Key Points
This is the first study to compare thromboembolism and mortality risk in CAD against a general population cohort.
Patients with CAD were at a significantly increased risk of death, especially during the first 5 years after diagnosis.
Abstract
Cold agglutinin disease (CAD) is a rare form of autoimmune hemolytic anemia with limited epidemiological and clinical data. We used the Danish National Patient Registries to examine CAD occurrence and risk of thromboembolic events (TEs) and mortality in CAD patients compared with a matched cohort from the general population in Denmark. We identified 72 patients diagnosed with CAD and 720 matched controls between 1999 and 2013. For 2013, the most recent year of study, crude incidence of CAD was 0.18 per 100 000 inhabitants per year and prevalence was 1.26 per 100 000 inhabitants. Risk of TEs was higher in the CAD patient cohort than in the comparison cohort at 1 year (7.2% of CAD patients had TEs vs 1.9% of comparisons), 3 years (9.0% vs 5.3%), and 5 years (11.5% vs 7.8%) after the index date. The median survival was 8.5 years. CAD patients had increased mortality compared with the general population cohort (adjusted hazard ratio [aHR], 1.84; 95% confidence interval [CI], 1.10-3.06; P = .020), with the highest mortality observed during the first 5 years after diagnosis (aHR, 2.27; 95% CI, 1.32-3.89; P = .003). Mortality rates 1 and 5 years after diagnosis were 17% and 39% in the CAD group vs 3% and 18% in the comparison cohort, respectively. CAD is a rare illness characterized by increased risk of TEs and mortality.
Introduction
Cold agglutinin disease (CAD) accounts for 15% to 25% of autoimmune hemolytic anemia (AIHA) cases.1,2 It is characterized by lysis of red blood cells (RBCs) induced by cold agglutinins (CAs; ie, immunoglobulin M autoantibodies that cause agglutination of RBCs), most often at temperatures of 0°C to 4°C.3 These CAs bind to RBC surface antigen 1 and activate the classical complement pathway via the C1 complex, triggering a cascade of events that result predominantly in extravascular and to a lesser extent in intravascular hemolysis.2,4 The critical role of the C1 complex in CAD was demonstrated in vitro by Shi et al5 in 2014 and later reaffirmed in a phase 1 trial, in which anti-C1 antibody sutimlimab (formerly BIVV009) rapidly ameliorated hemolytic anemia in patients with CAD.6
CA-mediated AIHA most often occurs as primary CAD, which is today considered a clonal lymphoproliferative bone marrow disorder.4,7 Causes of secondary CA-mediated AIHA include specific infections (ie, Mycoplasma pneumoniae, Epstein-Barr virus) and aggressive lymphoma. In recent literature, the term CA syndrome (CAS) has been reserved for this secondary, heterogeneous, and smaller group, which has not systematically been distinguished from CAD in older publications or diagnostic classification systems.3,4,8
Information on incidence, prevalence, and clinical course is limited. A retrospective population-based study in Norway reported incidence of 1 case per million persons per year, prevalence of 16 cases per million persons, and median survival of 12.5 years from disease onset.9
Primary CAD has traditionally been perceived as a benign disorder with a chronic nonlethal course.9 However, various forms of hemolytic anemia are known to have increased risk of thromboembolism,10,11 a potentially life-threatening event with a 1-year case fatality rate of up to 20%. A recent retrospective study using a large US clinical claims data set (Optum) showed a correlation between CAD and thromboembolic events (TEs).12 To date, there have been no studies comparing CAD patients with a general population cohort, limiting our understanding of associated risks and complications. We therefore examined the incidence and prevalence of CAD in Denmark and compared risk of TEs and mortality with that of the Danish general population.
