Key Points
TLI-ATG allows for outpatient allogeneic transplantation with a low risk of GVHD and NRM in patients ineligible for more intensive regimens.
Durable remissions were seen across various hematologic malignancies, with particularly favorable outcomes for heavily pretreated lymphomas.
Abstract
Nonmyeloablative total lymphoid irradiation and antithymocyte globulin (TLI-ATG) conditioning is protective against graft-versus-host disease (GVHD), while retaining graft-versus-tumor activity across various hematologic malignancies. We report our comprehensive experience using TLI-ATG conditioning in 612 patients with hematologic malignancies who underwent allogeneic transplantation at Stanford University from 2001 to 2016. All patients received granulocyte colony-stimulating factor–mobilized peripheral blood grafts and cyclosporine and mycophenolate mofetil for GVHD prophylaxis. The median age was 60 years (range, 21-78), with a median follow-up of 6.0 years (range, 1.0-16.4). Common diagnoses included acute myeloid leukemia (AML; n = 193), myelodysplastic syndrome (MDS; n = 94), chronic lymphocytic leukemia (CLL; n = 80), non-Hodgkin lymphoma (NHL; n = 175), and Hodgkin lymphoma (HL; n = 35). Thirty-four percent of patients had a comorbidity index ≥3, 30% had a high to very high disease risk index, and 56% received unrelated donor grafts, including 15% with HLA-mismatched donors. Ninety-eight percent underwent transplant in the outpatient setting, and 57% were never hospitalized from days 0 through 100. The 1-year rates of nonrelapse mortality (NRM), grade II-IV acute GVHD, and extensive chronic GVHD were 9%, 14%, and 22%, respectively. The 4-year estimates for overall and progression-free survival were 42% and 32% for AML, 30% and 21% for MDS, 67% and 43% for CLL, 68% and 45% for NHL, and 78% and 49% for HL. Mixed chimerism correlated with the risk of relapse. TLI-ATG conditioning was well tolerated, with low rates of GVHD and NRM. Durable remissions were observed across hematologic malignancies, with particularly favorable outcomes for heavily pretreated lymphomas. Several efforts are underway to augment donor chimerism and reduce relapse rates while maintaining the favorable safety and tolerability profile of this regimen.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for high-risk or refractory hematologic malignancies, but many patients are not eligible for myeloablative conditioning because of advanced age or comorbidities. Reduced-intensity conditioning (RIC) regimens have expanded the population of patients eligible to undergo allogeneic HCT, but are limited by the risk of disease relapse and nonrelapse mortality (NRM), most commonly due to graft-versus-host disease (GVHD). Prior studies using fludarabine-based RIC regimens have demonstrated rates of acute GVHD (grades II-IV) of 20% to 60%, chronic GVHD of 30% to 70%, and NRM of 5% to 30% at 1 year, with significant variation based on patient age, comorbidities, and donor source.1-5 In contrast to RIC, nonmyeloablative regimens are even less intensive, do not require donor stem cell support to mitigate cytopenias, and rely primarily on the graft-versus-tumor (GVT) effect for tumor eradication and disease control.6-8 Nonmyeloablative regimens allow for further reduction in early toxicity and NRM at the expense of a higher risk of relapse and may be desirable for older, frailer patients ineligible for more intensive regimens.9
Our group previously developed a nonmyeloablative conditioning regimen of total lymphoid irradiation and antithymocyte globulin (TLI-ATG) based on murine studies that demonstrated a protective effect against GVHD.10,11 In the murine model, TLI-ATG conditioning skewed residual host T-cell subsets to favor invariant natural killer T (NKT) cells.12,13 Numerous studies have demonstrated that invariant NKT cells are protective against GVHD, mediated by their production of interleukin-4, which polarizes donor T cells toward a T helper 2 cell phenotype and promotes expansion of regulatory T cells.14-19 TLI-ATG conditioning was designed for patients who are ineligible for more intensive regimens because of advanced age or comorbidities or who are unlikely to benefit from additional high-dose cytotoxic therapy because of chemorefractory disease, including failure of prior autologous HCT.
Collectively, prior studies by our group and others, using TLI-ATG, have demonstrated a favorable safety profile with a low risk at 1 year of acute GVHD (grade II-IV) of 2% to 13%, chronic GVHD of 18% to 36%, and NRM of 3% to 9%.10,11,20,21 Despite the low risk of GVHD, GVT activity is evident, with durable remissions observed in patients with acute and chronic leukemias,10,11 myelodysplastic syndrome (MDS), myeloproliferative neoplasms,22 and Hodgkin (HL) and non-Hodgkin (NHLs) lymphomas after failure of autologous HCT.23,24 In a randomized phase II trial from the Belgian Hematological Society comparing TLI-ATG to low-dose total body irradiation (TBI, 2 Gy) and fludarabine, TLI-ATG was associated with a lower risk of GVHD and NRM and a higher risk of relapse, leading to equivalent overall survival (OS) at 4 years.21 Herein, we report our single-center experience using TLI-ATG conditioning in a large cohort of patients (N = 612) with hematologic malignancies, who underwent transplant over a 15-year period. These data allow for a comprehensive assessment of the advantages and limitations of TLI-ATG and identification of subgroups that may derive the greatest benefit from this regimen.
