Key Points
Responses and survival with venetoclax for “real-world” AML patients were promising but inferior to those treated in a clinical trial.
Compared with induction, response rates are as high as would be predicted and venetoclax patients had a lower than expected early death rate.
Abstract
Venetoclax is approved for older untreated acute myeloid leukemia (AML) patients. Venetoclax was available prior to approval off-label. We assessed our single-institution off-label experience with venetoclax/azacitidine, comparing outcomes with a clinical trial cohort that administered this regimen at the same institution. Thirty-three untreated AML patients unfit or unwilling to receive induction chemotherapy and prescribed venetoclax/azacitidine off-trial were retrospectively analyzed and compared with 33 patients who received the same therapy on trial. Outcomes were compared, and comparisons were made to a theoretical scenario in which off-trial patients received induction. Digital droplet polymerase chain reaction evaluated measurable residual disease (MRD). Off-trial venetoclax was attainable in nearly all patients for whom this was desired. The complete remission (CR)/CR with incomplete blood count recovery rate was 63.3% for off-trial patients who received treatment and 84.9% for trial patients (P = .081). The median overall survival for off-trial patients who received treatment was 381 days (95% confidence interval [CI], 174, not reached) vs 880 days (95% CI, 384, not reached) for trial patients (P = .041). Prior exposure to hypomethylating agents was associated with worse outcomes. Response rates with venetoclax/azacitidine were not inferior to a theoretical scenario in which patients received induction, and early death rates were less than expected with induction. MRD negativity was achievable. Newly diagnosed AML patients treated in a “real-world” scenario with off-trial venetoclax/azacitidine had inferior outcomes compared with patients treated in the setting of a clinical trial. Additionally, this therapy may be as effective, and less toxic, when compared with induction chemotherapy.
Introduction
Historically, acute myeloid leukemia (AML) patients have had poor outcomes. Most younger patients who receive induction chemotherapy achieve a remission, but the majority die of the disease or related causes.1 The median age at diagnosis is 68 years2 ; most older patients are poor candidates for induction chemotherapy and experience inferior outcomes.3 Low-intensity therapies allow for responses in a minority of older patients with relatively brief overall survival (OS).4,5 Recently, based on promising early phase clinical trial data,6,7 the selective B-cell lymphoma 2 (BCL-2) inhibitor venetoclax was approved by the US Food and Drug Administration (FDA) with low-intensity chemotherapy for patients ≥75 years of age, or younger patients unsuitable for induction chemotherapy. In other settings, nonclinical trial “real-world” experiences with new therapies have differed from clinical trial results.8,,-11 Because venetoclax was FDA-approved for chronic lymphocytic leukemia in 2016, it was obtainable prior to its AML approval “off-label.” This presented a unique opportunity to evaluate the off-label AML venetoclax experience and compare it with clinical trial results from the same institution, providing the ability to manage expectations for venetoclax post–FDA approval.
Methods
The University of Colorado Institutional Review Board approved a request to retrospectively analyze AML patients prescribed venetoclax off-label. Data from patients at the same institution who consented to a trial of venetoclax/azacitidine (NCT02203773), previously reported,12 were updated and reanalyzed. Comparisons between off-label (also referred to as “off-trial”) patients and predicted outcomes, had those patients been treated with induction chemotherapy (using the AML-SCORE calculator13 ), were made.
Patient population
Thirty-three patients with nonacute promyelocytic AML who had not received therapy for AML, were not candidates for or did not wish to participate in the clinical trial, and had a provider who attempted to obtain venetoclax were reviewed. Venetoclax could not be obtained for 1 patient. Venetoclax was acquired for 2 patients who died before receiving treatment. The first off-trial patient included in this analysis started treatment 13 December 2017; the last patient started treatment 28 November 2018. The first clinical trial patient included in this analysis started treatment 5 January 2015 and the study was closed to accrual 15 June 2018. Baseline characteristics for the 30 off-trial patients who received venetoclax and took at least 1 dose are summarized (Table 1).
