Key Points
In a whole blood assay, ICs cause neutrophil activation and degranulation.
Individuals have a fixed susceptibility to neutrophil activation by ICs.
Abstract
Immune complexes (ICs) can trigger inflammation and thrombosis, in part, by activating neutrophils. Much attention has focused on the serologic characteristics of ICs and Fc receptors associated with cellular activation, but few studies have examined host susceptibility to neutrophil activation by ICs. Here, we use a novel whole blood system to investigate the ability of ICs to cause neutrophil activation and degranulation. Using monoclonal anti-platelet factor 4/heparin (PF4/heparin), anti-protamine/heparin antibodies, patient-derived anti-PF4/heparin antibodies, and heat-aggregated immunoglobulin G as model ICs, we demonstrate that heparin-containing ICs cause robust, heparin-dependent neutrophil activation and degranulation which is mediated by both FcγRIIa and complement. Longitudinal testing over a 1-year period shows that an individual’s neutrophil response to ICs represents a fixed phenotype resulting in high, intermediate, or low reactivity. Examination of individuals at the extremes of reactivity (high vs low) shows that phenotypic variation resides in the cellular compartment and is correlated with host white blood cell count and absolute neutrophil count, but not age, sex, race, polymorphisms in neutrophil Fcγ receptors, or CR1, CR3, and Fcγ receptor expression on neutrophils. Together, these studies demonstrate that susceptibility to neutrophil activation by ICs is intrinsic to the host and is likely genetic in origin. These findings may be relevant to the heterogeneous clinical outcomes seen in patients with heparin-induced thrombocytopenia and other IC-mediated disorders and could potentially identify patients at high risk for thrombotic and inflammatory complications.
Introduction
A variety of prothrombotic disorders are characterized by circulating antigen/antibody immune complexes (ICs). Examples of ICs associated with thrombotic disorders include β2-glycoprotein I (β2-GPI) ICs in antiphospholipid syndrome (APS),1 ADAMTS-13–specific ICs in thrombotic thrombocytopenic purpura,2 and platelet factor 4/heparin (PF4/heparin) ICs in heparin-induced thrombocytopenia (HIT).3 These antibody-mediated diseases are associated with high morbidity and mortality (9% mortality rate for APS in a 10-year study4 and 10% mortality for HIT in a 14-year study5 ).
Although patients with IC-mediated disorders are predisposed to arterial and/or venous thrombosis, many do not develop overt thrombotic complications. For example, only a subset of patients with APS who have circulating β2-GPI ICs will develop thrombosis.1 Similarly, although all patients with HIT have anti-PF4/heparin antibodies,6 only 30% to 50% will develop arterial and/or venous thrombosis.5,7,8 Currently, there is no biomarker to predict which patients with IC-mediated disease will develop thrombosis.
Although the pathogenesis of IC-mediated thrombosis is not fully understood, recent studies indicate that neutrophil activation plays a major role. For example, neutrophils from patients with APS are predisposed to spontaneous activation and neutrophil extracellular trap (NET) release.9 In APS, release of NETs correlates with clinical manifestations10 by promoting thrombin generation9 and contributing to arterial and/or venous thrombosis.11 Likewise, in HIT, anti-PF4/heparin antibodies induce neutrophil activation resulting in increased cell-surface Mac-1 expression,12,13 enhanced adhesion to the endothelium,13,14 infiltration into venous thrombi,14 and release of NETs.14 Together, these studies demonstrate that neutrophil activation contributes to thrombotic and inflammatory complications in patients with IC-mediated disorders.12,-14
Despite increasing recognition that neutrophils are integral in the pathogenesis of thrombosis in IC-mediated disorders, little is known about variability in neutrophil function, both in health and disease. Previous studies that used healthy donors demonstrated quantitative differences in the surface density of various neutrophil antigens involved in complement-dependent cytotoxicity.15,16 In other studies of healthy donors, variable expression of the CD11b adhesion molecule on neutrophils was seen, both at baseline and after stimulation with phorbol 12-myristate 13-acetate (PMA), as well as differences in cell-associated oxidant content after PMA stimulation.16 Unlike expression of CD11b, oxidative burst did correlate, in part, with sex and race.16 Similarly, in another study of healthy donors, increased oxidative activity was noted in females.17 In a single study from van Mirre et al,18 variation in neutrophil responsiveness to ICs consisting of aggregated immunoglobulin G (IgG) was assessed and was found to be associated with the FcγRIIa:FcγRIIb2 ratio.
On the basis of these reported differences, we undertook studies to investigate donor heterogeneity to IC-induced neutrophil activation in a whole blood environment. By using model ICs of PF4/heparin, protamine/heparin (PRT/heparin), and heat-aggregated IgG, we developed a whole blood assay to quantify IC-induced neutrophil activation and degranulation. Our studies confirm donor heterogeneity, and by using biologically relevant ICs, we demonstrate that the neutrophil response to ICs represents a fixed phenotype for a given individual. Our findings suggest that the neutrophil activation response to ICs may potentially serve as a biomarker for disease susceptibility in IC-mediated disorders.
Methods
Reagents
A mouse monoclonal IgG2bκ anti-PF4/heparin antibody (KKO), a monoclonal IgG2bκ isotype control, a mouse monoclonal IgG3κ anti-PRT/heparin antibody (ADA),19 and recombinant human PF4 were isolated, as previously described.20,21 PMA, a monoclonal IgG3 isotype, and protamine sulfate were purchased from Sigma (St. Louis, MO), unfractionated heparin (UFH) from Fresenius Kabi (Lake Zurich, IL), enoxaparin from Aventis Pharmaceuticals (Paris, France), and a monoclonal antibody to CD32 (IV.3) from STEMCELL Technologies (Vancouver, BC, Canada). A C3 inhibitor (CP40), was generously donated by John Lambris and Edimara Reis. Anti-PF4/heparin antibodies in patients were detected by using an IgG-specific immunoassay (Zymutest HIA IgG; HYPHEN BioMed, Neuville-sur-Oise, France). Heat-aggregated IgG was prepared, as previously described.18
Patient samples
After informed consent (Duke University Medical Center IRB#Pro00012901), blood samples in 3.2% sodium citrate were obtained from patients with anti-PF4/heparin IgG antibodies (n = 27). After centrifugation, plasma was stored at −80°C. Samples from healthy donors were collected in acid-citrate-dextrose (n = 71; Duke IRB#Pro00010740). Healthy donors were excluded if they reported active infection, a chronic comorbid inflammatory condition, or antibiotic use. Whole blood from healthy donors was used within 1 hour of collection. Complete blood counts were obtained by using an automated hematology analyzer (Sysmex Corporation, Kobe, Japan).