Methods
Patients
We included all consecutive patients who underwent transplant at Stanford University and received TLI-ATG conditioning and allogeneic HCT from 1 December 2001 through 31 December 2016 for a hematologic malignancy, excluding patients with cutaneous T-cell lymphomas and multiple myeloma who were treated on separate protocols. All patients provided informed consent in accordance with the Declaration of Helsinki and were enrolled in transplant protocols approved by the Stanford University Institutional Review Board or on treatment plans. The censoring date was the last clinic visit before 30 June 2018, allowing for a minimum follow-up greater than 1 year for all living patients. Patients selected for this regimen were either ineligible for or unlikely to benefit from myeloablative conditioning, because of advanced age, comorbidities, or chemorefractory disease, including failure of autologous HCT. Patients with a Karnofsky performance status <50%, left ventricular ejection fraction <30%, pulmonary diffusion capacity <35%, or decompensated cirrhosis or who were pregnant were not eligible.
Transplant regimen
TLI-ATG conditioning was administered as previously described.10,11 In brief, rabbit ATG (Sanofi Genzyme, Boston, MA) was infused at 1.5 mg/kg for 5 consecutive days, beginning on day −11 before HCT. TLI was administered daily in 0.8-Gy fractions for 8 doses between days −11 and −7 and days −4 and −2, and 2 additional 0.8-Gy fractions were administered on day −1, for a total dose of 8 Gy. In accordance with a protocol amendment, patients treated after 31 May 2009 received 1.2-Gy fractions, scheduled as above, for a total dose of 12 Gy, in an effort to improve engraftment and chimerism. The radiation fields used have been described.10 All patients received granulocyte colony-stimulating factor–mobilized peripheral blood stem cells on day 0 and cyclosporine and mycophenolate mofetil for GVHD prophylaxis. In the absence of GVHD, cyclosporine was tapered to discontinuation between days 100 and 180. Mycophenolate mofetil was discontinued at day 28 in patients with HLA-matched related donors (MRDs) and was tapered between days 42 and 96 for patients with unrelated donors. All patients were monitored for cytomegalovirus (CMV) and Epstein-Barr virus viremia with serum polymerase chain reaction assays, and all patients received antimicrobial prophylaxis according to institutional guidelines. CMV and Epstein-Barr virus reactivation were treated according to institutional standards.
Evaluation of donor chimerism
DNA genotyping of polymorphic markers encoding short tandem repeats was used to quantify donor chimerism at days +30, +60, +90, +180, and +365. Donor chimerism was evaluated in whole blood and cell subsets by using immunomagnetic beads (Dynal Biotech/Invitrogen, Waltham, MA) coated with monoclonal antibodies against CD3, CD15, CD19, and CD56. Full donor chimerism was defined as achievement of ≥95% CD3+ cells in the peripheral blood by day +180. Primary graft failure was defined as failure to attain 5% donor CD3+ cells, and mixed chimerism was defined as donor CD3+ cells ranging from 5% to 94%.25 Donor lymphocyte infusions (DLIs) were given at the discretion of the attending physician, to treat disease relapse, but were not used to convert mixed chimerism to full donor chimerism in the absence of disease progression.