Baseline characteristics of patients treated at a single institution with off-trial venetoclax and azacitidine and patients who received venetoclax and azacitidine in the setting of a clinical trial at the same single institution
| . | Off-trial venetoclax patients without prior hypomethylating agent who received treatment . | Off-trial venetoclax patients with prior hypomethylating agent who received treatment . | All off-trial venetoclax patients who received treatment . | Clinical trial patients . | Off-trial vs clinical trial,*P . |
|---|---|---|---|---|---|
| N | 26 | 4 | 30 | 33 | |
| Median age, y | 72 | 71 | 72 | 75 | .073 |
| Baseline white blood cell count, ×109/L | 2.8 | 7.7 | 3.3 | 2.5 | .440 |
| Median baseline blast, % | 59 | 26 | 55 | 40 | .334 |
| Prior hypomethylating agent, n (%) | 0 (0) | 4 (100) | 4 (13.3) | 0 (0) | .046 |
| Antecedent hematologic disorder, n (%) | 2 (7.7) | 4 (100) | 6 (20.0) | 10 (30.3) | .397 |
| Treatment-related AML, n (%) | 5 (19.2) | 0 (0) | 5 (16.7) | 6. (18.2) | 1.000 |
| Cytogenetic risk group, n (%) | |||||
| Intermediate | 15 (57.7) | 0 (0) | 15 (50.0) | 20 (60.6) | .516 |
| Unfavorable | 10 (38.5) | 1 (25) | 11 (36.7) | 12 (36.4) | |
| Unknown | 1 (3.9) | 2 (50) | 3 (10.0) | 1 (3) | |
| Complex cytogenetics, n (%) | 8 (30.8) | 1 (25) | 9 (30.0) | 5 (15.2) | .175 |
| Monosomal karyotype, n (%) | 4 (15.4) | 1 (25) | 5 (16.7) | 5 (15.2) | .860 |
| FLT3 ITD, n (%) | 5 (19.2) | 0 (0) | 5 (16.7) | 5 (15.2) | .265 |
| IDH1/IDH2, n (%) | 5 (19.2) | 0 (0) | 5 (16.7) | 12 (36.4) | .095 |
| ASXL1, n (%) | 9 (34.6) | 2 (50) | 11 (36.7) | 5 (15.2) | .081 |
| TP53, n (%) | 3 (11.5) | 2 (50) | 5 (16.7) | 3 (9.1) | .462 |
| ELN risk group, n (%) | |||||
| Adverse | 17 (65.4) | 3 (75) | 20 (66.7) | 18 (54.5) | .143 |
| Intermediate | 2 (7.7) | 1 (25) | 3 (10) | 10 (30.3) | |
| Favorable | 7 (26.9) | 0 (0) | 7 (23.3) | 5 (15.2) | |
| Would have been eligible for clinical trial, n (%) | 9 (34.6) | 0 (0) | 9 (30) | N/A | N/A |
| . | Off-trial venetoclax patients without prior hypomethylating agent who received treatment . | Off-trial venetoclax patients with prior hypomethylating agent who received treatment . | All off-trial venetoclax patients who received treatment . | Clinical trial patients . | Off-trial vs clinical trial,*P . |
|---|---|---|---|---|---|
| N | 26 | 4 | 30 | 33 | |
| Median age, y | 72 | 71 | 72 | 75 | .073 |
| Baseline white blood cell count, ×109/L | 2.8 | 7.7 | 3.3 | 2.5 | .440 |
| Median baseline blast, % | 59 | 26 | 55 | 40 | .334 |
| Prior hypomethylating agent, n (%) | 0 (0) | 4 (100) | 4 (13.3) | 0 (0) | .046 |
| Antecedent hematologic disorder, n (%) | 2 (7.7) | 4 (100) | 6 (20.0) | 10 (30.3) | .397 |
| Treatment-related AML, n (%) | 5 (19.2) | 0 (0) | 5 (16.7) | 6. (18.2) | 1.000 |
| Cytogenetic risk group, n (%) | |||||
| Intermediate | 15 (57.7) | 0 (0) | 15 (50.0) | 20 (60.6) | .516 |
| Unfavorable | 10 (38.5) | 1 (25) | 11 (36.7) | 12 (36.4) | |
| Unknown | 1 (3.9) | 2 (50) | 3 (10.0) | 1 (3) | |
| Complex cytogenetics, n (%) | 8 (30.8) | 1 (25) | 9 (30.0) | 5 (15.2) | .175 |
| Monosomal karyotype, n (%) | 4 (15.4) | 1 (25) | 5 (16.7) | 5 (15.2) | .860 |
| FLT3 ITD, n (%) | 5 (19.2) | 0 (0) | 5 (16.7) | 5 (15.2) | .265 |
| IDH1/IDH2, n (%) | 5 (19.2) | 0 (0) | 5 (16.7) | 12 (36.4) | .095 |
| ASXL1, n (%) | 9 (34.6) | 2 (50) | 11 (36.7) | 5 (15.2) | .081 |
| TP53, n (%) | 3 (11.5) | 2 (50) | 5 (16.7) | 3 (9.1) | .462 |
| ELN risk group, n (%) | |||||
| Adverse | 17 (65.4) | 3 (75) | 20 (66.7) | 18 (54.5) | .143 |
| Intermediate | 2 (7.7) | 1 (25) | 3 (10) | 10 (30.3) | |
| Favorable | 7 (26.9) | 0 (0) | 7 (23.3) | 5 (15.2) | |
| Would have been eligible for clinical trial, n (%) | 9 (34.6) | 0 (0) | 9 (30) | N/A | N/A |
ASXL1, additional sex combs like 1; ELN, European LeukemiaNetwork; FLT3, FMS-like tyrosine kinase 3; IDH, isocitrate dehydrogenase; ITD, internal tandem duplication; N/A, not available; TP53, tumor protein 53.
Comparison of off-trial patients with clinical trial patients includes all off-trial patients, with or without hypomethylating agent.
Acquisition of venetoclax
When a patient was identified as a candidate for off-label venetoclax, a prescription was sent to the medication access center and processed through pharmacy benefits. In some cases, the prescription was covered; it was dispensed if the copay was affordable. If the prescription was covered but the copay was unaffordable, grants/patient assistance programs were pursued. If venetoclax was denied, an appeal was submitted; if accepted, copay issues were managed as just described (in this section). If denied, assistance programs were pursued.
Treatment, monitoring, and follow-up
During cycle 1, patients were hospitalized for intrapatient dose escalation of oral venetoclax (100 mg, 200 mg, 400 mg on days 1, 2, and 3, respectively, as previously reported6 ). Patients then self-administered 400 mg venetoclax daily, for 28-day cycles. Azacitidine 75 mg/m2 was administered IV or subcutaneously days 1 to 7. IV hydration and an oral uric acid–lowering agent constituted tumor lysis syndrome prophylaxis. Serum chemistries, uric acid, and creatinine were assessed every 6 hours during dose escalation. Antifungal prophylaxis was not used. If a strong CYP3A inhibitor was required after dose escalation, venetoclax was decreased from 400 mg to 100 mg; no adjustments were made for moderate CYP3A inhibitors.