Whole blood neutrophil degranulation assays
Whole blood assays were performed to determine the ability of ICs to activate and degranulate neutrophils. Myeloperoxidase (MPO), lactoferrin, and matrix metallopeptidase 9 (MMP-9) were quantified as markers of primary (azurophilic), secondary (specific), and tertiary (gelatinase) granule release, respectively. Unless specified, the following concentrations were used: KKO or isotype control antibody (25 µg/mL), PF4 (25 µg/mL), and UFH (1 U/mL). For all assays, 100 µL of healthy donor whole blood was incubated with buffer or KKO, with or without PF4, and with or without UFH, which were added sequentially. For patient samples, seropositive patient plasma or healthy donor control plasma (1:10 dilution for both) was added to whole blood alone or with antigens. After 30 minutes at 37°C and after centrifugation, neutrophil-specific granules were measured in plasma. MPO and MMP-9 were measured using commercial human immunoassay kits (R&D Systems, Minneapolis, MN). Lactoferrin was measured using a sandwich enzyme-linked immunoassay (supplemental Methods). Results were measured at 450nm (Spectramax 384 PLUS; Molecular Devices, Sunnyvale, CA) and analyzed by using SoftMax PRO software (Molecular Devices).
For assays using patient plasma, results are reported as percent granule release. This allows for correction of variability in background plasma granule levels and expresses results as a percentage of maximum granule release, defined by whole blood incubated with KKO-PF4/heparin ICs. Thus, final results are calculated as: % granule release = ([granules released with patient plasma + PF4/heparin] − [granules released with patient plasma])/(granules released with KKO + PF4/heparin).
PF4-dependent P-selectin expression assay
To identify platelet activation induced by anti-PF4/heparin antibodies, the PF4-dependent P-selectin expression assay (PEA) was performed, as previously described (supplemental Methods).22 P-selectin expression on CD41+ platelets was recorded using a BD FACSCanto Flow Cytometer (BD Biosciences). Analyses were performed using FCS Express (version 5.01.0080, DeNovo Software, Glendale, CA). Following published methods,22 results were calculated as the percentage of maximum P-selectin expression from thrombin receptor-activating peptide–treated platelets, with results ≥24% considered positive.22
Statistical analysis
Data are expressed as mean ± standard deviation. Variability in donor responses is depicted as median with interquartile range to better represent distribution and to categorize donors into quartiles. Demographics were compared by using Kruskal-Wallis tests for continuous variables and χ2 tests for dichotomous variables. Differences in MMP-9 release among different conditions were tested with analysis of variance. Correlations of MMP-9 response and donor variables were examined with Spearman’s correlation coefficient for continuous variables or analysis of variance for categorical variables. Analyses were performed using Prism version 8.0.2 (Graphpad Software, La Jolla, CA) and SAS version 9.4 (SAS Institute, Cary, NC). Results are reported using a 2-sided P < .05 to indicate statistical significance. All results are representative of at least 3 independent experiments using a minimum of 3 healthy donors.
Results
Whole blood assay for assessing neutrophil activation and degranulation by ICs
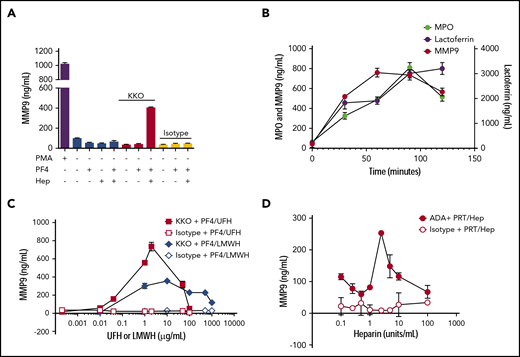
Previous studies have shown that HIT ICs activate and degranulate purified neutrophils isolated after gradient centrifugation of whole blood.13 In replicating these studies, we found that neutrophil isolation resulted in cellular activation, degranulation, and depletion (data not shown). To circumvent this, we developed an assay to examine neutrophil activation in whole blood using KKO (a monoclonal anti-PF4/heparin antibody) and exogenous antigen (PF4/heparin). After incubating whole blood with PMA (positive control) or antigens in combination with antibodies (Figure 1A), we saw that whole blood incubated with buffer, antigen alone, KKO with or without PF4, or isotype did not elicit MMP-9 release, whereas incubation with KKO-PF4/heparin ICs resulted in significant MMP-9 release. Consistent with previous studies,12,13 we show that neutrophil activation by KKO-PF4/heparin ICs causes release of all 3 neutrophil granule populations. As shown in Figure 1B, KKO-PF4/heparin ICs induce release of MPO (representative of primary granules), lactoferrin (secondary granules), and MMP-9 (tertiary granules), when compared with whole blood incubated with buffer, PF4/heparin, or isotype control (data not shown). As seen in Figure 1B, MMP-9 is the most readily exocytosed granule. Given that MMP-9 release is robust and rapid and results in a stable releasate under freeze/thaw conditions (data not shown), we used MMP-9 as an end point for the remaining studies as a marker of neutrophil activation and degranulation.
Heparin-containing ICs induce heparin-dependent neutrophil activation. (A) KKO-PF4/heparin ICs cause neutrophil degranulation and MMP-9 release. First, 100 µL of whole blood from a healthy donor was incubated with buffer, PMA (500 nM), KKO (25 µg/mL), or IgG2b isotype control (25 µg/mL) in the presence of the following antigens alone or in combination: PF4 (25 µg/mL) or heparin (Hep; 1 U/mL). After incubation for 30 minutes at 37°C, samples were centrifuged at 300g for 5 minutes. Plasma was removed, and MMP-9 was quantified. (B) KKO-PF4/heparin ICs cause release of all neutrophil granule populations. KKO (25 µg/mL) along with PF4 (25 µg/mL) and heparin (1 U/mL) was added to whole blood. After incubation for varying amounts of time (0-120 minutes), release of MMP-9, lactoferrin, or MPO was measured in plasma. (C) KKO-PF4/heparin IC–induced neutrophil activation is heparin/low molecular weight heparin (LMWH) dependent. Whole blood was incubated with KKO (25 µg/mL) along with PF4 (25 µg/mL) and UFH or LMWH at varying concentrations (ranging from 0 to 1000 µg/mL) or IgG2b isotype control (25 µg/mL) along with PF4 (25 µg/mL) and varying levels of UFH/LMWH. After 30 minutes, release of MMP-9 was measured in the plasma. (D) ADA-PRT/heparin ICs also induce heparin-dependent MMP-9 release. Whole blood was incubated with a monoclonal anti-PRT/heparin antibody (ADA 50 µg/mL) along with PRT (25 µg/mL) and varying amounts of heparin (0-100 U/mL). After 30 minutes, MMP-9 released in plasma was measured. Data are representative of 3 independent experiments and results using whole blood from 3 healthy donors. Results are expressed as mean ± standard deviation values for triplicate wells.