Study end points
The study end points included OS, progression-free survival (PFS), GVHD-free/relapse-free survival (GRFS), and the cumulative incidences of disease relapse, NRM, and acute and chronic GVHD. OS was defined as the time to death from any cause after transplant, and PFS was defined as the first observation of relapse, progression, or death. GRFS was defined as the first observation of relapse, progression, death, grade III-IV acute GVHD, or chronic GVHD requiring immunosuppression.26 Relapse included progressive disease in patients with measurable disease at the time of transplant. NRM was defined as death from any cause other than disease progression or relapse. Acute and chronic GVHD were graded according to consensus criteria.27,28
Statistical analysis
For time-to-event analyses, the Kaplan-Meier method was used to estimate the probabilities of OS, PFS, and GRFS. The cumulative incidences of relapse, NRM, and acute and chronic GVHD were estimated by the competing risk method. Relapse was treated as a competing risk for NRM, and death was treated as a competing risk for relapse. Log-rank tests were used to detect differences between groups. The Fisher exact test was used to detect differences in chimerism distributions between patients who relapsed or never relapsed. Cox regression analysis was used to identify factors associated with OS, and the Fine-Gray method was used to identify factors associated with NRM and relapse. All P values are 2-tailed, with values of P < .05 considered significant. Statistical analyses were performed using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
All patients (N = 612) underwent allogeneic HCT with TLI-ATG conditioning at a median age of 60 years (range, 21-78 years), with a median follow-up of 6.0 years (range, 1.0-16.4) for all living patients (Table 1). Of those patients, 341 have been reported in prior publications, but with limited follow-up.10,11,22-24,29 Diagnoses included acute myeloid leukemia (AML; n = 193), MDS (n = 94), myelofibrosis (n = 9), chronic myeloid leukemia (CML; n = 12), acute lymphoblastic leukemia (ALL; n = 14), chronic lymphocytic leukemia (CLL; n = 80), NHL (n = 175), and HL (n = 35). Forty-four percent of patients had HLA-MRDs, 41% had HLA-matched unrelated donors (MUDs; matching at 10 of 10 alleles), and 15% had partially HLA-mismatched unrelated donors (MMUDs), matching at 8 to 9 of 10 alleles (including 3% mismatched at HLA-DQB1 only). Thirty-four percent of patients had an HCT comorbidity index (HCT-CI) ≥ 3,30 and 30% had a high or very high disease risk index (DRI).31 Of 210 lymphoma patients, 116 (55%) had failure of prior autologous HCT.
Patient and disease characteristics
| Characteristic . | Data (N = 612) . |
|---|---|
| Age and follow-up, median (range), y | |
| Age at time of transplant | 60 (21-78) |
| Follow-up | 6.0 (1.0-16.4) |
| Diagnosis, n (%) | |
| AML | 193 (32) |
| De novo AML | 139 (23) |
| Secondary AML* | 54 (9) |
| MDS† | 94 (15) |
| Myelofibrosis | 9 (1) |
| CML | 12 (2) |
| ALL | 14 (2) |
| CLL | 80 (13) |
| NHL | 175 (29) |
| Diffuse large B-cell lymphoma | 66 (11) |
| Mantle cell lymphoma | 53 (9) |
| Follicular lymphoma | 22 (4) |
| Other B-cell lymphoma‡ | 12 (2) |
| T-cell lymphoma§ | 22 (4) |
| HL | 35 (6) |
| Lymphoma with prior autologous HCT | 116/210 (55) |
| Donor, n (%) | |
| HLA-MRD | 271 (44) |
| HLA-MUD | 250 (41) |
| HLA-MMUD | 89 (15) |
| HLA-MMRD | 2 (0.3) |
| DRI, n (%) | |
| Low risk | 140 (23) |
| Intermediate risk | 289 (47) |
| High risk | 167 (27) |
| Very high risk | 16 (3) |
| CI, n (%) | |
| HCT-CI 0 | 178 (29) |
| HCT-CI 1 | 112 (18) |
| HCT-CI 2 | 113 (18) |
| HCT-CI 3 | 104 (17) |
| HCT-CI ≥4 | 105 (17) |
| Donor and recipient sex, n (%) | |
| Male donor, male recipient | 195 (32) |
| Male donor, female recipient | 143 (23) |
| Female donor, male recipient | 161 (26) |
| Female donor, female recipient | 113 (18) |
| CMV serologic status, n (%) | |
| Donor and/or recipient seropositive | 444 (73) |
| Donor and recipient seronegative | 168 (27) |
| Characteristic . | Data (N = 612) . |
|---|---|
| Age and follow-up, median (range), y | |
| Age at time of transplant | 60 (21-78) |
| Follow-up | 6.0 (1.0-16.4) |
| Diagnosis, n (%) | |
| AML | 193 (32) |
| De novo AML | 139 (23) |
| Secondary AML* | 54 (9) |
| MDS† | 94 (15) |
| Myelofibrosis | 9 (1) |
| CML | 12 (2) |
| ALL | 14 (2) |
| CLL | 80 (13) |
| NHL | 175 (29) |
| Diffuse large B-cell lymphoma | 66 (11) |
| Mantle cell lymphoma | 53 (9) |
| Follicular lymphoma | 22 (4) |
| Other B-cell lymphoma‡ | 12 (2) |
| T-cell lymphoma§ | 22 (4) |
| HL | 35 (6) |
| Lymphoma with prior autologous HCT | 116/210 (55) |
| Donor, n (%) | |
| HLA-MRD | 271 (44) |
| HLA-MUD | 250 (41) |
| HLA-MMUD | 89 (15) |
| HLA-MMRD | 2 (0.3) |
| DRI, n (%) | |
| Low risk | 140 (23) |
| Intermediate risk | 289 (47) |
| High risk | 167 (27) |
| Very high risk | 16 (3) |
| CI, n (%) | |
| HCT-CI 0 | 178 (29) |
| HCT-CI 1 | 112 (18) |
| HCT-CI 2 | 113 (18) |
| HCT-CI 3 | 104 (17) |
| HCT-CI ≥4 | 105 (17) |
| Donor and recipient sex, n (%) | |
| Male donor, male recipient | 195 (32) |
| Male donor, female recipient | 143 (23) |
| Female donor, male recipient | 161 (26) |
| Female donor, female recipient | 113 (18) |
| CMV serologic status, n (%) | |
| Donor and/or recipient seropositive | 444 (73) |
| Donor and recipient seronegative | 168 (27) |
MMRD, mismatched related donor.