Methods
Data sources
Denmark has a universal tax-supported health care system and medical registries that provide complete follow-up information, allowing for highly accurate disease and mortality ascertainment.13 Data in the Danish National Patient Registry (DNPR), which covers all Danish hospitals, provide population-based information to evaluate disease occurrence, burden, and clinical course in CAD patients.13,14
Setting and design
Residents of Denmark between 1999 and 2013, approximately 6 million people, were studied. CAD patients were identified from the DNPR using the International Statistical Classification of Diseases and Related Health Problems (10th revision; ICD-10) code D59.1A, which is specific to CA-mediated AIHA and distinct from warm or mixed AIHA. Date of the first inpatient or outpatient encounter for CAD was used as the index date. Use of ICD-10 was introduced in Denmark in 1994.13
Patients were observed from diagnosis (1999 at earliest) until end of study follow-up on 31 December 2013 or date of outcome of interest (death or TE), whichever occurred first. For each patient with CAD, 10 individuals from the general population identified through the CRS and without a CAD diagnosis on the index date were matched on year of birth, sex, and region of residence.18
Variable selection
Medical histories of patients with diagnosis codes specific for CAD and their comparisons were abstracted from the DNPR, and current vital status was ascertained from the CRS. Information abstracted from the medical registry, including demographic and clinical variables, was used to characterize the study cohorts. TEs (venous and arterial) were identified using the ICD-10 codes provided in supplemental Table 1. In patients with >1 TE coded, only the first TE was used for analysis to avoid overcounting.
Analyses for mortality and TEs were adjusted for comorbidities using CCI score. Multivariate models were built using Cox regression analyses to remove any potential confounding effects of CCI on the relationship between CAD and risk of TEs or mortality.
Statistical analysis
Incidence and prevalence of CAD in Denmark were calculated for individual years from 1999 to 2013 as rates per 100 000 inhabitants using the entire Danish population. Frequency and proportion of CAD patients and general population comparators were characterized by age at diagnosis, sex, year of diagnosis/index, region of residence, comorbidities, and duration of follow-up.
Risks of first TE and mortality at end of 2013 and at 1, 3, and 5 years after index were computed using a cumulative incidence function for competing risk data and Kaplan-Meier function, respectively. Hazard ratios (HRs), 95% confidence intervals (CIs), and P values were calculated using stratified Cox regression analysis to compare the CAD cohort with the matched comparison cohort. P < .05 was considered statistically significant. Cox regression HRs were adjusted for comorbidities using CCI scores.
To attempt to evaluate mortality specifically among patients with primary CAD, sensitivity analyses were conducted by excluding patients with an additional diagnosis code for any kind of non-Hodgkin lymphoma, myeloma, chronic lymphocytic leukemia, Waldenström macroglobulinemia, or certain infections known to be associated with secondary CAS (eg, Epstein-Barr virus, cytomegalovirus, and M pneumoniae). Patients with codes for infections would only be excluded if the infection were diagnosed on the same date or during the same hospitalization as the initial CAD diagnosis. Comparison patients who were matched to excluded patients were removed for this analysis. Any additional patients from the comparison group (even if not matched to excluded CAD patients) who had any of the associated secondary diagnoses were also removed. The ICD codes used to exclude these patients are provided in supplemental Table 2. Although we also had information on TEs for this group, the number of patients with TEs was too small to make the analyses informative.
Results
Study cohorts
We identified 72 patients diagnosed with CAD between 1999 and 2013 and matched them to a general population comparison cohort of 720 individuals. Descriptive characteristics of the 2 cohorts are presented in Table 1. Median age in both cohorts was 72 years, as a result of age matching. There was a preponderance of female patients in both cohorts (58.3%). Nearly two-thirds of patients were diagnosed between 2009 and 2013, and 36% were from the capital region of Denmark. Median duration of follow-up was similar in the 2 cohorts (3.1 years for CAD patients and 2.9 years for matched comparisons).