Heparin-containing ICs induce heparin-dependent neutrophil activation. (A) KKO-PF4/heparin ICs cause neutrophil degranulation and MMP-9 release. First, 100 µL of whole blood from a healthy donor was incubated with buffer, PMA (500 nM), KKO (25 µg/mL), or IgG2b isotype control (25 µg/mL) in the presence of the following antigens alone or in combination: PF4 (25 µg/mL) or heparin (Hep; 1 U/mL). After incubation for 30 minutes at 37°C, samples were centrifuged at 300g for 5 minutes. Plasma was removed, and MMP-9 was quantified. (B) KKO-PF4/heparin ICs cause release of all neutrophil granule populations. KKO (25 µg/mL) along with PF4 (25 µg/mL) and heparin (1 U/mL) was added to whole blood. After incubation for varying amounts of time (0-120 minutes), release of MMP-9, lactoferrin, or MPO was measured in plasma. (C) KKO-PF4/heparin IC–induced neutrophil activation is heparin/low molecular weight heparin (LMWH) dependent. Whole blood was incubated with KKO (25 µg/mL) along with PF4 (25 µg/mL) and UFH or LMWH at varying concentrations (ranging from 0 to 1000 µg/mL) or IgG2b isotype control (25 µg/mL) along with PF4 (25 µg/mL) and varying levels of UFH/LMWH. After 30 minutes, release of MMP-9 was measured in the plasma. (D) ADA-PRT/heparin ICs also induce heparin-dependent MMP-9 release. Whole blood was incubated with a monoclonal anti-PRT/heparin antibody (ADA 50 µg/mL) along with PRT (25 µg/mL) and varying amounts of heparin (0-100 U/mL). After 30 minutes, MMP-9 released in plasma was measured. Data are representative of 3 independent experiments and results using whole blood from 3 healthy donors. Results are expressed as mean ± standard deviation values for triplicate wells.
Anti-PF4/heparin antibodies bind to PF4/heparin complexes over a narrow range of heparin concentrations, which coincides with concentrations of heparin needed for optimal PF4/heparin ultralarge complex (ULC) formation.23 To determine whether neutrophil degranulation induced by KKO is heparin dependent, whole blood was incubated with PF4 and increasing amounts of heparin (0-100 U/mL UFH or 0-1000 µg/mL enoxaparin). As shown in Figure 1C, optimal neutrophil activation and MMP-9 release is seen when whole blood is incubated with KKO along with PF4 and heparin at 1 U/mL (∼2 µg/mL) or 10 µg/mL enoxaparin. A subsequent decline in MMP-9 release is seen with increasing concentrations of heparin or enoxaparin, resulting in loss of MMP-9 release with 100 U/mL (∼200 µg/mL) heparin and 100 µg/mL enoxaparin, demonstrating that KKO-PF4/heparin–induced neutrophil activation in our whole blood assay is heparin/low molecular weight heparin dependent.
We next determined whether other ULCs can also induce neutrophil activation in our whole blood assay. Like KKO, ADA (a monoclonal anti-PRT/heparin antibody19 ) forms large, heparin-dependent ICs.23 To determine whether ADA-PRT/heparin ICs can similarly cause neutrophil degranulation, whole blood was incubated with ADA or isotype and protamine, along with varying concentrations of heparin (0-100 U/mL). As shown in Figure 1D and supplemental Figure 1, ADA-PRT/heparin ICs also induce heparin-dependent neutrophil activation with release of all 3 granule populations.
Patient-derived anti-PF4/heparin antibodies induce heparin-dependent neutrophil activation
We next examined the effects of patient-derived, polyclonal anti-PF4/heparin antibodies in our neutrophil activation and degranulation assay. Plasma from seropositive patients (Figure 2A; HIT patient 1 [HIT1] and HIT patient 2 [HIT2]) was used as a source of polyclonal anti-PF4/heparin antibodies. As shown in Figure 2A, when compared with buffer or PF4 alone, significantly more MMP-9 was released when patient plasma was added with PF4/heparin complexes at an optimal stoichiometric ratio (PF4:heparin = 1.7:1; HIT1: P = .02; HIT2: P < .0001 relative to all other conditions). To demonstrate the time course of neutrophil activation by patient-derived antibodies, whole blood was incubated with seropositive patient plasma (1:10 dilution) and PF4/heparin for varying amounts of time. As shown in Figure 2B, ICs formed with patient-derived anti-PF4/heparin antibodies induce mobilization of all 3 granule compartments resulting in robust degranulation compared with patient plasma alone or control plasma with PF4/heparin (data not shown). Consistent with previous reports,12 neutrophil activation by HIT antibodies in whole blood was heparin dependent (Figure 2C). Taken together, using monoclonal and polyclonal heparin-dependent antibodies, we show that these ICs trigger neutrophil activation in the presence of optimal antigen conformation and elicit release from all 3 neutrophil granule compartments.
ICs containing patient-derived anti-PF4/heparin antibodies induce robust, heparin-dependent neutrophil degranulation. (A) ICs containing patient-derived anti-PF4/heparin antibodies cause MMP-9 release. Whole blood was incubated with buffer or KKO (25 µg/mL) along with PF4 (25 µg/mL) and heparin (1 U/mL), with plasma from 2 individual HIT patients (HIT1 and HIT2; 1:10 dilution), or plasma from a normal healthy donor (Control, 1:10 dilution) in the presence of buffer or PF4 with or without heparin. Released MMP-9 was measured by enzyme-linked immunosorbent assay (ELISA). Data shown are representative of whole blood from 3 healthy donors. (B) ICs containing patient-derived anti-PF4/heparin antibodies cause release of all neutrophil granule populations. Plasma from a patient with anti-PF4/heparin antibodies (diluted 1:10) was added to whole blood from a healthy donor, along with PF4 (25 µg/mL) and heparin (1 U/mL). After incubation for varying amounts of time (0-120 minutes), granules were measured in plasma. Data shown are representative of whole blood from 2 healthy donors. (C) ICs containing patient-derived anti-PF4/heparin antibodies induce heparin/LMWH-dependent neutrophil activation. Whole blood from a healthy donor was incubated with plasma from a patient with anti-PF4/heparin antibodies or with plasma from a healthy donor as a control (both at a 1:10 dilution) along with PF4 (25 µg/mL) and varying amounts of UFH or LMWH (0-1000 µg/mL). After 30 minutes, MMP-9 release was measured in plasma. Data shown are representative of whole blood from 3 healthy donors. All data are representative of at least 3 independent experiments. Results are expressed as mean ± standard deviation values for triplicate wells.
ICs containing patient-derived anti-PF4/heparin antibodies induce robust, heparin-dependent neutrophil degranulation. (A) ICs containing patient-derived anti-PF4/heparin antibodies cause MMP-9 release. Whole blood was incubated with buffer or KKO (25 µg/mL) along with PF4 (25 µg/mL) and heparin (1 U/mL), with plasma from 2 individual HIT patients (HIT1 and HIT2; 1:10 dilution), or plasma from a normal healthy donor (Control, 1:10 dilution) in the presence of buffer or PF4 with or without heparin. Released MMP-9 was measured by enzyme-linked immunosorbent assay (ELISA). Data shown are representative of whole blood from 3 healthy donors. (B) ICs containing patient-derived anti-PF4/heparin antibodies cause release of all neutrophil granule populations. Plasma from a patient with anti-PF4/heparin antibodies (diluted 1:10) was added to whole blood from a healthy donor, along with PF4 (25 µg/mL) and heparin (1 U/mL). After incubation for varying amounts of time (0-120 minutes), granules were measured in plasma. Data shown are representative of whole blood from 2 healthy donors. (C) ICs containing patient-derived anti-PF4/heparin antibodies induce heparin/LMWH-dependent neutrophil activation. Whole blood from a healthy donor was incubated with plasma from a patient with anti-PF4/heparin antibodies or with plasma from a healthy donor as a control (both at a 1:10 dilution) along with PF4 (25 µg/mL) and varying amounts of UFH or LMWH (0-1000 µg/mL). After 30 minutes, MMP-9 release was measured in plasma. Data shown are representative of whole blood from 3 healthy donors. All data are representative of at least 3 independent experiments. Results are expressed as mean ± standard deviation values for triplicate wells.
