | 3 (21.4) | ||
| Secondary to MDS | 8 (57.1) | ||
| Secondary to MPD | 3 (21.4) | ||
| ELN score, n (%) | |||
| Favorable | 1 (4.2) | ||
| Intermediate | 14 (58.3) | ||
| Adverse | 9 (37.5) | ||
| ECOG PS, n (%) | |||
| 0 | 9 (34.6) | 9 (37.5) | 0 (0.0) |
| 1 | 14 (53.8) | 12 (50.0) | 2 (100.0) |
| 2 | 3 (11.5) | 3 (12.5) | 0 (0.0) |
| BM blast percentage | |||
| Median (range) | 83.0 (22-100) | 83.0 (22-100) | 68.5 (48-89) |
| 20%-30%, n (%) | 1 (3.8) | 1 (4.2) | 0 (0.0) |
| 31%-50%, n (%) | 4 (15.4) | 3 (12.5) | 1 (50.0) |
| >50%, n (%) | 19 (73.1) | 18 (75.0) | 1 (50.0) |
| White blood cells, ×109/L | |||
| Median | 7.2 | 8.2 | 1.8 |
| Range | 0.8-104 | 0.8-104 | 1.7-2.0 |
| . | All patients, N = 26 . | AML, n = 24 . | ALL, n = 2 . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 76.5 (26-90) | 76.0 (26-88) | 84.5 (79-90) |
| Age ≥75, n (%) | 17 (65.4) | 15 (62.5) | 2 (100.0) |
| Age ≥80, n (%) | 9 (34.6) | 8 (33.3) | 1 (50.0) |
| Sex, n (%) | |||
| M | 14 (53.8) | 12 (50.0) | 2 (100.0) |
| F | 12 (46.2) | 12 (50.0) | 0 (0.0) |
| Leukemia type, n (%) | |||
| AML | 24 (92.3) | ||
| ALL | 2 (7.7) | ||
| AML status, n (%) | |||
| Refractory | 6 (25.0) | ||
| Relapse | 4 (16.7) | ||
| Newly diagnosed, unfit for standard therapy | 14 (58.3) | ||
| De novo | 3 (21.4) | ||
| Secondary to MDS | 8 (57.1) | ||
| Secondary to MPD | 3 (21.4) | ||
| ELN score, n (%) | |||
| Favorable | 1 (4.2) | ||
| Intermediate | 14 (58.3) | ||
| Adverse | 9 (37.5) | ||
| ECOG PS, n (%) | |||
| 0 | 9 (34.6) | 9 (37.5) | 0 (0.0) |
| 1 | 14 (53.8) | 12 (50.0) | 2 (100.0) |
| 2 | 3 (11.5) | 3 (12.5) | 0 (0.0) |
| BM blast percentage | |||
| Median (range) | 83.0 (22-100) | 83.0 (22-100) | 68.5 (48-89) |
| 20%-30%, n (%) | 1 (3.8) | 1 (4.2) | 0 (0.0) |
| 31%-50%, n (%) | 4 (15.4) | 3 (12.5) | 1 (50.0) |
| >50%, n (%) | 19 (73.1) | 18 (75.0) | 1 (50.0) |
| White blood cells, ×109/L | |||
| Median | 7.2 | 8.2 | 1.8 |
| Range | 0.8-104 | 0.8-104 | 1.7-2.0 |
F, female; HMA, hypomethylating agents; M, male; MPD, myeloproliferative disorders.
Twenty-two patients (84.6%) were ineligible for standard induction/salvage chemotherapy, due to either age ≥75 years (65.4%) or comorbidities (19.2%).
The baseline median BM blast percentage was 83.0 (range 22 to 100). Nine patients with AML (37.5%) had adverse ELN cytogenetic risk score, whereas 14 patients (58.3%) and 1 patient (4.2%) had an intermediate or favorable score, respectively.
In the subgroup of 11 patients with newly diagnosed AML, either de novo or secondary to MDS, unfit for standard induction, the median age was 78.0 years (range 70 to 88); 8 patients (72.7%) had AML secondary to MDS, and 5 patients (45.5%) were previously treated with hypomethylating agents (HMA) for MDS. The median BM blast percentage was 80.0% (range 22% to 100%); 6 patients (54.5%) had adverse ELN cytogenetic score, and 4 patients (36.4%) and 2 patients (18.2%) had intermediate or favorable ELN score, respectively (Table 3).