Descriptive characteristics of the Danish CAD cohort and matched comparison cohort
| . | CAD patients . | Matched comparison cohort . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| All | 72 | 100.00 | 720 | 100.00 |
| Sex | ||||
| Female | 42 | 58.33 | 420 | 58.33 |
| Male | 30 | 41.67 | 300 | 41.67 |
| Age at diagnosis, y | ||||
| 19-59 | 17 | 23.61 | 170 | 23.61 |
| 60-69 | 13 | 18.06 | 130 | 18.06 |
| 70-79 | 27 | 37.50 | 276 | 38.33 |
| 80-89 | 15 | 20.83 | 144 | 20.00 |
| Mean (SD) | 68.5 (14.9) | 68.5 (14.7) | ||
| Median (range) | 72.2 (20.3-89.6) | 72.2 (19.8-89.8) | ||
| Year of diagnosis/match date | ||||
| 1999-2003 | 5 | 6.94 | 50 | 6.94 |
| 2004-2008 | 22 | 30.56 | 220 | 30.56 |
| 2009-2013 | 45 | 62.50 | 450 | 62.50 |
| Region of residence at diagnosis | ||||
| North Denmark | 5 | 6.94 | 50 | 6.94 |
| Central Denmark | 13 | 18.06 | 130 | 18.06 |
| Southern Denmark | 19 | 26.39 | 190 | 26.39 |
| Capital region | 26 | 36.11 | 260 | 36.11 |
| Zealand | 9 | 12.50 | 90 | 12.50 |
| Duration of follow-up, y | ||||
| Mean (SD) | 3.4 (2.9) | 3.8 (3.1) | ||
| Median (range) | 3.1 (0.0-14.3) | 2.9 (0.0-14.3) | ||
| . | CAD patients . | Matched comparison cohort . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| All | 72 | 100.00 | 720 | 100.00 |
| Sex | ||||
| Female | 42 | 58.33 | 420 | 58.33 |
| Male | 30 | 41.67 | 300 | 41.67 |
| Age at diagnosis, y | ||||
| 19-59 | 17 | 23.61 | 170 | 23.61 |
| 60-69 | 13 | 18.06 | 130 | 18.06 |
| 70-79 | 27 | 37.50 | 276 | 38.33 |
| 80-89 | 15 | 20.83 | 144 | 20.00 |
| Mean (SD) | 68.5 (14.9) | 68.5 (14.7) | ||
| Median (range) | 72.2 (20.3-89.6) | 72.2 (19.8-89.8) | ||
| Year of diagnosis/match date | ||||
| 1999-2003 | 5 | 6.94 | 50 | 6.94 |
| 2004-2008 | 22 | 30.56 | 220 | 30.56 |
| 2009-2013 | 45 | 62.50 | 450 | 62.50 |
| Region of residence at diagnosis | ||||
| North Denmark | 5 | 6.94 | 50 | 6.94 |
| Central Denmark | 13 | 18.06 | 130 | 18.06 |
| Southern Denmark | 19 | 26.39 | 190 | 26.39 |
| Capital region | 26 | 36.11 | 260 | 36.11 |
| Zealand | 9 | 12.50 | 90 | 12.50 |
| Duration of follow-up, y | ||||
| Mean (SD) | 3.4 (2.9) | 3.8 (3.1) | ||
| Median (range) | 3.1 (0.0-14.3) | 2.9 (0.0-14.3) | ||
SD, standard deviation.
Incidence and prevalence
Incidence of CAD diagnosis rose from 0.018 per 100 000 inhabitants per year in 1999 to 0.175 per 100 000 inhabitants per year in 2013. Similarly, prevalence rose from 0.018 per 100 000 inhabitants in 1999 to 1.261 per 100 000 inhabitants in 2013. For the most recent 5 years of study (2009-2013), cumulative incidence and prevalence were 0.74 per 100 000 inhabitants and 1.184 per 100 000 inhabitants, respectively (compared with 0.086 and 0.086 per 100 000 inhabitants for the first 5 years of the study [1999-2003]).