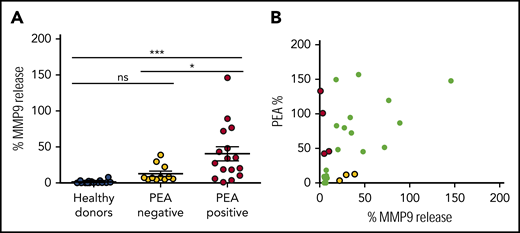
Neutrophil activation induced by KKO-PF4/heparin ICs is distinct from platelet activation
Functional anti-PF4/heparin antibodies are capable of activating platelets, thereby contributing to thrombosis in HIT. To determine whether the ability of anti-PF4/heparin antibodies to activate neutrophils coincides with the ability to activate platelets, we used plasma from patients with anti-PF4/heparin antibodies (n = 27) and compared results in our MMP-9 assay to those in the PEA, a flow cytometry-based platelet activation assay with high sensitivity (96%) and specificity (85%).22 As shown in Figure 3A, seropositive patients were segregated by PEA results: 11 patients had nonfunctional anti-PF4/heparin antibodies (PEA-negative, yellow) and 16 patients had platelet-activating antibodies (PEA-positive, red). As seen in Figure 3A, after adding PF4/heparin, plasma from healthy donors (n = 16) and from PEA-negative patients induced minimal MMP-9 release (1.3% ± 2.2% MMP-9 release for healthy donors vs 12.8% ± 11.7% from PEA-negative patients; P = not significant). In contrast, plasma from PEA-positive patients along with PF4/heparin caused significant neutrophil activation and MMP-9 release (healthy donors: 40.5% ± 38.7% [P < .0005] vs PEA-positive patients; P < .05 for PEA-negative vs PEA-positive patients). Although platelet and neutrophil activation are congruent for most patients (Figure 3B; r = 0.42; P = .03), they were not concordant in all. As shown in Figure 3B, 25% of patients (4 of 16) have antibodies that are platelet activating but not neutrophil activating (red). Likewise, 27% of patients (3 of 11) have anti-PF4/heparin antibodies that are neutrophil activating but not platelet activating (yellow). These studies demonstrate that although most functional anti-PF4/heparin antibodies are broadly cellular activating (able to elicit both platelet and neutrophil activation), cellular activation of platelets or neutrophils by ICs are likely distinct processes.
Platelet activation and neutrophil activation induced by anti-PF4/heparin ICs are distinct processes. (A) Most functional anti-PF4/heparin antibodies are capable of both platelet and neutrophil activation. Whole blood from a healthy donor was incubated with plasma from individual healthy donors (n = 16), plasma from patients with anti-PF4/heparin antibodies who tested negative in the PEA (n = 11), or with plasma from seropositive patients who were PEA positive (n = 16; all at 1:10 dilution), along with PF4 (25 µg/mL) and heparin (1 U/mL). After 30 minutes of incubation, MMP-9 release was measured in plasma. Data shown are representative of 3 independent experiments. *P < .05; ***P < .0005. Results are expressed as mean ± standard deviation. (B) Platelet activation and neutrophil activation are distinct processes. PEA results in patients with anti-PF4/heparin antibodies (n = 27) are plotted as a function of percent MMP-9 release (Spearman r = 0.42; P = .03). Patients who test positive in the PEA but negative in the MMP-9 assay are shown in red. Patients who test positive in the MMP-9 assay but negative in the PEA are shown in yellow. ns, not significant.
Platelet activation and neutrophil activation induced by anti-PF4/heparin ICs are distinct processes. (A) Most functional anti-PF4/heparin antibodies are capable of both platelet and neutrophil activation. Whole blood from a healthy donor was incubated with plasma from individual healthy donors (n = 16), plasma from patients with anti-PF4/heparin antibodies who tested negative in the PEA (n = 11), or with plasma from seropositive patients who were PEA positive (n = 16; all at 1:10 dilution), along with PF4 (25 µg/mL) and heparin (1 U/mL). After 30 minutes of incubation, MMP-9 release was measured in plasma. Data shown are representative of 3 independent experiments. *P < .05; ***P < .0005. Results are expressed as mean ± standard deviation. (B) Platelet activation and neutrophil activation are distinct processes. PEA results in patients with anti-PF4/heparin antibodies (n = 27) are plotted as a function of percent MMP-9 release (Spearman r = 0.42; P = .03). Patients who test positive in the PEA but negative in the MMP-9 assay are shown in red. Patients who test positive in the MMP-9 assay but negative in the PEA are shown in yellow. ns, not significant.
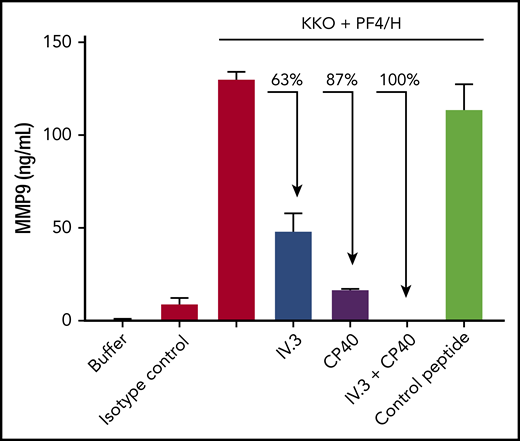
KKO-PF4/heparin–induced neutrophil activation is mediated by cellular FcγRIIa and complement
IC-specific degranulation requires incorporation of complement followed by binding to neutrophil Fcγ and complement receptors.24 To understand the mechanism of anti-PF4/heparin IC-induced neutrophil activation in our assay, we examined the role of FcγRIIa, which mediates platelet25 and neutrophil activation,26 and complement, which is activated by ICs.24 As shown in Figure 4, when whole blood is pre-incubated with IV.3 (a monoclonal antibody to human CD32 which blocks cellular FcγRIIa), there is a 63% decline in KKO-PF4/heparin–induced neutrophil degranulation. Similarly, when whole blood is pre-incubated with CP40 (a peptide inhibitor of C3),27 there is an 87% decline in KKO-PF4/heparin-induced MMP-9 release. When whole blood is pre-incubated with both IV.3 and CP40, there is complete inhibition of KKO-PF4/heparin–induced neutrophil degranulation, which demonstrates a synergistic effect of FcγRIIa and complement in neutrophil activation by HIT ICs.