Patients with an antecedent myeloid malignancy or therapy-related myeloid neoplasm.
Includes patients with chronic myelomonocytic leukemia (n = 4) and MDS/myeloproliferative neoplasm unclassifiable (n = 2).
Includes patients with marginal zone lymphoma (n = 7) and lymphoplasmacytic lymphoma (n = 5).
Includes patients with angioimmunoblastic T-cell lymphoma (n = 8), peripheral T-cell lymphoma not otherwise specified (n = 6), anaplastic large cell lymphoma (n = 5), subcutaneous panniculitis-like T-cell lymphoma (n = 2), and extranodal NKT-cell lymphoma (n = 1).
Engraftment and chimerism
Durable donor hematopoietic cell engraftment occurred in 95% of patients, whereas graft failure or rejection occurred in 5% of patients and varied by donor source (3%, 7%, and 8% for MRDs, MUDs, and MMUDs, respectively). On days +30, +90, +180, and +365, the median proportions of donor-derived CD3+ cells in the blood were 87%, 92%, 98%, and 100%, respectively. Excluding patients with graft failure or rejection (n = 33) or missing data (n = 10), 58% of patients achieved full donor chimerism by day +180, and 42% had mixed chimerism (<95% donor CD3+ cells by day +180). Increasing the dose of TLI from 8 to 12 Gy had no impact on engraftment or chimerism.
Tolerability and hospitalization
Ninety-eight percent of patients underwent allogeneic HCT in the outpatient setting, and 57% of patients were never hospitalized from days 0 to 100 (Table 2). Of the 43% of patients who were hospitalized within 100 days after transplant, the median number of hospitalizations was 1 (range, 1-5), with a median length of stay of 5 days (range, 1-155). The most common causes of hospitalization were documented infections in 120 patients (20%) or febrile neutropenia without an identified infectious source in 94 patients (15%). Only 16 patients (3%) were hospitalized for acute GVHD.
Hospitalization after allogeneic transplantation
| . | n (%) . |
|---|---|
| Hospitalization after allogeneic transplantation | |
| Patients hospitalized between day 0 and day +100, n (%)* | 263 (43) |
| Median hospitalizations per patient (range), n | 1 (1-5) |
| Median length of hospitalization (range), d | 5 (1-155) |
| Reasons for hospitalization, n (%) | |
| Documented viral, bacterial, or fungal infection | 120 (20) |
| Fever without identified infectious source | 94 (15) |
| Medication related (eg, cyclosporine toxicity, ATG infusion reaction) | 25 (4) |
| Neurologic complaint (eg, altered mental status, syncope, seizure) | 23 (4) |
| Gastrointestinal complaint (eg, abdominal pain, nausea, diarrhea without source) | 21 (3) |
| Management of disease relapse | 18 (3) |
| Acute GVHD | 16 (3) |
| Cardiac complaint (eg, chest pain, arrhythmia, congestive heart failure) | 15 (2) |
| Other | 15 (2) |
| Electrolyte abnormality | 7 (1) |
| Musculoskeletal (eg, fracture, musculoskeletal pain) | 6 (1) |
| Pulmonary complaint (eg, shortness of breath, cough without source) | 5 (1) |
| Endocrine complaint (eg, hyperglycemia, adrenal insufficiency) | 4 (1) |
| . | n (%) . |
|---|---|
| Hospitalization after allogeneic transplantation | |
| Patients hospitalized between day 0 and day +100, n (%)* | 263 (43) |
| Median hospitalizations per patient (range), n | 1 (1-5) |
| Median length of hospitalization (range), d | 5 (1-155) |
| Reasons for hospitalization, n (%) | |
| Documented viral, bacterial, or fungal infection | 120 (20) |
| Fever without identified infectious source | 94 (15) |
| Medication related (eg, cyclosporine toxicity, ATG infusion reaction) | 25 (4) |
| Neurologic complaint (eg, altered mental status, syncope, seizure) | 23 (4) |
| Gastrointestinal complaint (eg, abdominal pain, nausea, diarrhea without source) | 21 (3) |
| Management of disease relapse | 18 (3) |
| Acute GVHD | 16 (3) |
| Cardiac complaint (eg, chest pain, arrhythmia, congestive heart failure) | 15 (2) |
| Other | 15 (2) |
| Electrolyte abnormality | 7 (1) |
| Musculoskeletal (eg, fracture, musculoskeletal pain) | 6 (1) |
| Pulmonary complaint (eg, shortness of breath, cough without source) | 5 (1) |
| Endocrine complaint (eg, hyperglycemia, adrenal insufficiency) | 4 (1) |
This number excludes hospitalizations lasting less than 24 hours, to expedite a procedure (eg, endoscopy or planning scan for radiotherapy).