Mortality
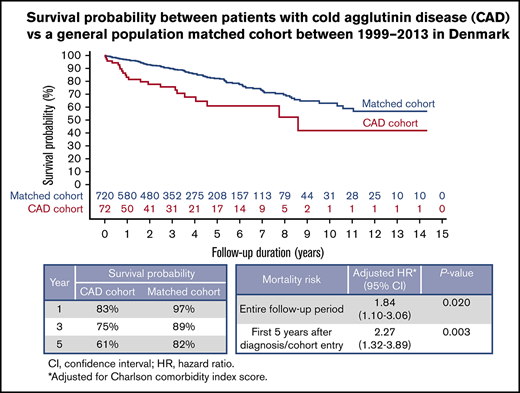
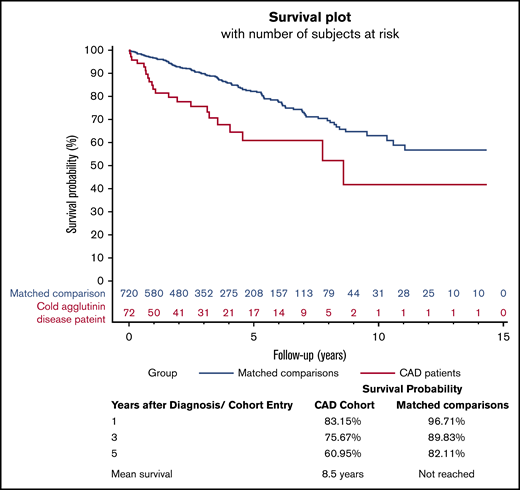
There were 22 deaths among patients diagnosed with CAD and 114 deaths among individuals from the general population matched comparison cohort during the study period. Median overall survival among CAD patients was 8.5 years, and their mortality was increased throughout the study period (Figure 1). Mortality rate 1 year after the index date was 17% for CAD patients vs 3% for individuals in the comparison cohort (Figure 1). Corresponding mortality rates were 25% vs 11% after 3 years and 39% vs 18% after 5 years, respectively (Figure 1).
Kaplan-Meier survival curve for CAD patients and matched general population comparison cohort.
Kaplan-Meier survival curve for CAD patients and matched general population comparison cohort.
CCI score adjusted HR (aHR) for mortality among CAD patients was 1.84 (95% CI, 1.10-3.06; P = .020; Table 2). Highest mortality was observed during the first 5 years after diagnosis (aHR, 2.27; 95% CI, 1.32-3.89; P = .003 Table 2).
Mortality in CAD patients compared with individuals in general population matched comparison cohort
| Outcome . | Overall analysis for patients with CAD (n = 72) and matched controls (n = 720) . | Sensitivity analysis for patients with primary CAD (n = 59) and matched controls (n = 585) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAD cohort patient deaths, n (%) . | Control cohort patient deaths, n (%) . | Crude HR (95% CI) . | aHR* (95% CI) . | P . | CAD cohort patient deaths, n (%) . | Control cohort patient deaths, n (%) . | Crude HR (95% CI) . | aHR* (95% CI) . | P . | |
| Mortality (entire follow-up) | 22 (30.6) | 114 (15.8) | 2.69 (1.66-4.35) | 1.84 (1.10-3.06) | .020 | 18 (30.5) | 93 (15.8) | 3.02 (1.76-5.17) | 2.35 (1.34-4.13) | .003 |
| First 5 y after diagnosis/study entry | 20 (27.8) | 82 (11.4) | 3.20 (1.92-5.33) | 2.27 (1.32-3.89) | .003 | 16 (27.1) | 65 (11.0) | 3.55 (1.99-6.33) | 3.00 (1.64-5.48) | .0004 |
| Outcome . | Overall analysis for patients with CAD (n = 72) and matched controls (n = 720) . | Sensitivity analysis for patients with primary CAD (n = 59) and matched controls (n = 585) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAD cohort patient deaths, n (%) . | Control cohort patient deaths, n (%) . | Crude HR (95% CI) . | aHR* (95% CI) . | P . | CAD cohort patient deaths, n (%) . | Control cohort patient deaths, n (%) . | Crude HR (95% CI) . | aHR* (95% CI) . | P . | |
| Mortality (entire follow-up) | 22 (30.6) | 114 (15.8) | 2.69 (1.66-4.35) | 1.84 (1.10-3.06) | .020 | 18 (30.5) | 93 (15.8) | 3.02 (1.76-5.17) | 2.35 (1.34-4.13) | .003 |
| First 5 y after diagnosis/study entry | 20 (27.8) | 82 (11.4) | 3.20 (1.92-5.33) | 2.27 (1.32-3.89) | .003 | 16 (27.1) | 65 (11.0) | 3.55 (1.99-6.33) | 3.00 (1.64-5.48) | .0004 |
Match variables included year of birth, sex, and region of residence. Model also included adjustment for CCI score.