Baseline characteristics of subjects with newly diagnosed AML (de novo or secondary to MDS), unfit for standard therapy
| . | Variables, n = 11 . |
|---|---|
| Age, y | |
| Median (range) | 78.0 (70-88) |
| Age ≥75, n (%) | 8 (72.7) |
| Age ≥80, n (%) | 5 (45.5) |
| Sex, n (%) | |
| M | 6 (54.5) |
| F | 5 (45.5) |
| AML type, n (%) | |
| De novo | 3 (27.3) |
| Secondary to MDS | 8 (72.7) |
| Prior HMA treatment of MDS | 5 (45.5) |
| ELN score, n (%) | |
| Favorable | 1 (9.1) |
| Intermediate | 4 (36.4) |
| Adverse | 6 (54.5) |
| ECOG PS, n (%) | |
| 0 | 4 (36.4) |
| 1 | 5 (45.5) |
| 2 | 2 (18.2) |
| BM blast percentage | |
| Median (range) | 80.0 (22-100) |
| 20%-30%, n (%) | 1 (9.1) |
| 31%-50%, n (%) | 3 (27.3) |
| >50%, n (%) | 7 (63.6) |
| White blood cells, ×109/L | |
| Median | 4.3 |
| Range | 0.8-61.8 |
| . | Variables, n = 11 . |
|---|---|
| Age, y | |
| Median (range) | 78.0 (70-88) |
| Age ≥75, n (%) | 8 (72.7) |
| Age ≥80, n (%) | 5 (45.5) |
| Sex, n (%) | |
| M | 6 (54.5) |
| F | 5 (45.5) |
| AML type, n (%) | |
| De novo | 3 (27.3) |
| Secondary to MDS | 8 (72.7) |
| Prior HMA treatment of MDS | 5 (45.5) |
| ELN score, n (%) | |
| Favorable | 1 (9.1) |
| Intermediate | 4 (36.4) |
| Adverse | 6 (54.5) |
| ECOG PS, n (%) | |
| 0 | 4 (36.4) |
| 1 | 5 (45.5) |
| 2 | 2 (18.2) |
| BM blast percentage | |
| Median (range) | 80.0 (22-100) |
| 20%-30%, n (%) | 1 (9.1) |
| 31%-50%, n (%) | 3 (27.3) |
| >50%, n (%) | 7 (63.6) |
| White blood cells, ×109/L | |
| Median | 4.3 |
| Range | 0.8-61.8 |
Three patients did not complete the first 6-day course. One patient (cohort 3) was withdrawn from the study at the discretion of the treating physician due to exacerbation of preexisting pneumonia. Among the 2 other patients (both from cohort 6), one did not complete the treatment due to grade 5 toxicity (tumor lysis syndrome) and the other did not complete the treatment due to DLT (pneumonitis). Two patients, aged 80 and 81 years in cohorts 2 and 6, respectively, were treated with 2 induction courses of BST-236.
PK
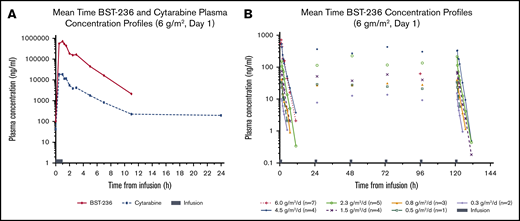
PK of BST-236 and its metabolite, cytarabine, were determined following daily drug infusions, as detailed in “Methods.” During 1-hour infusion, plasma BST-236 concentrations rapidly increased, reaching 77% of the maximal level by 30 minutes. Following infusion completion, concentrations rapidly declined in an apparent biphasic manner, reaching negligible levels (≤5% of the peak) at 6 to 10 hours after infusion end (Figure 1A).
PK of BST-236 and cytarabine. (A) Mean time-concentration profiles of BST-236 and cytarabine following a single BST-236 infusion administration (day 1) for a patient receiving 6 g/m2. (B) Mean time-concentration profiles of BST-236 following repeated BST-236 infusion administration (days 1 to 6) for all treatment groups (n = 26).
PK of BST-236 and cytarabine. (A) Mean time-concentration profiles of BST-236 and cytarabine following a single BST-236 infusion administration (day 1) for a patient receiving 6 g/m2. (B) Mean time-concentration profiles of BST-236 following repeated BST-236 infusion administration (days 1 to 6) for all treatment groups (n = 26).