GVHD and NRM
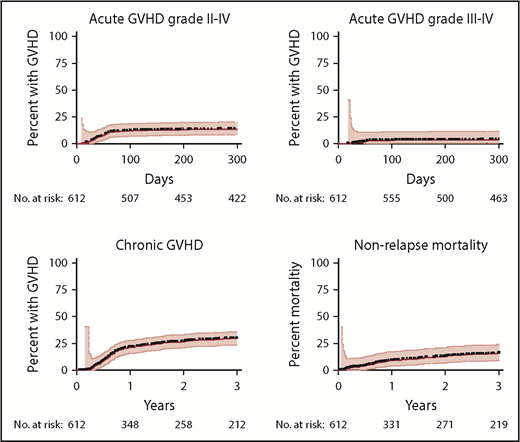
At 1 year, acute GVHD grades II-IV (Figure 1A), and III-IV (Figure 1B) occurred in 12% and 3% of patients, respectively, by day +100, and in 14% and 4% of patients, respectively, at 1 year. The cumulative incidence of extensive chronic GVHD was 22% at 1 year and 27% at 2 years (Figure 1C). NRM was 9% at 1 year and 13% at 2 years (Figure 1D) and varied with age, HCT-CI, and donor source (supplemental Figure 1). The risk of acute GVHD grade II-IV varied by donor (6%, 14%, and 22% at day +100 for MRDs, MUDs, and MMUDs, respectively), but severe acute GVHD (grade III-IV) was rare (≤5%), regardless of donor source and the degree of HLA matching (supplemental Figure 2). Among the 205 long-term survivors (alive at 4 years after transplant), 170 patients (83%) were off all immunosuppression, and 35 patients (17%) remained on immunosuppression for chronic GVHD.
GVHD and NRM. Cumulative incidence estimates of acute GVHD (A, grade II-IV; B, grade III-IV), chronic GVHD (C, extensive), and NRM (D) are shown with 95% CI.
GVHD and NRM. Cumulative incidence estimates of acute GVHD (A, grade II-IV; B, grade III-IV), chronic GVHD (C, extensive), and NRM (D) are shown with 95% CI.
Patient outcomes by disease
The probabilities of OS and PFS for the 5 most common disease groups are shown in Figure 2. GRFS is shown by disease in supplemental Figure 3. Outcomes for common disease subgroups are shown in Table 3 and summarized by disease in the following sections.
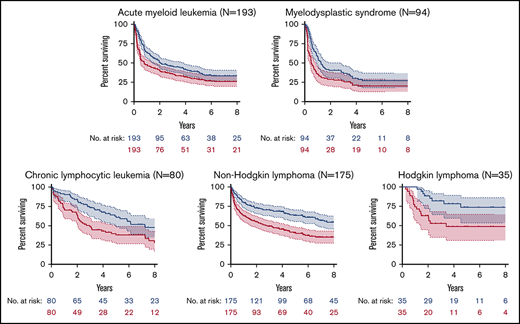
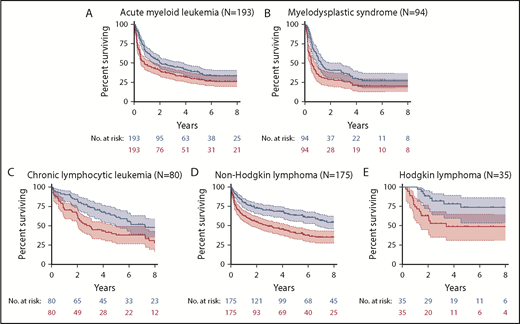
OS and PFS. Kaplan-Meier estimates of OS (blue) and PFS (red) are shown with 95% CI for AML (A), MDS (B), CLL (C), NHL (D), and HL (E).