Subsequent cycles of venetoclax/azacitidine were repeated as appropriate, without dose escalation or inpatient monitoring. Azacitidine dose modifications were made according to the package label. Discontinuation of azacitidine, and dose modification/discontinuation of venetoclax, occurred at the discretion of the treating physician. All patients initiated treatment at the University of Colorado; 5 received the majority of their postcycle 1 treatment elsewhere. Outside hospital records were requested, obtained, and reviewed. No patients were lost to follow-up.
Response assessments
Institutional recommendations to obtain bone marrow biopsies at baseline and after cycles 1, 4, 10, and every 6 months thereafter were made. Response assessments were retrospectively assessed using the 2017 European LeukemiaNetwork (ELN) critiera14 ; progression was defined according to the International Working Group.15 After cycle 1, patients in morphologic remission without residual disease by flow cytometry could have a maximum 14-day period off therapy; some with neutropenia were administered growth factors during this period. Patients with improvements in blood counts during this 14-day period, with or without growth factor support, had responses upgraded, from morphologic leukemia-free state (MLFS) to complete remission (CR) with incomplete blood count recovery (CRi) or CR, or from CRi to CR. Patients with no objective response by their second cycle of therapy were not offered further cycles of therapy.
Genomic testing
For the initial bone marrow biopsy, the QIAamp DNA Mini kit was used for genomic extraction, and the RainDance ThunderBolt Myeloid next-generation sequencing (NGS) panel investigating 49 recurrent mutations in AML was used. Variant allelic frequencies (VAFs) ≥5% were reported. Where possible, subsequent biopsies had measurable residual disease (MRD) assessments performed by droplet digital PCR (ddPCR) analysis (assays were obtained [Bio-Rad] or designed), based on diagnostic mutations. As many genes as feasible were used for ddPCR analysis on subsequent bone marrow aspirates for MRD purposes; DNMT3A, TET2, and ASXL1 were excluded from MRD analysis.16 All assays were validated; testing was performed in duplicate. Bio-Rad QuantaSoft software performed data analysis with MRD reported as VAF; VAF ≤0.01% was defined as MRD−.
Statistical assessments
Analyses were conducted to compare off-trial and clinical trial patients. Among off-trial patients, we hypothesized that use of a prior hypomethylating agent may have been a variable of interest and we performed additional analyses of the off-trial group based on prior hypomethylating agent exposure. Univariate logistic regression models were used with CR/CRi vs all other responses/nonresponses, relapse status, and alive/dead status as dependent variables. Odds ratios were calculated for independent variables of interest. Response duration and OS were calculated using Kaplan-Meier methods. Median follow-up times were calculated using reverse Kaplan-Meier. Univariate Cox regression assessed the impact of the variables on response duration/OS. The Fisher exact test compared response rates. The validated AML SCORE calculator13 predicted the chance of achieving CR and risk of early death for each patient; these results were then compared with actual outcomes. The area under the curve (AUC) statistic obtained from a receiver operator curve of the data assessed the model fit by comparing to an AUC = 0.50. The Youden J-test statistic selected an optimal cutoff value for CR/early death.
Results
One patient who was not able to obtain venetoclax opted for supportive care and died 72 days after diagnosis. Two patients died before treatment was initiated, 7 and 10 days after diagnosis. Baseline characteristics of all previously untreated AML patients who received at least 1 day of treatment with off-trial venetoclax/azacitidine (N = 30) are listed (Table 1). The median age was 72 years; 6 of 30 (20%) evolved from an antecedent hematological condition, 11 of 30 (37%) had unfavorable risk cytogenetics, 5 of 30 (17%) had a monosomal karyotype, and 20 of 30 (67%) had adverse risk by ELN criteria. A detailed summary of baseline characteristics, clinical outcomes, and the reason each patient was not enrolled in the trial (NCT02203773) is shown (Table 2). The most common reasons patients were treated off-trial were: unavailability of the study (N = 7), treatment with a hypomethylating agent for an antecedent hematological condition (N = 4), other active malignancy (N = 4), age <65 years (N = 3), chronic respiratory disease requiring continuous oxygen (N = 2), unable to provide consent (N = 2), core-binding factor cytogenetic abnormality (N = 2), and logistical challenges with participation in a trial (N = 2). Compared with the trial cohort, off-trial patients were more likely to have been treated with a hypomethylating agent (P = .046); there were otherwise no significant disease-related differences between cohorts (Table 1).