BST-236 mean terminal elimination half-life was ∼1.25 hours. By 6 to 10 hours from infusion completion, essentially all of BST-236 was cleared. At 24 hours postdose (next predose), the drug concentration in all samples was below the quantification limit, and no day-to-day accumulation was observed (Figure 1B). The increase in BST-236 exposure was more than dose-proportional, potentially due to the estimated higher clearance rate at lower BST-236 dose levels.
The time-concentration profile of cytarabine followed the same pattern as that of the BST-236 profile, although concentration levels were at different scales (Figure 1A): the metabolite-to-parent (cytarabine to BST-236) ratio was 0.05 (ie, plasma concentrations of cytarabine were ∼20-fold lower than of BST-236). This ratio was similar for various administered BST-236 doses. Cytarabine mean terminal elimination half-life ranged from 1 hour at the lower BST-236 doses to 4.5 hours at the highest dose. At all BST-236 dose levels, most of cytarabine was cleared from the plasma by 10 hours post-administration. However, at the higher BST-236 dose levels (≥1.5 g/m2), some subjects had negligible quantifiable cytarabine plasma levels (≤1% of Cmax) at 24 hours postinfusion that contributed to the longer terminal elimination half-life; however, cytarabine did not accumulate following multiple BST-236 administrations (data not shown). Similar to BST-236 exposure, cytarabine exposure increased with BST-236 dose increase in a more than dose-proportional manner.
Safety
BST-236 treatment was well tolerated. Treatment-emergent AEs (TEAEs) are presented in Table 4. Grade 3 or higher TEAEs reported in ≥2 patients were mainly hematological or infectious. No cerebral or cerebellar toxicity and no renal failure were reported. No mucositis events were reported other than a single case of grade 2 mouth ulceration at the lowest tested dose.
TEAEs occurring in ≥10% of patients
| . | All doses (N = 26), n (%) . | ||||
|---|---|---|---|---|---|
| Preferred term . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . | Total . |
| Febrile neutropenia | 8 (31) | 6 (23) | 0 (0) | 0 (0) | 14 (53) |
| Hypokalemia | 10 (38) | 0 (0) | 0 (0) | 0 (0) | 10 (38) |
| Constipation | 9 (35) | 0 (0) | 0 (0) | 0 (0) | 9 (35) |
| Neutropenia | 0 (0) | 6 (23) | 3 (12) | 0 (0) | 9 (35) |
| Diarrhea | 7 (27) | 1 (4) | 0 (0) | 0 (0) | 8 (31) |
| Dyspnea | 4 (15) | 2 (8) | 1 (4) | 0 (0) | 7 (27) |
| Fluid overload | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 6 (23) |
| Vomiting | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 6 (23) |
| Anemia | 1 (4) | 5 (19) | 0 (0) | 0 (0) | 6 (23) |
| Edema peripheral | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 6 (23) |
| Chills | 5 (19) | 0 (0) | 0 (0) | 0 (0) | 5 (19) |
| Hypocalcemia | 5 (19) | 0 (0) | 0 (0) | 0 (0) | 5 (19) |
| Pneumonia | 0 (0) | 3 (12) | 0 (0) | 2 (8) | 5 (19) |
| Epistaxis | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Headache | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Insomnia | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Edema | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Pancytopenia | 1 (4) | 3 (12) | 0 (0) | 0 (0) | 4 (15) |
| Rash | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Thrombocytopenia | 0 (0) | 0 (0) | 3 (12) | 1 (4) | 4 (15) |
| Asthenia | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Back pain | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Catheter site hematoma | 2 (8) | 1 (4) | 0 (0) | 0 (0) | 3 (12) |
| Cough | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Hematoma | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Hepatic enzyme increased | 2 (8) | 1 (4) | 0 (0) | 0 (0) | 3 (12) |
| Hypomagnesaemia | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Nausea | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Tumor lysis syndrome | 0 (0) | 2 (8) | 0 (0) | 1 (4) | 3 (12) |
| Upper respiratory tract infection | 2 (8) | 1 (4) | 0 (0) | 0 (0) | 3 (12) |
| . | All doses (N = 26), n (%) . | ||||
|---|---|---|---|---|---|