KKO-PF4/heparin IC-induced neutrophil degranulation is mediated by FcγRII and complement. Whole blood was incubated with buffer, IgG2b isotype control, or KKO (25 µg/mL) along with PF4 (25 µg/mL) and heparin (H; 1 U/mL). Or whole blood was pre-incubated for 30 minutes with IV.3 (10 µg/mL) or CP40 (25 µM) alone or in combination, or with control peptide (25 µM) before the addition of KKO-PF4/heparin. After 30 minutes, release of MMP-9 was measured in plasma. Results are expressed as mean ± standard deviation values for triplicate wells. Data shown are representative of 3 independent experiments performed in whole blood from 3 healthy donors.
KKO-PF4/heparin IC-induced neutrophil degranulation is mediated by FcγRII and complement. Whole blood was incubated with buffer, IgG2b isotype control, or KKO (25 µg/mL) along with PF4 (25 µg/mL) and heparin (H; 1 U/mL). Or whole blood was pre-incubated for 30 minutes with IV.3 (10 µg/mL) or CP40 (25 µM) alone or in combination, or with control peptide (25 µM) before the addition of KKO-PF4/heparin. After 30 minutes, release of MMP-9 was measured in plasma. Results are expressed as mean ± standard deviation values for triplicate wells. Data shown are representative of 3 independent experiments performed in whole blood from 3 healthy donors.
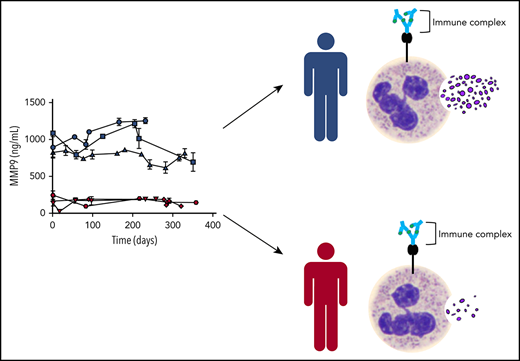
The neutrophil response to KKO-PF4/heparin ICs varies among healthy donors and represents a fixed phenotype
Knowing that heterogeneity in healthy donor neutrophil function exists,15,-17 we routinely tested whole blood from various donors to ensure that our observations were not the result of using samples from a single individual. Figure 5A depicts the aggregate results of 71 healthy donors in our MMP-9 assay. In these studies, minimal MMP-9 release was noted when whole blood was incubated with buffer, PF4/heparin, or isotype with PF4/heparin (Figure 5A; P = not significant between groups). In contrast, significant degranulation was seen with KKO-PF4/heparin ICs (P < .0001), with marked donor variability (range, 104.1-1166 ng/mL). To determine whether donor responses fluctuated over time, we identified several donors with extreme phenotypes (Figure 5A; high responders in blue defined as MMP-9 release >584.4 ng/mL [above the third quartile (Q3)] and low responders in red defined as <225.7 ng/mL [<Q1]) and examined their responses over time. As shown in Figure 5B, repeated testing over 1 year demonstrates that individuals have a fixed phenotype in response to KKO-PF4/heparin ICs: donors have neutrophils that have either persistently high reactivity to KKO-PF4/heparin ICs (blue) or persistently low reactivity (red). To determine whether this phenotype is specific only to MMP-9 or if the phenotype is representative of overall susceptibility to neutrophil degranulation, we tested samples from 10 representative high-reactivity patients (blue) and 10 representative low-reactivity patients (red) to determine whether their phenotype was retained with MPO (primary granule) or lactoferrin (secondary granule). As shown in Figure 5C, donors who released high amounts of MMP-9 in response to KKO-PF4/heparin ICs also released higher amounts of MPO and lactoferrin compared with low-reactivity donors. To determine whether this phenotype was generalizable to other ICs, we tested high- and low-reactivity donors and measured their responses to ADA-PRT/heparin ICs. As shown in Figure 5D, the neutrophil activation phenotype is preserved with ADA-PRT/heparin ICs. Finally, to determine whether the observed phenotype is a result of the presence of PF4 and/or heparin, neutrophil degranulation in response to heat-aggregated IgG was measured in high- and low-reactivity donors. As shown in Figure 5E, the neutrophil activation phenotype remains true with aggregated IgG. Together, these studies demonstrate that the observed neutrophil activation phenotype represents an individual’s susceptibility to IC-mediated degranulation, rather than a singular response to a specific antigen/antibody complex.
The neutrophil response to KKO-PF4/heparin ICs varies among individuals and represents a fixed phenotype. (A) The neutrophil response to anti-PF4/heparin ICs in healthy donors is heterogeneous. Whole blood from healthy donors (n = 71) was incubated with buffer, PF4/heparin (PF4 25 µg/mL; heparin [H] 1 U/mL) alone or in the presence of KKO or isotype control (Iso, 25 µg/mL), and MMP-9 was measured in plasma. Median and interquartile range are plotted, with individuals >Q3 depicted in blue and individuals <Q1 in red. (B) The neutrophil response to anti-PF4/heparin ICs represents a fixed phenotype. Whole blood from 3 healthy donors who had been designated as high responders (blue) and from 3 donors who had been designated as low responders (red) were repeatedly sampled over a course of 358 days. Whole blood from all donors was incubated with KKO-PF4/heparin ICs, and released MMP-9 was measured in plasma. Results are expressed as mean ± standard deviation values for triplicate wells. (C) The neutrophil activation phenotype is consistent among all 3 neutrophil granule populations. Samples from 10 representative high responders (blue) and 10 representative low responders (red) from panel A were tested for MPO (top) or lactoferrin (bottom) release. (D) The neutrophil activation phenotype is preserved with ADA-PRT/heparin ICs. Whole blood from a designated high responder and a low responder was incubated with ADA (50 µg/mL) along with protamine (25 µg/mL) and varying amounts of UFH (0-100 U/mL). After 30 minutes, MMP-9 release was measured in plasma. Data are representative of 3 high-responding and 3 low-responding donors. Results are expressed as mean ± standard deviation values for triplicate wells. (E) The neutrophil activation phenotype is preserved with heat-aggregated (agg) IgG. Whole blood from 3 representative high responders (blue) and 3 representative low responders (red) was incubated with buffer or heat-aggregated IgG (100 µg/mL). After 3 hours of incubation, MMP-9 release was measured in plasma.