Baseline information for each treated off-trial patient and an explanation for why each was not enrolled in a venetoclax clinical trial
| Pt . | Age, y . | Prior HMA . | AHD . | Cytogenetic risk group . | Baseline mutational profile . | Best response . | Predicted CR% based on AML SCORE . | Death within 60 d . | Predicted early death % based on AML SCORE13 . | Reason not enrolled in a clinical trial . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | No | Yes | Intermediate | IDH2, SRSF2, TET2 | CRi | 54.8 | No | 19.6 | Study unavailable |
| 2 | 72 | No | No | Intermediate | NPM1, TET2, GATA2 | CR | 73.8 | No | 26.1 | Study unavailable |
| 3 | 82 | No | No | Unfavorable | None | Resistant disease | 13.1 | No | 55.9 | Other malignancy |
| 4 | 59 | No | No | Intermediate | FLT3 TKD, NPM1 | CR | 70.5 | No | 14 | Age <65 y |
| 5 | 85 | No | No | Unknown | IDH2, NRAS, SRSF2, SMC1A | CR | 41.7 | No | 34.2 | Renal insufficiency |
| 6 | 68 | No | No | Unfavorable | ASXL1, RUNX1, TET2 | Resistant disease | 20.7 | No | 38.1 | Other malignancy |
| 7 | 73 | No | No | Intermediate | RUNX1, PHF6 | CR | 58.9 | No | 26.1 | Study unavailable |
| 8 | 79 | No | Yes | Unfavorable | JAK2, TP53, BCORL1 | CR | 21.2 | No | 39 | Evolved from MPN |
| 9 | 79 | No | No | Intermediate | IDH2, ASXL1, RUNX1, SRSF2, FLT3, RAD21, PHF6 | CR | 48.7 | No | 26.9 | Study unavailable |
| 10 | 70 | No | No | Intermediate | NPM1, FLT3 TKD, DNMT3A | CR | 58.6 | No | 17.4 | Study unavailable |
| 11 | 63 | No | No | Unfavorable | ASXL1, NRAS, TET2 | Resistant disease | 38 | Yes | 25.1 | Chronic respiratory disease requiring continuous oxygen use |
| 12 | 61 | No | No | Intermediate | ASXL1, SRSF2, STAG2, ZRSR2, RUNX1 | CRi | 78.5 | No | 8.9 | Active hepatitis C |
| 13 | 75 | No | No | Unfavorable | ASXL1, DNMT3A, RUNX1, U2AF1, BCORL1, PHF6, CBL, TET2 | Resistant disease | 30.5 | Yes | 39 | Study unavailable |
| 14 | 66 | No | No | Intermediate | TP53, CEBPA, ASXL1, SRSF2, STAG2 | Uninterpretable | 57.9 | No | 16.8 | Other malignancy |
| 15 | 68 | No | No | Intermediate | NPM1, DNMT3A, FLT3 ITD, IDH2, RUNX1 | CRi | 37 | No | 47.1 | Chronic respiratory disease requiring continuous oxygen use |
| 16 | 48 | No | No | Intermediate | NPM1, NRAS, SMC1A | N/A | N/A | Yes | N/A | Age <65 y |
| 17 | 73 | No | No | Intermediate | ASXL1, RUNX1, WT1, CEBPA, EZH2, TET2 | CR | 52.6 | No | 34.2 | Unable to provide informed consent |
| 18 | 68 | No | No | Intermediate | FTL3 ITD, DNMT3A, NPM1, | CRi | 51.7 | No | 33.1 | Unable to provide informed consent |
| 19 | 72 | No | No | Intermediate | NPM1, FLT3-ITD, DNMT3A | CR | 51.7 | No | 33.1 | Study unavailable |
| 20 | 83 | No | No | Unfavorable | TET2, PTPN11 | CR | 71.5 | No | 30.4 | Core-binding factor |
| 21 | 81 | No | No | Intermediate | FLT3 ITD, CEBPA, TET2, NPM1 | CRi | 59.6 | No | 26.9 | Logistics of travel |
| 22 | 72 | No | No | Intermediate | ASXL1, SETBP1, PHF6, MLL | MLFS | 58.9 | No | 26.1 | Active infection |
| 23 | 68 | No | No | Unfavorable | TP53 | CRi | 30.5 | No | 39 | Logistics of travel |
| 24 | 79 | No | No | Unfavorable | DNMT3A, IDH1 | CR | 16.8 | No | 47.5 | Other malignancy |
| 25 | 74 | No | No | Unfavorable | None | CR | 71.5 | No | 30.4 | Core-binding factor |
| 26 | 33 | No | No | Unfavorable | ASXL1 | CR | N/A | No | 19.6 | Age <65 y |
| 27 | 78 | Yes | Yes | Unknown | SRSF2, STAG2, CEBPA, ETV6 | Resistant disease | 30.4 | No | 34.2 | Prior hypomethylator |
| 28 | 71 | Yes | Yes | Unknown | ASXL1, SRSF2, STAG2, TET2 | MLFS | 36.1 | No | 26.1 | Prior hypomethylator |
| 29 | 68 | Yes | Yes | Unknown | JAK2, TP53, ASXL1, EZH2, KDM6A | N/A | 40.5 | Yes | 22.6 | Prior hypomethylator |
| 30 | 71 | Yes | Yes | Unfavorable | TP53, EZH2 | MLFS | 23.1 | No | 34.1 | Prior hypomethylator |
| Pt . | Age, y . | Prior HMA . | AHD . | Cytogenetic risk group . | Baseline mutational profile . | Best response . | Predicted CR% based on AML SCORE . | Death within 60 d . | Predicted early death % based on AML SCORE13 . | Reason not enrolled in a clinical trial . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | No | Yes | Intermediate | IDH2, SRSF2, TET2 | CRi | 54.8 | No | 19.6 | Study unavailable |
| 2 | 72 | No | No | Intermediate | NPM1, TET2, GATA2 | CR | 73.8 | No | 26.1 | Study unavailable |
| 3 | 82 | No | No | Unfavorable | None | Resistant disease | 13.1 | No | 55.9 | Other malignancy |
| 4 | 59 | No | No | Intermediate | FLT3 TKD, NPM1 | CR | 70.5 | No | 14 | Age <65 y |
| 5 | 85 | No | No | Unknown | IDH2, NRAS, SRSF2, SMC1A | CR | 41.7 | No | 34.2 | Renal insufficiency |