| Preferred term . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . | Total . |
| Febrile neutropenia | 8 (31) | 6 (23) | 0 (0) | 0 (0) | 14 (53) |
| Hypokalemia | 10 (38) | 0 (0) | 0 (0) | 0 (0) | 10 (38) |
| Constipation | 9 (35) | 0 (0) | 0 (0) | 0 (0) | 9 (35) |
| Neutropenia | 0 (0) | 6 (23) | 3 (12) | 0 (0) | 9 (35) |
| Diarrhea | 7 (27) | 1 (4) | 0 (0) | 0 (0) | 8 (31) |
| Dyspnea | 4 (15) | 2 (8) | 1 (4) | 0 (0) | 7 (27) |
| Fluid overload | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 6 (23) |
| Vomiting | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 6 (23) |
| Anemia | 1 (4) | 5 (19) | 0 (0) | 0 (0) | 6 (23) |
| Edema peripheral | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 6 (23) |
| Chills | 5 (19) | 0 (0) | 0 (0) | 0 (0) | 5 (19) |
| Hypocalcemia | 5 (19) | 0 (0) | 0 (0) | 0 (0) | 5 (19) |
| Pneumonia | 0 (0) | 3 (12) | 0 (0) | 2 (8) | 5 (19) |
| Epistaxis | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Headache | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Insomnia | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Edema | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Pancytopenia | 1 (4) | 3 (12) | 0 (0) | 0 (0) | 4 (15) |
| Rash | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (15) |
| Thrombocytopenia | 0 (0) | 0 (0) | 3 (12) | 1 (4) | 4 (15) |
| Asthenia | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Back pain | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Catheter site hematoma | 2 (8) | 1 (4) | 0 (0) | 0 (0) | 3 (12) |
| Cough | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Hematoma | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Hepatic enzyme increased | 2 (8) | 1 (4) | 0 (0) | 0 (0) | 3 (12) |
| Hypomagnesaemia | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Nausea | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Tumor lysis syndrome | 0 (0) | 2 (8) | 0 (0) | 1 (4) | 3 (12) |
| Upper respiratory tract infection | 2 (8) | 1 (4) | 0 (0) | 0 (0) | 3 (12) |
MTD was defined at the highest dose tested (6 g/m2 per day) due to 1 DLT event of pneumonitis (which was assessed by the study safety committee as pneumonia). Two patients died” during the 6-day treatment course: one from pneumonitis and one from tumor lysis syndrome. The 30-day mortality rate for the entire study population was 8 of 26 (30.7%; 95% confidence interval [CI] 13.0 to 48.5), including the 2 patients who died during treatment, 4 patients who died of disease progression, 1 patient who died from infection, and 1 sudden death from an unknown cause occurred 8 days posttreatment in a patient with remarkable history of cardiovascular diseases. The 60-day mortality rate was 11 of 26 (42.3%; 95% CI 23.3 to 61.3), including 2 additional patients who died of disease progression and 1 from pneumonia.
Except for 1 DLT event in cohort 6, no dose-related increase in AE frequency or severity was observed.
In the subgroup of patients with newly diagnosed AML, either de novo or secondary to MDS, unfit for standard induction, the 30-day mortality rate was 2 of 11 (18.2%; 95% CI, 0.0 to 41.0). The 60-day mortality rate was 3 of 11 (27.3%; 95% CI, 1.0 to 53.6).
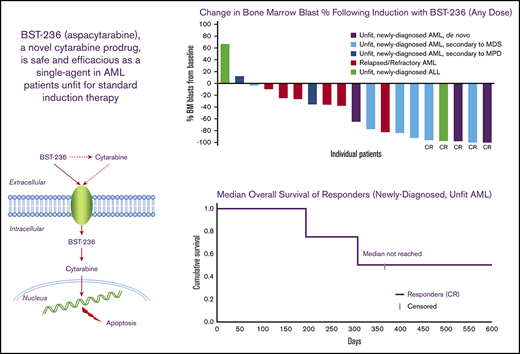
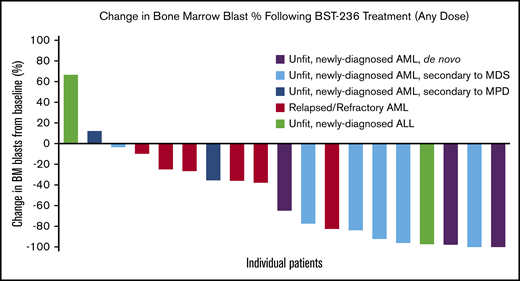
Response
BM blast reduction was observed in most patients (Figure 2). The ORR in the ITT population, at all doses combined, was 29.6% (95% CI, 9.9 to 44.0), and the CR/CRp rate was 19.2% (95% CI, 4.1 to 34.4). None of the 10 patients with relapsed/refractory AML or 3 patients with AML secondary to MPD reached CR, CRi, or CRp. In the subgroup with newly diagnosed AML, either de novo or secondary to MDS, the CR/CRp rate was 36.4% (95% CI, 12.4 to 68.4), with 3 patients reaching CR and 1 patient reaching CRp. All CR/CRps were sustained until the end of study follow-up. In the patients who reached CR, the median time to achieve ANC >1 × 109/L and platelet count >100 × 109/L was 37 (31 to 62) and 41.5 (26 to 62), respectively. One of the 2 patients with ALL (age 90 years) also reached a durable CR.