Sensitivity analyses for mortality in primary CAD
The diagnostic code for CAD includes both patients with primary CAD and those with CAD secondary to an underlying disorder (CAS). To attempt to evaluate the risk of mortality specifically among patients with primary CAD, sensitivity analyses were conducted by excluding patients who had additional diagnostic codes known to be associated with secondary CAS, such as lymphoma (supplemental Table 2). Of 72 patients with CAD, 13 were excluded from the CAD patient cohort. Diagnoses for these excluded patients were chronic lymphocytic leukemia, Waldenström macroglobulinemia, small lymphocytic lymphoma, nonfollicular diffuse lymphoma, and multiple myeloma. No patients were excluded because of codes for infections. Comparison patients who were matched to excluded patients were also removed from these analyses. Five additional patients from the comparison group (not directly matched to excluded CAD patients) were also found to have a diagnosis associated with secondary CAS and were therefore excluded.
Among the remaining 59 CAD patients whose disease was presumed to be primary, there were 18 deaths. Median overall survival for this group was 8.5 years. aHR for mortality was 2.35 (95% CI, 1.34-4.13; P = .003), and in line with the prior overall analysis, it was highest during the first 5 years after diagnosis (aHR, 3.00; 95% CI, 1.64-5.48; P = .0004; Table 2).
Thromboembolic events
There were 8 unique TEs among 7 individuals in the CAD group and 55 unique TEs among 51 individuals in the comparison cohort. Incidence rate of TEs was 30.4 (95% CI, 14.5-63.8) per 1000 person-years in CAD patients, compared with 18.6 (95% CI, 14.2-24.5) per 1000 person-years in the matched comparison group. Risk of TEs was higher in the CAD patient cohort than in the comparison cohort 1 year (7.2% of CAD patients had TEs vs 1.9% of comparisons), 3 years (9.0% vs 5.3%), and 5 years (11.5% vs 7.8%) after the index date. Corresponding aHR was 1.65 (95% CI, 0.69-3.95; P = .257).
Discussion
In this population-based study, we confirmed that CAD is a rare illness and found incidence and prevalence in 2013 to be consistent with prior reports.9 The lower incidence and prevalence observed in early years likely reflects an initial lack of awareness of the CAD entity and less detailed diagnostic workup in AIHA, rather than a true increase in CAD cases over time.
Traditionally, CAD has been considered a chronic illness without significant risks, but information on mortality is limited. A study describing 89 CAD patients from the Mayo Clinic reported median survival of 10.6 years,2 whereas in the Norwegian study, it was 12.5 years.9 Because there have not been any studies comparing mortality in CAD patients with a matched cohort, there are no published data on survival probabilities. Using data from the DNPR and CRS, we were able to compare CAD patients with a general population matched cohort. In addition to finding increased mortality, our data indicated that this risk starts early on, with 39% of CAD patients expected to be deceased within the first 5 years from diagnosis. This is contrary to the traditional belief that as a chronic disease, complications may occur later. In current real-world clinical practice, CAD may be initially managed with supportive measures only, such as cold avoidance and observation. One possible explanation for the early risk of death may be the lack of early therapeutic interventions used in these patients.
The sensitivity analysis for primary CAD demonstrated that presence of a malignancy was not driving the increased mortality. Because there are no separate diagnostic codes for primary CAD and secondary CAS, it is possible that we under- or overestimated the CAS cases. Furthermore, the exclusion of patients with certain diagnoses (in particular C88.0 [Waldenström macroglobulinemia] and C83X [nonfollicular lymphoma]) could have also excluded patients with primary CAD, because the typical primary CAD-associated lymphoproliferative bone marrow disorder can be difficult to distinguish from lymphoplasmacytic lymphoma and marginal zone lymphoma.7 However, the fact that the overall mortality analysis, which was corrected for confounding from existing comorbidities using validated regression models, and the sensitivity analysis, which effectively removed any patients with lymphoma, both showed similar results suggests that CAD itself is associated with reduced survival.