OS and PFS. Kaplan-Meier estimates of OS (blue) and PFS (red) are shown with 95% CI for AML (A), MDS (B), CLL (C), NHL (D), and HL (E).
Patient characteristics and outcomes for common disease groups and subgroups
| Disease group or subgroup . | n . | Median age (range), y . | Median HCT-CI (range) . | MRD, % . | Median follow-up, y . | 4-y OS, % . | 4-y PFS, % . |
|---|---|---|---|---|---|---|---|
| AML | 193 | 62 (23-78) | 2 (0-9) | 46 | 5.83 | 42 | 32 |
| Cytogenetic/molecular risk | |||||||
| Intermediate risk | 119 | 61 (29-74) | 2 (0-6) | 49 | 6.52 | 48 | 36 |
| Adverse risk* | 74 | 63 (23-78) | 2 (0-9) | 42 | 3.99 | 32 | 24 |
| Remission status | |||||||
| CR1 | 146 | 62 (39-73) | 2 (0-9) | 47 | 5.66 | 44 | 33 |
| CR2 | 32 | 62 (23-74) | 1 (0-6) | 47 | 7.54 | 37 | 31 |
| Beyond CR2 | 15 | 64 (45-78) | 2 (0-4) | 33 | 3.92 | 27 | 13 |
| MDS | 94 | 64 (37-74) | 3 (0-6) | 41 | 4.64 | 30 | 21 |
| IPSS-R risk | |||||||
| Intermediate risk | 27 | 63 (50-74) | 2 (0-5) | 37 | 4.40 | 57 | 41 |
| High or very high risk | 67 | 64 (37-72) | 3 (0-6) | 42 | 5.05 | 20 | 14 |
| Blast % at time of HCT | |||||||
| <5% blasts | 69 | 63 (37-74) | 3 (0-6) | 45 | 4.64 | 34 | 27 |
| ≥5% blasts | 25 | 64 (50-72) | 3 (0-5) | 28 | 5.12 | 19 | 8 |
| CLL | 80 | 56 (31-77) | 1 (0-5) | 39 | 7.76 | 67 | 43 |
| Cytogenetic risk | |||||||
| Standard risk | 33 | 54 (31-66) | 1 (0-4) | 39 | 9.01 | 72 | 51 |
| High risk† | 47 | 58 (38-77) | 2 (0-5) | 41 | 6.75 | 63 | 36 |
| Remission status | |||||||
| CR | 17 | 55 (34-68) | 1 (0-3) | 24 | 7.49 | 66 | 55 |
| PR/SD/PD | 63 | 56 (31-77) | 1 (0-5) | 44 | 8.02 | 67 | 39 |
| NHL | 175 | 56 (27-72) | 2 (0-8) | 49 | 6.23 | 68 | 45 |
| Lymphoma subtype | |||||||
| Indolent B-cell lymphoma‡ | 34 | 54 (35-69) | 1 (0-7) | 59 | 6.04 | 82 | 61 |
| DLBCL | 66 | 56 (27-72) | 2 (0-8) | 48 | 5.85 | 70 | 44 |
| Mantle cell lymphoma | 53 | 61 (41-71) | 2 (0-6) | 43 | 10.0 | 58 | 31 |
| T-cell lymphoma | 22 | 56 (28-72) | 2 (0-6) | 45 | 4.10 | 63 | 59 |
| Remission status | |||||||
| CR | 96 | 58 (28-72) | 2 (0-7) | 50 | 6.02 | 71 | 49 |
| PR/SD/PD | 79 | 54 (27-72) | 1 (0-8) | 47 | 8.05 | 64 | 42 |
| HL | 35 | 29 (21-59) | 1 (0-6) | 40 | 4.97 | 78 | 49 |
| Disease group or subgroup . | n . | Median age (range), y . | Median HCT-CI (range) . | MRD, % . | Median follow-up, y . | 4-y OS, % . | 4-y PFS, % . |
|---|---|---|---|---|---|---|---|
| AML | 193 | 62 (23-78) | 2 (0-9) | 46 | 5.83 | 42 | 32 |
| Cytogenetic/molecular risk | |||||||
| Intermediate risk | 119 | 61 (29-74) | 2 (0-6) | 49 | 6.52 | 48 | 36 |
| Adverse risk* | 74 | 63 (23-78) | 2 (0-9) | 42 | 3.99 | 32 | 24 |
| Remission status | |||||||
| CR1 | 146 | 62 (39-73) | 2 (0-9) | 47 | 5.66 | 44 | 33 |
| CR2 | 32 | 62 (23-74) | 1 (0-6) | 47 | 7.54 | 37 | 31 |
| Beyond CR2 | 15 | 64 (45-78) | 2 (0-4) | 33 | 3.92 | 27 | 13 |
| MDS | 94 | 64 (37-74) | 3 (0-6) | 41 | 4.64 | 30 | 21 |