Change in BM blast percentage following BST-236 treatment (any dose). Representation of the maximal change in BM blast percentage from baseline to day 14 or day 30 BM examination of each treatment course per each assessable patient.
Change in BM blast percentage following BST-236 treatment (any dose). Representation of the maximal change in BM blast percentage from baseline to day 14 or day 30 BM examination of each treatment course per each assessable patient.
In newly diagnosed patients with AML, various baseline clinical or biological prognostic factors, such as BM blasts, cytogenetics, molecular markers, prior MDS, and white blood cells at diagnosis, were similar for responders and nonresponders.
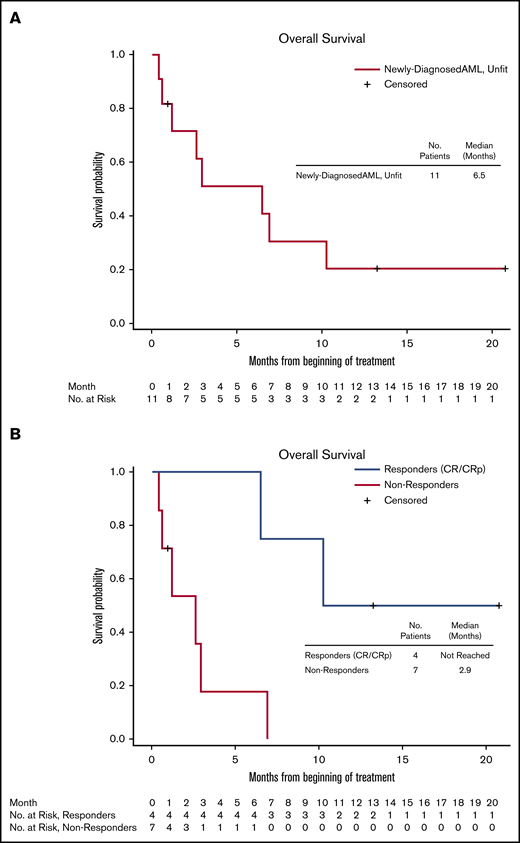
The median OS of the entire study population was 2.83 months (95% CI, 0.73 to 5.86), whereas the median OS in the newly diagnosed subgroup was 6.5 months (95% CI, 0.6 to 10.26) (Figure 3A). The median OS of the patients who achieved CR/CRp was not reached at 20 months, the time of database lock (lower bound of 95% CI was 6.5 months, upper bound not reached at the time of database lock) (Figure 3B). Individual outcomes of the subgroup of 11 newly diagnosed patients with AML are presented in Table 5.
OS of newly diagnosed patients with AML (de novo and secondary to MDS), unfit for standard therapy. (A) All patients. (B) Responders (CR/CRp) and nonresponders.
OS of newly diagnosed patients with AML (de novo and secondary to MDS), unfit for standard therapy. (A) All patients. (B) Responders (CR/CRp) and nonresponders.