Patients with hemolytic anemia have been reported to be at greater risk for TEs.11,19,,-22 A systematic review and meta-analysis reported elevated risk of TEs in patients with AIHA.10 In a recent retrospective cohort study of 308 patients with primary AIHA (27% of whom were diagnosed with CAD), Barcellini et al23 found that 33 patients (11%) had a record of TEs, occurrence of which was associated with severe onset (hemoglobin levels ≤8 g/dL and high median lactate dehydrogenase levels) and previous splenectomy. The recent Optum study identified 814 patients with the term CAD repeatedly mentioned in their records, with up to 10 matched comparisons for each, and found increased risk of cerebral, venous, and arterial TEs.12 Similarly, our results demonstrated higher cumulative incidence of TEs in patients with CAD. The corresponding aHR was elevated; however, it did not reach statistical significance. This may be due to the small sample size, but other reasons cannot be ruled out. Our findings, coupled with the Optum results, suggest that it is important for health care providers to be aware of potential TE risk in these patients to ensure timely consideration of mitigation strategies.
Several issues should be considered when interpreting our results. The main strength of our study is its population-based design with complete follow-up, limiting the risk of selection bias. Also, cardiovascular and comorbidity diagnoses have high accuracy in the DNPR.14,24,25 Diagnosis of CAD has not yet been validated in the patient registry; however, any misclassification would probably underappreciate the relative risk estimates.26 A limitation of the study is the lack of clinical detail and information on hereditary or acquired risk factors for TEs and mortality. Because no laboratory information was available in this registry, we could not correlate hemoglobin levels and hemolysis markers with our results.14 However, because of the rarity of this condition, population-level studies, with their limitations, may help us to better understand the disease course and elucidate some of the complications that these patients may develop.
In summary, the results of this population-level comparison study support the increased risk of TEs described in recent reports, and we found that patients with CAD also have increased mortality. Patients with CAD should be carefully monitored, and potential clinical complications should be evaluated promptly. Prospective data collection, such as in a CAD-specific patient registry, would help overcome most of the limitations encountered in this study.
Acknowledgments
Aarhus University received funds from EpidStrategies, a Division of ToxStrategies, Inc., Rockville, MD. EpidStrategies was funded by Bioverativ, a Sanofi company, and by the Program for Clinical Research Infrastructure. Editorial assistance for the development of this paper was provided by JK Associates, Inc., a member of the Fishawack Group of Companies, and was funded by Sanofi.
Authorship
Contribution: L.C.B. participated in study design, reviewed the analyses, wrote the manuscript, and approved the final version of the manuscript; A.G.O. participated in the study design, obtained and managed the data, interpreted the analyses, revised the manuscript, and approved of the final version of the manuscript; A. Rosenthal participated in study design, reviewed the analyses, revised the manuscript, and approved the final version of the manuscript; B.Ö. obtained and managed the data, performed the analyses, edited the manuscript, and approved the final version of the manuscript; J.P.F. participated in study design, reviewed the analyses, revised the manuscript, and approved the final version of the manuscript; J.M.A. reviewed the analyses, edited the manuscript, and approved the final version of the manuscript; A. Röth reviewed the analyses, revised the manuscript, and approved the final version of the manuscript; and S.B. reviewed the analyses and extensively revised the paper.
Conflict-of-interest disclosure: S.B. has received research support from Mundipharma, lecture honoraria from Alexion, Sanofi, and Janssen-Cilag, and travel support from Alexion, Apellis, and Celgene and has been an advisory board member for Sanofi; L.C.B. and J.P.F. are employees of EpidStrategies, a Division of ToxStrategies, Inc.; A. Rosenthal and J.M.A. are employees of Sanofi; and A. Röth has received research support from Alexion and Roche, lecture honoraria from Alexion, Novartis, and Sanofi, and travel support from Alexion and has been an advisory board member for Alexion and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Jon P. Fryzek, EpidStrategies, 9601 Medical Center Dr, Rockville, MD 20850; e-mail: jfryzek@epidstrategies.com.
The full-text version of this article contains a data supplement.