| 6 | 68 | No | No | Unfavorable | ASXL1, RUNX1, TET2 | Resistant disease | 20.7 | No | 38.1 | Other malignancy |
| 7 | 73 | No | No | Intermediate | RUNX1, PHF6 | CR | 58.9 | No | 26.1 | Study unavailable |
| 8 | 79 | No | Yes | Unfavorable | JAK2, TP53, BCORL1 | CR | 21.2 | No | 39 | Evolved from MPN |
| 9 | 79 | No | No | Intermediate | IDH2, ASXL1, RUNX1, SRSF2, FLT3, RAD21, PHF6 | CR | 48.7 | No | 26.9 | Study unavailable |
| 10 | 70 | No | No | Intermediate | NPM1, FLT3 TKD, DNMT3A | CR | 58.6 | No | 17.4 | Study unavailable |
| 11 | 63 | No | No | Unfavorable | ASXL1, NRAS, TET2 | Resistant disease | 38 | Yes | 25.1 | Chronic respiratory disease requiring continuous oxygen use |
| 12 | 61 | No | No | Intermediate | ASXL1, SRSF2, STAG2, ZRSR2, RUNX1 | CRi | 78.5 | No | 8.9 | Active hepatitis C |
| 13 | 75 | No | No | Unfavorable | ASXL1, DNMT3A, RUNX1, U2AF1, BCORL1, PHF6, CBL, TET2 | Resistant disease | 30.5 | Yes | 39 | Study unavailable |
| 14 | 66 | No | No | Intermediate | TP53, CEBPA, ASXL1, SRSF2, STAG2 | Uninterpretable | 57.9 | No | 16.8 | Other malignancy |
| 15 | 68 | No | No | Intermediate | NPM1, DNMT3A, FLT3 ITD, IDH2, RUNX1 | CRi | 37 | No | 47.1 | Chronic respiratory disease requiring continuous oxygen use |
| 16 | 48 | No | No | Intermediate | NPM1, NRAS, SMC1A | N/A | N/A | Yes | N/A | Age <65 y |
| 17 | 73 | No | No | Intermediate | ASXL1, RUNX1, WT1, CEBPA, EZH2, TET2 | CR | 52.6 | No | 34.2 | Unable to provide informed consent |
| 18 | 68 | No | No | Intermediate | FTL3 ITD, DNMT3A, NPM1, | CRi | 51.7 | No | 33.1 | Unable to provide informed consent |
| 19 | 72 | No | No | Intermediate | NPM1, FLT3-ITD, DNMT3A | CR | 51.7 | No | 33.1 | Study unavailable |
| 20 | 83 | No | No | Unfavorable | TET2, PTPN11 | CR | 71.5 | No | 30.4 | Core-binding factor |
| 21 | 81 | No | No | Intermediate | FLT3 ITD, CEBPA, TET2, NPM1 | CRi | 59.6 | No | 26.9 | Logistics of travel |
| 22 | 72 | No | No | Intermediate | ASXL1, SETBP1, PHF6, MLL | MLFS | 58.9 | No | 26.1 | Active infection |
| 23 | 68 | No | No | Unfavorable | TP53 | CRi | 30.5 | No | 39 | Logistics of travel |
| 24 | 79 | No | No | Unfavorable | DNMT3A, IDH1 | CR | 16.8 | No | 47.5 | Other malignancy |
| 25 | 74 | No | No | Unfavorable | None | CR | 71.5 | No | 30.4 | Core-binding factor |
| 26 | 33 | No | No | Unfavorable | ASXL1 | CR | N/A | No | 19.6 | Age <65 y |
| 27 | 78 | Yes | Yes | Unknown | SRSF2, STAG2, CEBPA, ETV6 | Resistant disease | 30.4 | No | 34.2 | Prior hypomethylator |
| 28 | 71 | Yes | Yes | Unknown | ASXL1, SRSF2, STAG2, TET2 | MLFS | 36.1 | No | 26.1 | Prior hypomethylator |
| 29 | 68 | Yes | Yes | Unknown | JAK2, TP53, ASXL1, EZH2, KDM6A | N/A | 40.5 | Yes | 22.6 | Prior hypomethylator |
| 30 | 71 | Yes | Yes | Unfavorable | TP53, EZH2 | MLFS | 23.1 | No | 34.1 | Prior hypomethylator |
AHD, antecedent hematological disorder; MPN, myeloproliferative neoplasm; PHA, prior hypomethylating agent; Pt, patient.
Median time to receive approval for venetoclax was 4.5 days (0-28 days); median time to start therapy from its request was 6.5 days (0-34 days). Twelve requests (40%) for venetoclax were made on behalf of outpatients; the remainder were inpatient.
Toxicity and deaths
One hundred sixty-two adverse events (AEs) were documented; most common were neutropenia (N = 28), anemia (N = 27), thrombocytopenia (N = 27), neutropenic fever (N = 14), pneumonia (N = 9), and fatigue (N = 9). Possibly related less than grade 1 events are listed (Table 3). Early death (within 60 days) occurred in 4 of 30 patients (13%), all due to disease progression. There were 11 deaths a median of 113 days (9-394 days) after treatment; 9 were from disease progression and 2 were from infectious complications unrelated to treatment.
Grade 2 or greater possibly related AEs
| Event . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Neutropenia | 12 | 16 | 0 | 0 |
| Anemia | 8 | 19 | 0 | 0 |
| Thrombocytopenia | 22 | 5 | 0 | 0 |
| Renal insufficiency | 1 | 0 | 0 | 0 |
| Fatigue | 5 | 0 | 0 | 0 |
| Nausea | 5 | 2 | 0 | 0 |
| Emesis | 2 | 1 | 0 | 0 |
| Event . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Neutropenia | 12 | 16 | 0 | 0 |
| Anemia | 8 | 19 | 0 | 0 |
| Thrombocytopenia | 22 | 5 | 0 | 0 |
| Renal insufficiency | 1 | 0 | 0 | 0 |
| Fatigue | 5 | 0 | 0 | 0 |
| Nausea | 5 | 2 | 0 | 0 |
| Emesis | 2 | 1 | 0 | 0 |
Clinical outcomes
Overall responses, defined as CR, CRi, partial remission, or MLFS, are summarized (Table 4). Two patients died prior to completion of cycle 1 and are regarded as nonresponders. A third patient had metastatic lung cancer that rendered the biopsy uninterpretable and was also regarded as a nonresponder.