Individual baseline characteristics and outcome of subjects with newly diagnosed AML (de novo or secondary to MDS), unfit for standard therapy
| BST-236 dose, g/m2 per d . | No. of induction courses . | Age, y . | ECOG PS . | Prior MDS . | No. of prior HMA courses . | ELN cytogenetic risk score . | Molecular aberration . | Cytogenetics . | Baseline BM blasts, % . | Baseline PB blasts, % . | Baseline WBC, ×109/L . | Best response . | Survival, d . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.8 | 2 | 80 | 1 | No | 0 | Adverse | FLT3+, NPM1+ | NK | 80 | 13 | 7.06 | CRp | 308 |
| 1.5 | 1 | 76 | 0 | Yes | 0 | Intermediate | IDH1+, NMP1+ | NK | 81 | 24 | 7.26 | Withdrawn* | 36 |
| 1.5 | 1 | 80 | 0 | Yes | 26 | Adverse | None | 5q− (+ complex karyotype) | 36 | 0 | 0.75 | Refractory | 13 |
| 2.3 | 1 | 83 | 2 | Yes | 0 | Intermediate | None | NK | 90 | ND | 0.75 | PR | 79 |
| 2.3 | 1 | 73 | 0 | Yes | 5 | Intermediate | None | NK | 58 | 53 | 2.41 | Refractory | 18 |
| 2.3 | 1 | 78 | 0 | No | 0 | Adverse | FLT3+ | NK | 43 | 30 | 2.50 | Refractory | 28 |
| 2.3 | 1 | 70 | 1 | Yes | 5 | Intermediate | None | NK | 22 | 9 | 1.86 | CR | 623 |
| 4.5 | 1 | 72 | 1 | Yes | 0 | Adverse | FLT3+, NPM1+ | NK | 85 | ND | 11.00 | Refractory | 207 |
| 4.5 | 1 | 77 | 2 | Yes | 10 | Adverse | None | Del 7 | 30 | ND | 4.30 | CR | 397 |
| 6 | 1 | 88 | 1 | Yes | 9 | Favorable | NPM1+ | NK | 100 | 85 | 61.82 | Refractory | 88 |
| 6 | 2 | 81 | 1 | No | 0 | Adverse | None | Complex karyotype | 100 | 40 | 24.55 | CR | 195 |
| BST-236 dose, g/m2 per d . | No. of induction courses . | Age, y . | ECOG PS . | Prior MDS . | No. of prior HMA courses . | ELN cytogenetic risk score . | Molecular aberration . | Cytogenetics . | Baseline BM blasts, % . | Baseline PB blasts, % . | Baseline WBC, ×109/L . | Best response . | Survival, d . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.8 | 2 | 80 | 1 | No | 0 | Adverse | FLT3+, NPM1+ | NK | 80 | 13 | 7.06 | CRp | 308 |
| 1.5 | 1 | 76 | 0 | Yes | 0 | Intermediate | IDH1+, NMP1+ | NK | 81 | 24 | 7.26 | Withdrawn* | 36 |
| 1.5 | 1 | 80 | 0 | Yes | 26 | Adverse | None | 5q− (+ complex karyotype) | 36 | 0 | 0.75 | Refractory | 13 |
| 2.3 | 1 | 83 | 2 | Yes | 0 | Intermediate | None | NK | 90 | ND | 0.75 | PR | 79 |
| 2.3 | 1 | 73 | 0 | Yes | 5 | Intermediate | None | NK | 58 | 53 | 2.41 | Refractory | 18 |
| 2.3 | 1 | 78 | 0 | No | 0 | Adverse | FLT3+ | NK | 43 | 30 | 2.50 | Refractory | 28 |
| 2.3 | 1 | 70 | 1 | Yes | 5 | Intermediate | None | NK | 22 | 9 | 1.86 | CR | 623 |
| 4.5 | 1 | 72 | 1 | Yes | 0 | Adverse | FLT3+, NPM1+ | NK | 85 | ND | 11.00 | Refractory | 207 |
| 4.5 | 1 | 77 | 2 | Yes | 10 | Adverse | None | Del 7 | 30 | ND | 4.30 | CR | 397 |
| 6 | 1 | 88 | 1 | Yes | 9 | Favorable | NPM1+ | NK | 100 | 85 | 61.82 | Refractory | 88 |
| 6 | 2 | 81 | 1 | No | 0 | Adverse | None | Complex karyotype | 100 | 40 | 24.55 | CR | 195 |
ND, not done; NK, normal karyotype; PB, peripheral blood; PR, partial response; WBC, white blood cells.
Withdrawn on day 2 due to pneumonia.