The neutrophil response to KKO-PF4/heparin ICs varies among individuals and represents a fixed phenotype. (A) The neutrophil response to anti-PF4/heparin ICs in healthy donors is heterogeneous. Whole blood from healthy donors (n = 71) was incubated with buffer, PF4/heparin (PF4 25 µg/mL; heparin [H] 1 U/mL) alone or in the presence of KKO or isotype control (Iso, 25 µg/mL), and MMP-9 was measured in plasma. Median and interquartile range are plotted, with individuals >Q3 depicted in blue and individuals <Q1 in red. (B) The neutrophil response to anti-PF4/heparin ICs represents a fixed phenotype. Whole blood from 3 healthy donors who had been designated as high responders (blue) and from 3 donors who had been designated as low responders (red) were repeatedly sampled over a course of 358 days. Whole blood from all donors was incubated with KKO-PF4/heparin ICs, and released MMP-9 was measured in plasma. Results are expressed as mean ± standard deviation values for triplicate wells. (C) The neutrophil activation phenotype is consistent among all 3 neutrophil granule populations. Samples from 10 representative high responders (blue) and 10 representative low responders (red) from panel A were tested for MPO (top) or lactoferrin (bottom) release. (D) The neutrophil activation phenotype is preserved with ADA-PRT/heparin ICs. Whole blood from a designated high responder and a low responder was incubated with ADA (50 µg/mL) along with protamine (25 µg/mL) and varying amounts of UFH (0-100 U/mL). After 30 minutes, MMP-9 release was measured in plasma. Data are representative of 3 high-responding and 3 low-responding donors. Results are expressed as mean ± standard deviation values for triplicate wells. (E) The neutrophil activation phenotype is preserved with heat-aggregated (agg) IgG. Whole blood from 3 representative high responders (blue) and 3 representative low responders (red) was incubated with buffer or heat-aggregated IgG (100 µg/mL). After 3 hours of incubation, MMP-9 release was measured in plasma.
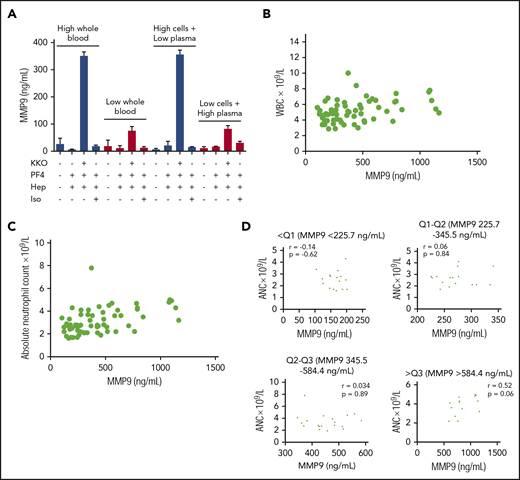
Neutrophil activation phenotype is determined, in part, by neutrophil count
Building on the observation that anti-PF4/heparin ICs induce degranulation via cellular FcγRIIa and complement, we next examined the contribution of cellular and plasma components to the activation phenotype. For this, mixing studies of ABO-compatible high- and low-reactivity donors were performed using the plasma fraction from a high-reactivity donor with the cellular fraction from a low-reactivity donor and vice-versa. Mixed plasma and cellular fractions were incubated with KKO-PF4/heparin ICs. As shown in Figure 6A, the neutrophil activation phenotype is defined primarily by the cellular fraction for each donor. Next, we determined whether the neutrophil activation phenotype correlates with host variables, including white blood cell count (WBC) and absolute neutrophil count (ANC). As shown in Figure 6B-C, WBC and ANC obtained on the day of testing are generally correlated with MMP-9 release (WBC: r = 0.39; P = .001; ANC: r = 0.49; P < .0001). However, the ANC does not fully explain an individual’s susceptibility to neutrophil activation by ICs. As shown in Figure 6D, when healthy donors are separated into quartiles based on reactivity to KKO-PF4/heparin ICs, there is no correlation between ANC and MMP-9 release (<Q1, P = .62; Q1-Q2, P = .84; Q2-Q3, P = .89; >Q3, P = .06). Likewise, host age, sex, and race do not correlate with MMP-9 release (data not shown). Finally, KKO-PF4/heparin–induced MMP-9 release was not affected by polymorphisms in donor FcγRIIa or in FcγRIIIb (supplemental Table 1) or by surface CR1 and CR3 expression (data not shown). Together, these studies demonstrate that although neutrophils are the source of MMP-9, additional genetic determinants likely contribute to the observed activation phenotype.
The degree of neutrophil activation induced by anti-PF4/heparin ICs is determined, in part, by neutrophil count. (A) The KKO-PF4/heparin IC activation phenotype is determined in the cellular fraction. Whole blood from an established high-reactivity donor and an established ABO-compatible low-reactivity donor was separated into their cellular and plasma fractions by centrifugation. After mixing the cellular and plasma fractions from high- and low-reactivity donors, reconstituted blood was incubated with buffer, PF4 (25 µg/mL), heparin (1 U/mL) alone or in the presence of KKO or isotype control (Iso, 25 µg/mL). MMP-9 release from reconstituted blood was compared with unmanipulated whole blood from the 2 donors. Results are expressed as mean ± standard deviation values for triplicate wells. (B) MMP-9 release in healthy donors is correlated with WBC count. WBCs from healthy donors (n = 71) is plotted as a function of KKO-PF4/heparin IC-induced MMP-9 release (Spearman r = 0.39; P = .001). (C) MMP-9 release in healthy donors is correlated with ANC. ANC in healthy donors (n = 71) is plotted as a function of KKO-PF4/heparin IC-induced MMP-9 release (Spearman r = 0.49; P < .0001). (D) Within healthy donor quartiles, MMP-9 release does not correlate with ANC. Healthy donors were divided into quartiles based on their KKO-PF4/heparin IC neutrophil response (as shown in Figure 5A). Within each quartile, ANC was plotted as a function of MMP-9. P = ns for all groups.
The degree of neutrophil activation induced by anti-PF4/heparin ICs is determined, in part, by neutrophil count. (A) The KKO-PF4/heparin IC activation phenotype is determined in the cellular fraction. Whole blood from an established high-reactivity donor and an established ABO-compatible low-reactivity donor was separated into their cellular and plasma fractions by centrifugation. After mixing the cellular and plasma fractions from high- and low-reactivity donors, reconstituted blood was incubated with buffer, PF4 (25 µg/mL), heparin (1 U/mL) alone or in the presence of KKO or isotype control (Iso, 25 µg/mL). MMP-9 release from reconstituted blood was compared with unmanipulated whole blood from the 2 donors. Results are expressed as mean ± standard deviation values for triplicate wells. (B) MMP-9 release in healthy donors is correlated with WBC count. WBCs from healthy donors (n = 71) is plotted as a function of KKO-PF4/heparin IC-induced MMP-9 release (Spearman r = 0.39; P = .001). (C) MMP-9 release in healthy donors is correlated with ANC. ANC in healthy donors (n = 71) is plotted as a function of KKO-PF4/heparin IC-induced MMP-9 release (Spearman r = 0.49; P < .0001). (D) Within healthy donor quartiles, MMP-9 release does not correlate with ANC. Healthy donors were divided into quartiles based on their KKO-PF4/heparin IC neutrophil response (as shown in Figure 5A). Within each quartile, ANC was plotted as a function of MMP-9. P = ns for all groups.