Overall response rates (ORRs) for patients by treatment group
| Group . | ORR, n/N (%) . | CR/CRi, n/N (%) . | CR rate, n/N (%) . |
|---|---|---|---|
| Off-trial venetoclax patients | 22/33 (66.7) | 19/33 (57.6) | 13/33 (39.4) |
| Off-trial venetoclax patients who received at least 1 day of therapy | 22/30 (73.3) | 19/30 (63.3) | 13/30 (43.3) |
| Off-trial venetoclax patients without prior hypomethylating agent | 20/26 (76.9) | 19/26 (73.1) | 12/26 (46.2) |
| Off-trial venetoclax patients with prior hypomethylating agent | 2/4 (50) | 0/4 (0) | 0/4 (0) |
| Clinical trial patients | 30/33 (90.9) | 28/33 (84.9) | 20/33 (60.6) |
| Group . | ORR, n/N (%) . | CR/CRi, n/N (%) . | CR rate, n/N (%) . |
|---|---|---|---|
| Off-trial venetoclax patients | 22/33 (66.7) | 19/33 (57.6) | 13/33 (39.4) |
| Off-trial venetoclax patients who received at least 1 day of therapy | 22/30 (73.3) | 19/30 (63.3) | 13/30 (43.3) |
| Off-trial venetoclax patients without prior hypomethylating agent | 20/26 (76.9) | 19/26 (73.1) | 12/26 (46.2) |
| Off-trial venetoclax patients with prior hypomethylating agent | 2/4 (50) | 0/4 (0) | 0/4 (0) |
| Clinical trial patients | 30/33 (90.9) | 28/33 (84.9) | 20/33 (60.6) |
Off-trial patients who received at least 1 day of therapy had a CR/CRi rate of 63.3% (Table 4); median number of cycles to achieve best response was 1 (1-7). This CR/CRi rate was lower, though not statistically, than the clinical trial group (84.9%) (Table 4) (P = .081). Off-trial patients who received no prior hypomethylating agent had a significantly better CR/CRi rate compared with off-trial patients who had received this treatment previously (73.1% vs 0%, respectively; P = .012). An additional analysis compared CR/CRi rates of patients who would not have been eligible for the study (N = 21), excluding those not enrolled for logistical reasons or because the study was not available (N = 9). This truly clinical trial–ineligible population had a CR/CRi rate of 52% (11 of 21), significantly lower than the 84.9% CR/CRi rate for the trial patients (P = .0136).
For response duration, median follow-up time was 595 days (95% confidence interval [CI], 328, 692): 191 days for off-trial patients who received at least 1 day of therapy (95% CI, 41, 333) and 692 days for clinical trial patients (95% CI, 595, 986). The median response duration for treated off-trial patients was 321 days (95% CI, 137, no upper bound) and the median response duration for trial patients has not been reached (Figure 1A; P = .122). For OS, the median follow-up time was 658 days (95% CI, 36, 1130): 256 days (95% CI, 98, 364) for treated off-trial patients and 977 days (95% CI, 670, 1340) for clinical trial patients. The median OS for treated off-trial patients was 381 days (95% CI, 174, not reached), and the median OS for trial patients was 880 days (95% CI, 384, not reached) (Figure 1B; P = .041).
Outcomes for clinical trial vs off-label patients. (A) Median response duration for off-trial venetoclax patients (blue) compared with clinical trial patients (red). (B) Median OS for off-trial venetoclax patients (blue) compared with clinical trial patients (red).
Outcomes for clinical trial vs off-label patients. (A) Median response duration for off-trial venetoclax patients (blue) compared with clinical trial patients (red). (B) Median OS for off-trial venetoclax patients (blue) compared with clinical trial patients (red).
Response prediction
Univariate analysis to determine whether baseline predictors for CR/CRi could be determined for off-trial patients who completed a minimum of 1 cycle with an interpretable response (N = 27) was performed. Baseline blast percentage (P = .0293), cytogenetic category (P = .0277), and the presence of an ASXL1 mutation (P = .0468) were predictive (supplemental Table 1). A similar analysis to ascertain whether predictors of relapse for responding patients (N = 22) could be determined showed only the presence of an antecedent hematological disorder to be predictive (P = .0397) (supplemental Table 2).
Theoretical comparison with intensive induction chemotherapy
Off-trial patients were deemed poor candidates for induction chemotherapy, or were unwilling to undergo this treatment. We were interested in comparing actual outcomes from venetoclax/azacitidine with how these patients would have responded to induction. We used the AML SCORE online calculator13 to evaluate each patients’ percentage chance of achieving a CR/CRi and risk of early death from induction chemotherapy, and compared expected and actual outcomes (Table 2). The AML SCORE was developed using outcomes from 1406 patients ≥60 years old who were treated with intensive induction chemotherapy and validated with an independent cohort of 801 similar patients. Using a χ2 test to assess the AUC, we found P = .0005 for the comparison of predicted vs actual CR/CRi, suggesting that off-trial patients did no worse than predicted with induction chemotherapy. Additionally, this test showed P = .337 for prediction of early death; 4 patients actually experienced this outcome, compared with 22 who had a >25% predicted chance with induction chemotherapy (the optimal cutoff determined by the Youden J-test statistic), suggesting that fewer patients experienced early death with venetoclax/azacitidine than would have been expected in the setting of a more intensive regimen. These models are shown in supplemental Figure 1.