Discussion
Although the survival of patients with AML has improved over the last 40 years, the estimated 5-year OS of all patients is still only 27.4%. Given a median age of 68 years at AML diagnosis with ∼30% of patients being at the age of 75 years or older (National Institutes of Health Surveillance, Epidemiology, and End Results data 1975 to 2015), it is not surprising that the long-term survival reported in large population-based datasets reaches only 4.5 to 6 months and 2 to 3 months in patients aged 66 to 75 and 76 to 89 years, respectively. This dismal long-term survival largely results from the fact that the majority of older adults are not offered any AML-directed treatment.10
Decisions regarding an appropriate treatment approach, either intensive, less intensive, or supportive in the population >65 years of age, are a matter of continuing debate. Given the paucity of prospective randomized studies comparing intensive (“7+3 like”) chemotherapy (IC) to less intensive therapy with HMA, low-dose cytarabine (LDAC), or best supportive care (BSC), large retrospective population-based data analyses repeatedly demonstrated an OS benefit of the more intensive approach. Moreover, despite treatment-related toxicity in patients receiving AML-directed intensive therapies, death rates appear to be significantly higher in the BSC arm (34% vs 19%).11,12 In nonrandomized AML studies, treatment with HMA or LDAC resulted in CR rates of 13% to 24% and 4% to 24% with median OS of 5.5 to 24.5 months and 3 to 17 months, respectively.13-20 A randomized phase 3 study compared treatment with HMA to either IC, LDAC, or BSC in a preselected population, demonstrating a 3.8-month benefit in OS with HMA compared with BSC. Notably, no such benefit was observed in treatment with HMA compared with IC or LDAC.21
BST-236 is a cytarabine prodrug designed to enable the delivery of high cytarabine doses with reduced systemic toxicity, thus offering IC to older and frail patients who are otherwise unfit for it. In this phase 1/2 study, BST-236 was found to be safe and well tolerated in this population, delivering up to 6 g/m2 per day of BST-236, equivalent to 4.1 g/m2 per day of native cytarabine, with no significant toxicities other than “on-target” hematological events.
The most frequent TEAEs observed in this study included cytopenia and infections, congruent with the drug's mode of action. Importantly, no cerebral or cerebellar toxicity, significant mucositis, renal failure, or even alopecia was reported. In addition, other AEs known to be associated with HiDAC, such as corneal disorder, necrotizing colitis, and skin exfoliation, were not reported in the study. Moreover, MTD was defined at the highest dose tested (6 g/m2 per day) due to 1 DLT event in 1 out of 7 patients treated with BST-236 in this cohort. Except for this DLT event, no dose-related increase in AE frequency or severity was observed. Given an apparently similar efficacy of 4.5 g/m2 per day and 6 g/m2 per day BST-236 doses, it was decided to proceed with the lower dose in the phase 2b study.
The reduced toxicity of BST-236 emerges from its prodrug construct design, which, when intact, is both inactive and protected from deamination. BST-236 PK analysis confirmed a prodrug profile with a 20:1 ratio of BST-236 to free cytarabine detected in the plasma at any dose. The Cmax value of cytarabine in patients receiving 1-hour infusion of 4.5 g/m2 BST-236 (containing the molar equivalent of HiDAC, 3 g/m2 of cytarabine) was 40 µM, similar to the Cmax of HiDAC with 3 g/m2 of free cytarabine administrated over 3 hours, but with much shorter exposure to peak levels. The AUC observed for cytarabine following exposure to BST-236, 91.7 µM/h, was twofold to threefold lower than the AUC reported for HiDAC treatment,22-24 confirming reduced systemic exposure to free cytarabine.
Although BST-236 treatment was associated with reduced extramedullary toxicity, it retained high antileukemic potency. The outcome of BST-236 therapy in this first-in-human phase 1/2a study compared favorably with the results obtained with HMA or LDAC, suggesting a clinical benefit as a first-line therapy in newly diagnosed patients with AML, either de novo or secondary to MDS, who are unfit for standard induction. These findings should be cautiously interpreted because randomized studies are not yet available. Moreover, because novel promising combination therapies, such as azacitidine/venetoclax, may also be applicable to this patient population, results of these studies should be taken into consideration when determining the most appropriate treatment regimen for this group.
In the present study, this group was characterized by very poor baseline characteristics. Median age was 78 years, with 72.7% of patients at age ≥75 years, and 45.5% of patients ≥80 years. The vast majority of patients (72.7%) had prior MDS, most of whom progressed to AML while on HMA treatment, a particularly poor prognosis indicator associated with significantly lower remission rates and OS.25 Despite the poor prognosis of this subgroup, the CR/CRp reached with all BST-236 doses combined was 36.4%, approximately twofold higher than the pooled CR rate of 15% reported with treatment with HMA or LDAC in a better-prognosis population with mainly de novo AML and no prior HMA treatment.
Importantly, responses to BST-236 were durable and correlated with increased survival. The median OS in this population was 6.5 months (95% CI, 0.6 to 10.26), and was not reached at 20 months among responders, while being only 2.9 months (95% CI, 0.43 to 6.9) among nonresponders. These results are encouraging, because they are comparable to a median OS of 6.3 months (95% CI, 5.1 to 7.6) reported for HMA or LDAC treatment in patients with better prognosis in terms of age, ELN cytogenetic score, previous hematological disorders, and prior HMA treatment.13,15-21 These outcomes are even more promising considering that the vast majority of patients (9 of 11) received only 1 cycle of BST-236.