| IPSS-R risk | |||||||
| Intermediate risk | 27 | 63 (50-74) | 2 (0-5) | 37 | 4.40 | 57 | 41 |
| High or very high risk | 67 | 64 (37-72) | 3 (0-6) | 42 | 5.05 | 20 | 14 |
| Blast % at time of HCT | |||||||
| <5% blasts | 69 | 63 (37-74) | 3 (0-6) | 45 | 4.64 | 34 | 27 |
| ≥5% blasts | 25 | 64 (50-72) | 3 (0-5) | 28 | 5.12 | 19 | 8 |
| CLL | 80 | 56 (31-77) | 1 (0-5) | 39 | 7.76 | 67 | 43 |
| Cytogenetic risk | |||||||
| Standard risk | 33 | 54 (31-66) | 1 (0-4) | 39 | 9.01 | 72 | 51 |
| High risk† | 47 | 58 (38-77) | 2 (0-5) | 41 | 6.75 | 63 | 36 |
| Remission status | |||||||
| CR | 17 | 55 (34-68) | 1 (0-3) | 24 | 7.49 | 66 | 55 |
| PR/SD/PD | 63 | 56 (31-77) | 1 (0-5) | 44 | 8.02 | 67 | 39 |
| NHL | 175 | 56 (27-72) | 2 (0-8) | 49 | 6.23 | 68 | 45 |
| Lymphoma subtype | |||||||
| Indolent B-cell lymphoma‡ | 34 | 54 (35-69) | 1 (0-7) | 59 | 6.04 | 82 | 61 |
| DLBCL | 66 | 56 (27-72) | 2 (0-8) | 48 | 5.85 | 70 | 44 |
| Mantle cell lymphoma | 53 | 61 (41-71) | 2 (0-6) | 43 | 10.0 | 58 | 31 |
| T-cell lymphoma | 22 | 56 (28-72) | 2 (0-6) | 45 | 4.10 | 63 | 59 |
| Remission status | |||||||
| CR | 96 | 58 (28-72) | 2 (0-7) | 50 | 6.02 | 71 | 49 |
| PR/SD/PD | 79 | 54 (27-72) | 1 (0-8) | 47 | 8.05 | 64 | 42 |
| HL | 35 | 29 (21-59) | 1 (0-6) | 40 | 4.97 | 78 | 49 |
Patients with complex cytogenetics, monosomy 5 or 7, del(17p), inv(3), t(3;3), or t(6;9) or mutated RUNX1, ASXL1, or TP53.
Patients with del(11q) or del(17p).
Follicular lymphoma (n = 22), marginal zone lymphoma (n = 7), and lymphoplasmacytic lymphoma (n = 5).
AML.
One hundred ninety-three patients had AML, with a median age at transplant of 62 years (range, 23-78) and a median follow-up of 5.8 years. Thirty-eight percent had adverse cytogenetic or molecular features, as defined by the 2017 European LeukemiaNet classification,32 and 28% had secondary AML with antecedent MDS or a therapy-related myeloid neoplasm. Seventy-six percent of patients were in first complete remission (CR1), 16% were in second complete remission (CR2), and 8% were beyond CR2. For the entire AML cohort, the 4-year OS and PFS estimates were 42% (95% confidence interval [CI], 33%-47%) and 32% (95% CI, 24%-37%), respectively. GRFS was 38% at 1 year. Adverse cytogenetic/molecular features were associated with inferior OS and PFS compared with patients with intermediate risk disease by the European LeukemiaNet classification (supplemental Figure 4). Disease status beyond CR2 was also associated with inferior OS and PFS, compared with patients in CR1 or CR2. Pretransplant MRD by flow cytometry was available for 16 patients who underwent transplant during 2015 and 2016. All 6 MRD-positive patients relapsed, compared with only 4 of 10 MRD-negative patients (supplemental Figure 5). The majority of relapses (62%) in the AML cohort occurred less than 6 months after transplant.
MDS.