Discussion
It is well established that ICs can elicit an array of neutrophil responses, including oxidative burst activity,28 phagocytosis,29 antibody-dependent cellular cytotoxicity,30 and degranulation with enzyme release.24 Previous studies using ICs composed of aggregated myeloma Ig’s31,32 or antigen-specific antibodies (eg, 5-iodo-4-hydroxy-3-nitrophenacetyl [NIP] and anti-NIP antibodies24,33 or human albumin and anti-albumin antibodies28 ) showed that specific features of an IC, such as antibody class or subclass, antigen:antibody ratio, ability to fix complement, and antigen epitope density, determine which neutrophil receptor(s) will be engaged and the nature of the neutrophil response. For example, to induce degranulation, IgG2 and IgG4 ICs require complement and bind to neutrophils predominantly via complement receptors (CR1 and CR3), whereas IgG1 and IgG3 bind primarily via Fc receptors (CD16 and CD32).24 The process of degranulation is also subclass-dependent. IgG3 ICs induce degranulation primarily via CD16 whereas ICs of the remaining subclasses induce degranulation primarily via CD32.24 Importantly, most previous studies have focused on defining the characteristics of a given IC which determine its ability to induce degranulation. To date, few studies have examined host characteristics which determine susceptibility to IC-induced degranulation.
The ability to identify individuals at higher risk for IC-induced neutrophil degranulation is important because neutrophil activation and degranulation contribute to disease pathogenicity. When neutrophils degranulate, they release proinflammatory components (eg, reactive oxygen species, leukotrienes, serine proteases, cytokines). These components can directly mediate tissue or vascular damage and can alter the activation status of platelets and/or endothelial cells.34 Indeed, neutrophil degranulation is used as an index of disease activity in many inflammatory disorders, such as rheumatoid arthritis,35 asthma, and chronic obstructive pulmonary disease,36 in which excessive degranulation is a characteristic of more severe disease.37 In prothrombotic disorders such as stroke and myocardial infarction, evidence of primary granule release in plasma is associated with higher morbidity and mortality.38 In recent studies, neutrophil degranulation has been shown to promote thrombosis by accelerating fibrin polymerization, increasing fibrin density within thrombi, and impairing fibrinolysis.39 Specific to HIT, Khairy et al,12 found that patients with HIT had elevated plasma MPO concentrations compared with healthy participants, patients with non-immune HIT, or patients with venous or arterial thrombosis, suggesting that degranulation may serve as a biomarker for HIT as well. However, these investigators did not measure the neutrophil-activating properties of anti-PF4/heparin antibodies ex vivo.
In this article, we describe a whole blood immunoassay that quantifies IC-mediated neutrophil degranulation to examine heterogeneity in responses among healthy donors. Using both a monoclonal anti-PF4/heparin antibody (Figure 1), polyclonal, patient-derived antibodies (Figure 2), and a monoclonal anti-PRT/heparin antibody (Figure 1D), we demonstrate that in a whole blood environment, these antibody-antigen complexes induce robust, IC-dependent neutrophil activation. We know that size and antigen:antibody ratio are important in determining the capability of an IC to activate neutrophils,28 and the ability of these heparin-containing ICs to induce neutrophil activation is likely due, in part, to their ability to form ULCs.23,40 This is consistent with the observation that neutrophil activation in our assay occurs over a narrow molar range of PF4 or PRT to heparin, which coincides with ULC formation23 (Figures 1C-D and 2C). This functional property is unlike single antigen-antibody complexes, such as those seen with monoclonal anti-PF4/PF4, polyclonal anti-PF4/PF4, or anti-ova/ova complexes which do not generate the larger molecular structures41 needed to cluster FcR, and consequently do not induce degranulation in our assay (data not shown). Our finding that anti-PF4/PF4 ICs are unable to induce degranulation is in contrast to a previous study by Xiao et al13 that demonstrated robust tertiary and secondary granule release in response to anti-PF4/PF4 ICs. However, it is important to note that limited comparisons can be made between the assay described by Xiao et al,13 which uses isolated neutrophils collected in EDTA, and our assay, which uses whole blood. In fact, our assay is exquisitely sensitive to EDTA, and collection of whole blood in EDTA completely abolishes neutrophil activation in our whole blood system, highlighting the critical role of divalent cations and complement in our system (data not shown). Nevertheless, to ensure that our observations are not a result of the presence of PF4 and/or heparin, neutrophil degranulation assays were performed with heat-aggregated IgG. As shown in Figure 5, differences in neutrophil reactivity remain constant regardless of the immune complex tested. Neutrophil activation in our whole blood assay is dependent on both FcγRIIa and complement (Figure 4), which is consistent with previous studies that demonstrate that antigen-antibody ICs incorporate complement and then bind to specific Fcγ and complement receptors to induce degranulation.24 These findings suggest that complement inhibition could be an effective therapeutic strategy in HIT, not only to inhibit neutrophil activation, but possibly to target other cellular effectors.
We used our assay to test a cohort of healthy donors over 1 year and demonstrated that although the degree of neutrophil degranulation in response to ICs is very heterogeneous, the response represents a fixed phenotype for a given individual (Figure 5B). In other words, some individuals have neutrophils that are always highly activated by ICs whereas others have neutrophils with persistently low reactivity. This phenotype is not determined by donor characteristics such as age, sex, and race. And although IC-mediated degranulation occurs through complement and Fc receptors, this phenotype is not determined by polymorphisms in FcγRIIa or FcγRIIIb (supplemental Table 1) or by quantitative differences in surface CR1, CR3, FcγRIIa, or FcγRIIIb (data not shown). As expected, the neutrophil activation phenotype is correlated, to a degree, with both total WBC and ANC (Figure 6B-C). This is consistent with observations from other diseases, including myeloproliferative neoplasms and sickle cell disease, in which leukocytosis is consistently linked to adverse outcomes such as thrombosis,42 cerebral infarction,43 and increased mortality.44,45 However, specific to our study, the ANC is not the sole determinant of an individual’s susceptibility to neutrophil activation by ICs. When healthy donors are subdivided into quartiles on the basis of KKO-PF4/heparin–induced MMP-9 release (Figure 5A), ANC does not correlate with MMP-9 (Figure 6D), indicating that other variables must contribute to the observed phenotype. At this time, the determinants of the neutrophil activation phenotype are unknown. Additional studies are needed to determine the underlying factor(s) behind the heterogeneous neutrophil response and to determine the exact contribution of each cellular component (eg, monocytes and lymphocytes). Furthermore, the precise role of complement, which is incorporated into antigen-antibody ICs during the process of degranulation,24 remains to be determined.
Our finding that individuals have a fixed response to ICs raises the idea of using the neutrophil activation phenotype to define patients with IC-mediated diseases who are at high risk for inflammatory or thrombotic outcomes. This concept of using variants in neutrophil function to identify patients at risk for disease is not new. For example, multiple single nucleotide polymorphisms have been found at the ITGAM locus that encode the α subunit of CR3, resulting in altered Fc receptor–mediated function, including decreased neutrophil adhesion and reduced phagocytosis. Individuals with these single nucleotide polymorphisms and altered neutrophil function are more susceptible to systemic lupus erythematosus.46 Similarly, our study noted marked heterogeneity in IC-mediated neutrophil degranulation. The full impact of this variability in neutrophil activation is currently unknown. However, in IC-mediated, prothrombotic disorders such as HIT, neutrophil activation has been firmly linked to pathology. Specific to HIT, neutrophil adhesion is critical during the initial phase of thrombosis. When neutrophils are recruited to the endothelial surface,47 neutrophil-platelet aggregate formation contributes to arterial thrombi,12 and NET release propagates arterial and venous thrombosis.14,48 Our findings suggest that the neutrophil activation phenotype could serve as a biomarker to identify patients at risk for IC-mediated thrombosis or other adverse outcomes, but this hypothesis will require future, prospective studies. Likewise, the possibility of using this assay to determine the cellular-activating properties of other antigen-antibody ICs remains to be determined (eg, such as in thrombotic thrombocytopenic purpura and APS, in which antibody-induced neutrophil activation contributes to pathophysiology of disease49,50 ).