MRD
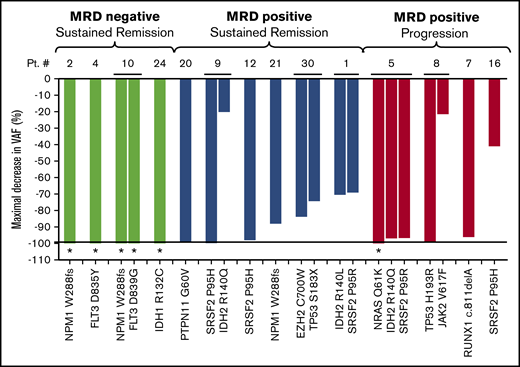
MRD analysis by ddPCR was performed on 14 off-trial patients who responded to therapy. MRD was not performed in the other 16 patients because they: (1) did not respond to therapy (N = 5); (2) had no detectable mutations on baseline NGS testing (n = 1); (3) did not have a ddPCR assay that could be designed for any mutations (N = 4); and/or (4) had no subsequent bone marrow biopsies performed at our institution (N = 6). Median follow-up for these 14 patients was 323 days. Best MRD response for these patients is shown in Figure 2. Four achieved MRD negativity by ddPCR; none have relapsed (Figure 2 green bars). Four with persistent MRD positivity by ddPCR relapsed (Figure 2 red bars). Six additional patients have persistent MRD positivity and remain in remission.
Best MRD outcome in 14 responding patients treated off-trial with venetoclax plus azacitidine using the ddPCR technique. Between 1 and 3 mutations identified on diagnostic NGS were monitored throughout therapy for patients who achieved a morphologic remission with venetoclax plus azacitidine, had baseline mutations or mutations that could be followed with ddPCR, and had available follow-up bone marrow samples (N = 14). The maximal decrease in VAF (normalized percentage of decrease in VAF from baseline) is shown on the y-axis; individual mutations for each patient are shown on the x-axis. Four patients (green bars) had MRD negativity and remain in remission. Five patients (red bars) were MRD+ and relapsed. Six patients (blue bars) were MRD+ but have not relapsed at the time of data censoring. *Individual mutations that were undetectable at best response (<0.01% VAF).
Best MRD outcome in 14 responding patients treated off-trial with venetoclax plus azacitidine using the ddPCR technique. Between 1 and 3 mutations identified on diagnostic NGS were monitored throughout therapy for patients who achieved a morphologic remission with venetoclax plus azacitidine, had baseline mutations or mutations that could be followed with ddPCR, and had available follow-up bone marrow samples (N = 14). The maximal decrease in VAF (normalized percentage of decrease in VAF from baseline) is shown on the y-axis; individual mutations for each patient are shown on the x-axis. Four patients (green bars) had MRD negativity and remain in remission. Five patients (red bars) were MRD+ and relapsed. Six patients (blue bars) were MRD+ but have not relapsed at the time of data censoring. *Individual mutations that were undetectable at best response (<0.01% VAF).
Discussion
Past experience has shown that patients treated in the setting of a clinical trial have better outcomes than the real-world experience with the same regimen, possibly due to differences in comorbidities, adherence to a protocol, the center in which patients are treated, or other factors.8,,-11,17 Because venetoclax was approved for chronic lymphocytic leukemia and was widely available for off-label use simultaneous with an AML clinical trial that showed early promise, it provided a unique experience to compare outcomes of patients treated on and off of the clinical trial with the same regimen at the same institution.
Primarily, we found that the CR/CRi rate for treated off-trial patients was lower than for patients treated on the trial; this was largely driven by the poor response rate seen in AML patients who had been treated for an antecedent hematological disorder with a hypomethylating agent; these patients were excluded from the clinical trial. The venetoclax with low-dose cytarabine study did allow newly diagnosed AML patients who had received a prior hypomethylating agent to enroll.18 Twenty-four of 82 (29%) had this exposure; the CR/CRi response rate in that study was 54%, compared with roughly 70% for patients on the venetoclax with hypomethylating agent study.6,18 Furthermore, only 33% of the 24 patients who had a hypomethylating agent responded to the venetoclax plus low-dose cytarabine combination, compared with 62% of the 58 patients who did not have a prior hypomethylating agent,18 numbers consistent with our off-trial experience with and without a prior hypomethylator. Intensive induction chemotherapy fragments and amplifies the leukemia stem cell (LSC) population19 ; venetoclax/azacitidine targets LSCs,12 and whether prior treatment with a hypomethylating agent similarly impacts LSCs, or whether prior treatment is a marker for more aggressive disease biology, is unclear. Starting a hypomethylating agent prior to venetoclax is likely to be a frequent occurrence in the real world as the drug approval and shipment of oral therapies can be delayed; however, these findings may prompt a reconsideration of the nonconcomitant initiation of these therapies.
Some off-trial patients were not eligible for the clinical trial; others would have been eligible but could not participate for logistical reasons, or, in some cases, there were no openings when a patient required treatment. When patients who met the eligibility criteria but were treated off-trial were removed from the analysis, enriching for the real-world experience post–venetoclax approval, there were more profound differences in response rates compared with trial patients. These findings may help practitioners and patients develop more reasonable expectations for the real-world activity of this regimen.
Although the decreased response duration for off-trial vs clinical trial patients did not reach statistical significance, a trend, with limited follow-up time, exists. The median OS was significantly shorter for patients treated off-trial, which would be expected for a group that had a lower response rate and more comorbidities than were seen in the clinical trial group. Adherence to a protocol, and receiving the majority of treatment in a single center with experience in administering this regimen, may also be differences that contributed to this finding.