In addition to the newly diagnosed patients with AML, among the 2 newly diagnosed patients with ALL, one (aged 90 years) reached a durable CR. However, the sample size of the ALL group does not allow drawing conclusions.
Ten relapsed/refractory patients with AML and 3 patients with AML secondary to MPD did not respond to BST-236 in this study. It should be noted that the majority of these patients were treated in lower-dose cohorts. Whether this is related to the disease refractoriness or to limited BST-236 efficacy in this setting is planned to be assessed in further studies.
New therapeutic options have recently emerged targeting older patients with AML. In a randomized study, CPX-351, a dual liposomal preparation of cytarabine and daunorubicin, given as induction and consolidation, has been found to significantly improve remission rates in older patients fit for standard induction, compared with a standard “7+3” regimen (47.7% vs 33.3%, respectively). This has translated into a modest but statistically significant increase in median OS in patients with secondary AML. Importantly, in that trial, CPX-351 treatment was given to patients at an age of up to 75 years who were fit for IC.26
Combinations of the BCL-2 inhibitor venetoclax with either LDAC or HMA in patients aged >65 years unfit for IC demonstrate tolerable toxicity and efficacy. CR rates of 21%, 37%, and 54% and ORR of 42%, 61%, and 62% were observed with the combination of venetoclax with LDAC, azacitidine, and decitabine, respectively.27,28 Notably, patients receiving prior HMA treatment for MDS, and excluded from the venetoclax + HMA study, benefited less from the venetoclax + LDAC combination.29 It is therefore particularly encouraging that response to BST-236 in this study was similar in patients who had multiple prior cycles of HMA and HMA-naive patients.
Overall, IC administration to older patients with AML is warranted to achieve durable responses and survival benefit, if the toxicity profile is acceptable. BST-236, a cytarabine prodrug, has demonstrated a favorable safety profile and significant efficacy in this phase 1/2 a study of older patients with AML unfit for IC. Patients with newly diagnosed AML, whether primary or secondary to MDS, have benefited most. The fact that patients over the age of 70 years, and particularly octogenarians, are able to tolerate intensive induction therapy with BST-236 without the common toxicities associated with HiDAC is remarkable and, if confirmed in a larger study, represents a truly novel approach in AML treatment. It also signifies a unique modality and a platform for possible combination therapies. A phase 2b multicenter study (#NCT03435848), using a BST-236 dose of 4.5 g/m2 per day as induction and consolidation therapy in this population, has been launched in an intent to confirm these encouraging results.
Acknowledgment
The authors gratefully acknowledge the assistance of Sonia Kamenetsky in the preparation of this manuscript.
Authorship
Contribution: T.Z. designed and performed research, wrote the paper, and approved the final version of the paper; R.R. performed research, interpreted the data, and approved the final version of the paper; L.A., M.K.-M., R.H., I.H., N.L., Y.O., N.A.H., O.N., S.T., and S.Y. performed research and approved the final version of the paper; S.G. designed research, interpreted the data, and approved the final version of the paper; L.F., S.T., and R.B.Y. designed research, interpreted the data, wrote the paper, and approved the final version of the paper; and J.M.R. designed research, wrote the paper, and approved the final version of the paper.
Conflict-of-interest disclosure: T.Z. and J.M.R. are consultants for BioSight Ltd. Y.O. received honoraria or consultation fees from Pfizer, Roche, AbbVie, and Astellas Pharma. S.G., L.F., S.T., and R.B.Y. are employees of BioSight Ltd. The remaining authors declare no competing financial interests.
Correspondence: Tsila Zuckerman, Department of Hematology and Bone Marrow Transplantation, Rambam Health Care Campus, 8, Ha’Aliya St, Haifa 3109601, Israel; e-mail: t_zuckerman@rambam.health.gov.il.
References
Author notes
Presented as an oral presentation at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
Individual participant data of study BST Phase 1-01 (#NCT02544438) are available. These data points include individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) An additional available document will be the study protocol. Data will be available to researchers who provide a methodologically sound proposal for individual participant data meta-analysis. The data will be available beginning 6 months and ending 24 months following article publication. To gain access, data requestors will need to sign a data access agreement. Proposals should be directed to liat@BioSight-pharma.com.
The full-text version of this article contains a data supplement.