Although our MMP-9 assay identifies most functional anti-PF4/heparin antibodies (Figure 3A), at this time, it is not a viable alternative to existing functional assays because of limited sensitivity and specificity. The logistic regression of our MMP-9 assay for predicting PEA results has an area under the curve of 74%. As provided by this model, the sensitivity (0.81) and specificity (0.73) are maximum when MMP-9 release is ≥11.7%. Although the MMP-9 and PEA results correlate well (Figure 3B; r = 0.42; P = .03), when using this cutoff, many patients test positive in the PEA but negative in the MMP-9 assay and vice versa. Further analysis reveals no significant difference between patients who tested PEA-positive/MMP-9–negative (n = 4) and patients who tested PEA-negative/MMP-9–positive (n = 3) in regard to age, sex, WBC count, platelet count, anti-PF4/heparin results in a polyspecific IgG/IgA/IgM enzyme-linked immunosorbent assay (ELISA) and in an IgG-specific ELISA, circulating and endogenous MMP-9 levels, 4T score, and thrombosis (data not shown). Although our assay can identify many patients with broadly cellular-activating antibodies, platelet and neutrophil activation remain 2 distinct processes. This may simply be a result of differences in cell surface receptors and engagement by ICs. For example, IC-mediated degranulation occurs via a combination of complement (CR1 and CR3) and Fc receptors (CD16 and CD32).24 In contrast, ICs activate platelets via CD32 (the sole Fc receptor on platelets), which acts synergistically with receptors for C1q51 and C5a.52 It is possible that some ICs, whether because of antigenic epitope density or because of the distribution of antibody isotypes for a given patient,24 bind preferentially to neutrophil-specific cell surface receptors. Consistent with this line of thought, we demonstrate that patients can have anti-PF4/heparin antibodies that induce neutrophil activation but not platelet activation and vice versa (Figure 3B). The reason for this biologic discrepancy is currently unknown, but it is consistent with emerging evidence that HIT ICs can directly activate neutrophils without requiring platelet activation.38 In a recent study,38 neutrophil activation with subsequent NET release was the primary driver of thrombosis in HIT, and this process was clearly distinct from the development of thrombocytopenia, which was mediated by HIT ICs binding to platelets.53 Specific to our study, full characterization of our cohorts will determine the significance of this variability in antibody function and will also determine whether dual positivity (anti-PF4/heparin antibodies that induce both platelet and neutrophil activation) predicts for adverse outcomes.
In summary, we describe a novel whole blood assay that directly measures IC-induced neutrophil activation and degranulation. In addition, we demonstrate that susceptibility to neutrophil activation by ICs is an inherent characteristic for a given individual. In doing so, we not only confirm the broad cellular-activating properties of anti-PF4/heparin antibodies but also provide a possible explanation for the variability in clinical outcomes seen in patients with IC-mediated diseases.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank John D. Lambris and Edimara S. Reis for providing CP40.
This work was supported by a National Institutes of Health, National Heart, Lung, and Blood Institute grant (K08HL127183) (G.M.L.).
Authorship
Contribution: G.M.L., M.D., G.M.A., S.K., and M.K. conceived of and designed the study, collected and assembled the data, analyzed and interpreted the data, wrote the manuscript, and gave final approval of the manuscript; and G.M.L. and G.M.A. provided study materials or patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Grace M. Lee, Division of Hematology, Duke University Medical Center, DUMC Box 3486, Room 356, Alex H. Sands Building, Research Dr, Durham, NC 27710; e-mail: grace.lee@duke.edu.


![The neutrophil response to KKO-PF4/heparin ICs varies among individuals and represents a fixed phenotype. (A) The neutrophil response to anti-PF4/heparin ICs in healthy donors is heterogeneous. Whole blood from healthy donors (n = 71) was incubated with buffer, PF4/heparin (PF4 25 µg/mL; heparin [H] 1 U/mL) alone or in the presence of KKO or isotype control (Iso, 25 µg/mL), and MMP-9 was measured in plasma. Median and interquartile range are plotted, with individuals >Q3 depicted in blue and individuals <Q1 in red. (B) The neutrophil response to anti-PF4/heparin ICs represents a fixed phenotype. Whole blood from 3 healthy donors who had been designated as high responders (blue) and from 3 donors who had been designated as low responders (red) were repeatedly sampled over a course of 358 days. Whole blood from all donors was incubated with KKO-PF4/heparin ICs, and released MMP-9 was measured in plasma. Results are expressed as mean ± standard deviation values for triplicate wells. (C) The neutrophil activation phenotype is consistent among all 3 neutrophil granule populations. Samples from 10 representative high responders (blue) and 10 representative low responders (red) from panel A were tested for MPO (top) or lactoferrin (bottom) release. (D) The neutrophil activation phenotype is preserved with ADA-PRT/heparin ICs. Whole blood from a designated high responder and a low responder was incubated with ADA (50 µg/mL) along with protamine (25 µg/mL) and varying amounts of UFH (0-100 U/mL). After 30 minutes, MMP-9 release was measured in plasma. Data are representative of 3 high-responding and 3 low-responding donors. Results are expressed as mean ± standard deviation values for triplicate wells. (E) The neutrophil activation phenotype is preserved with heat-aggregated (agg) IgG. Whole blood from 3 representative high responders (blue) and 3 representative low responders (red) was incubated with buffer or heat-aggregated IgG (100 µg/mL). After 3 hours of incubation, MMP-9 release was measured in plasma.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/3/19/10.1182_bloodadvances.2019000235/3/m_advancesadv2019000235f5.png?Expires=1767738547&Signature=AEtlKBdfy1grhNeYes3PoCnn1aBKs2wEBxQjity2BUN~nOH71DyK0jZYYCgZyKRmjIftMKL487GluuhYBXlaAbYqu-YincBuXKusACPSgfQFPLQ6IcY~Ut9~nCHPjMtxAEzcOg0tRGPnjrAnaWw22IQ5TLOAOCOySe4Sek9p65y8ubzGUQRa-BPhilRT0qfQ3jSTbctn-GcEci6tKqhdpT8fMb5FdcIw8YT9GOM6g6-9urzmNED0KCrCxz5pz9ulAMaxtlBNDB9vfTmHTCHYKL40oFN-Wb7g~zVGcL2St6e4wNeZ4b~CwU3Z30NnSeDNVNIS2zEVIev7opu63csMNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)