An enduring criticism of studies designed for “induction-ineligible” patients is that this determination is subjective. To address the concern that the true standard of care for these patients is induction chemotherapy, we undertook a “parallel universe” analysis, using the AML SCORE to predict outcomes based on validated baseline characteristics that may have occurred had patients been induced. To do this, we explored whether predictions using the model could perform better than a model that assigned a 50% probability of predicting response. A significant P value, therefore, indicates that a predictive model performs well, or better than chance. The AML SCORE model showed that responses from venetoclax/azacitidine were comparable to responses that would have been expected had the same patients received induction chemotherapy (P < .0005). However, the model did not perform better than a chance model in predicting early death (P = .337); there were fewer actual early deaths from venetoclax/azacitidine than would have been expected with induction. There are limitations with this type of analysis. Although the AML SCORE predicts the chance of achieving CR, in our patients there was no difference in response duration or OS when comparing CR vs CRi (P = .761 and .897, respectively), so the 2 response measures were used interchangeably. Using the model to predict CR only did not show a significant finding (P = .103), likely limited by the sample size. A controlled trial could be done to formally compare venetoclax/azacitidine with induction chemotherapy in older patients.
MRD assessment by ddPCR revealed similar patterns of molecular response previously reported for trial patients.12 ddPCR as a customizable MRD assay for patients with a wide variety of baseline mutations was feasible. Additionally, MRD− remissions with an extremely sensitive assay were possible in some patients with this low-intensity regimen. As we have shown previously,12 attainment of MRD negativity associates with durable remissions, and lack of achievement of this level of response may herald relapse. More efforts to understand the basis of increased and decreased sensitivity to this regimen are necessary.
Several other observations from this experience are notable. The trial excluded patients with cytogenetically favorable risk disease; although intensive chemotherapy is preferred in this setting, this is not possible when it afflicts older patients who are not induction candidates. We show here that CR is achievable with venetoclax/azacitidine in favorable-risk patients (N = 2). Additionally, 3 younger patients, who would typically be considered induction eligible due to their ages (33, 48, and 59 years of age), were either considered poor candidates (N = 1) or refused this treatment (N = 2) (Table 2). The 2 younger patients who were evaluable for response both achieved a CR.
When patients were found to be in morphologic remission without evidence of disease by flow cytometry, they were permitted to take 14 days off of therapy, with growth factor support if indicated. Some extenuating circumstances prolonged this delay, but the median time between the completion of cycle 1 and the initiation of cycle 2 was 13 days (5-40 days). If blood counts improved from the end-of-cycle bone marrow to the completion of the 14-day hold, responses were upgraded from MLFS to CRi/CR or CRi to CR. The definitions of CR/CRi were made in the context of induction chemotherapy,15 and lack of count recovery in this setting portends worse OS.20 However, when low-intensity therapies with myelosuppressive properties are continuously administered, the relevance of count recovery may differ. In our small sample set, there were no observable differences in response duration or OS for patients who achieved CR vs CRi; similar analyses should be performed on larger data sets. Therapeutic pauses, with or without growth factor support, should be standard and “upgraded” responses should be reported in future clinical trials.
This study had several limitations. Our use of the term real world may adequately describe the patient population that we expect to see in the post–venetoclax approval era, in contrast to patients who meet strict eligibility criteria in the setting of a clinical trial, but the expertise and experience at our center in using this regimen may not be applicable to the real world; this may have resulted in better than expected outcomes for our real-world patients. Several of the real-world patients would have been candidates for the clinical trial had it been available when they required treatment; the study was open to accrual during roughly one-half of the period in which we treated the off-trial patients in this analysis (182 of 348 days). In addition, the numbers were relatively small and the retrospective nature of data collection is not ideal. However, nearly all patients had assessment bone marrows performed at a single institution, which allows a higher confidence of the integrity of the response data reported.
In conclusion, the real-world experience with venetoclax/azacitidine is inferior to the clinical trial results, with lower response rates and OS. Any prior therapy for an antecedent hematologic malignancy might negatively impact the ability of a patient to optimally respond. A theoretical comparison with induction chemotherapy did not reveal lower-than-expected response rates but suggested it may be a less toxic treatment option.
Acknowledgments
The authors thank Jessica Pollyea who designed the visual abstract.
D.A.P. was supported by the Robert H. Allen Chair in Hematology Research, the University of Colorado Department of Medicine Outstanding Early Career Scholars Program, and the Leukemia & Lymphoma Society Scholar in Clinical Research Award. A.C.W. was supported by the St. Baldrick’s Foundation and the Denver chapter of Swim Across America.
Authorship
Contribution: A.C.W. collected data, performed analysis, helped write the manuscript, and edited the manuscript; J.A.G. cared for patients, helped write the manuscript, and edited the manuscript; E.P., M.N., J.T., S.C., J.K., L.L., C.B., K.H., J.T.S., J.R., M.D.E., B.S., V.R., S.S., and C.S. cared for patients and edited the manuscript; D.A. performed analysis, helped write the manuscript, and edited the manuscript; B.M.S. performed analysis and edited the manuscript; C.T.J. edited the manuscript; and D.A.P. collected data, performed analysis, cared for patients, and wrote and edited the manuscript.
Conflict-of-interest disclosure: D.A.P. receives research funding from AbbVie and serves on advisory boards for AbbVie, Pfizer, Gilead, Astellas, Agios, and Daiichi Sankyo. L.L. has served on advisory boards for Agios, Celgene, Incyte Corporation, Novartis, Takeda Oncology, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Daniel A. Pollyea, University of Colorado Anschutz Medical Campus, 1665 Aurora Ct, Mail Stop F754, Aurora, CO 80045; e-mail: daniel.pollyea@ucdenver.edu.
The full-text version of this article contains a data supplement.