Ninety-four patients had MDS, with a median age of 64 years (range, 37-74) and a median follow-up of 4.6 years. Seventy-one percent had high- or very high-risk disease at the time of diagnosis, as defined by the revised International Prognostic Scoring System (IPSS-R).33 Comorbidities were common (median HCT-CI, 3), including cardiovascular disease and prior cancers, with 21 patients (22%) diagnosed with therapy-related MDS. Twenty-seven percent of patients had ≥5% blasts at the time of transplantation. For the entire MDS cohort, the 4-year OS and PFS estimates were 30% (95% CI, 20%-39%) and 21% (95% CI, 13%-29%), respectively. GRFS was 26% at 1 year. Factors associated with inferior OS included high-/very-high-risk disease by IPSS-R score and therapy-related MDS (supplemental Figure 6). Factors associated with inferior PFS included high-/very-high-risk disease and ≥5% blasts at the time of transplantation. As with AML, the majority of relapses (75%) occurred less than 6 months after transplant.
CLL.
Eighty patients had CLL, with a median age of 56 years (range, 31-77) and a median follow-up of 7.8 years. Fifty-nine percent had high-risk cytogenetics, including del(11q) or del(17p). Twenty-one percent were in CR and 79% had residual disease at the time of HCT, including 73% with a partial response (PR) and 6% with stable disease (SD) or progressive disease (PD). The majority of patients underwent transplant before US Food and Drug Administration approval of ibrutinib and venetoclax or had progression or intolerance of these agents. For the entire CLL cohort, the 4-year OS and PFS estimates were 67% (95% CI, 54%-74%) and 43% (95% CI, 30%-52%), respectively. The median OS was 7.3 years after allogeneic HCT. GRFS was 50% at 1 year. Of the 63 patients with residual disease at the time of HCT, 37 (59%) converted to CR at a median of 5.3 months after transplant (supplemental Figure 7).
NHL.
One hundred seventy-five patients had indolent or aggressive NHLs, with a median age of 56 years (range, 27-72) and a median follow-up of 6.2 years. Thirty-four patients (19%) had indolent B-cell NHLs, including follicular, marginal zone, or lymphoplasmacytic lymphomas; 66 (38%) had diffuse large B-cell lymphoma (DLBCL); 53 (30%) had mantle cell lymphoma; and 22 (13%) had peripheral T-cell lymphomas. Forty-seven percent had failure of prior autologous HCT, and 45% had residual disease at the time of allogeneic HCT, including 39% with a PR and 6% with SD/PD. For the entire NHL cohort, the 4-year OS and PFS estimates were 68% (95% CI, 59%-73%) and 45% (95% CI, 36%-51%), respectively. The median OS was 10.6 years after allogeneic HCT. GRFS was 54% at 1 year. Stratified by NHL subtype, the 4-year OS and PFS estimates were 82% and 61% for indolent B-cell NHLs, 70% and 44% for DLBCL, 58% and 31% for mantle cell lymphoma, and 63% and 59% for peripheral T-cell lymphomas (supplemental Figure 8). Of the 79 patients with residual disease at the time of allogeneic HCT, 55 (70%) converted to CR at a median of 4.4 months after transplant. Late relapse (>4 years after transplant) occurred in 8 patients, predominantly those with mantle cell and other indolent lymphomas, but most of those patients returned to durable CRs with additional chemotherapy, radiotherapy, and/or DLI.
HL.
Thirty-five patients had HL, with a median age of 29 years (range, 21-59) and a median follow-up of 5 years. Thirty-three patients (94%) had failure of prior autologous HCT, and the other 2 had antecedent CLL with Hodgkin variant Richter transformation. Forty percent had received prior treatment with brentuximab vedotin. All patients underwent transplant before US Food and Drug Administration approval of nivolumab and pembrolizumab. Sixty percent had residual disease at the time of allogeneic HCT, including 51% with a PR and 9% with SD/PD. The 4-year OS and PFS estimates were 78% (95% CI, 58%-88%) and 49% (95% CI, 30%-63%), respectively, with median OS not reached. GRFS was 54% at 1 year. Of the 21 patients with residual disease at the time of allogeneic HCT, 17 (81%) converted to CR at a median of 3.0 months after transplant. Of the 16 patients who relapsed following allogeneic HCT, 6 returned to durable CR with additional chemotherapy, radiotherapy, and/or DLI. Thus, at the last follow-up, 24 patients (69%) were alive in CR.24
Factors associated with OS, NRM, and relapse
In a multivariate analysis, factors associated with inferior OS included age ≥65 years and a DRI of high/very high or intermediate risk compared with low risk (Table 4). NRM was significantly higher in patients who received unrelated donor grafts, and there was a trend toward increased NRM in patients with an HCT-CI ≥ 3. Factors associated with a higher risk of relapse included a DRI of high/very high and mixed chimerism, whereas the development of acute or chronic GVHD and sex mismatching between donors and recipients was associated with a lower risk of relapse. There was a trend toward lower relapse in recipients of HLA-MMUDs, but this was offset by the higher risk of NRM. The dose of TLI (8 vs 12 Gy) had no impact on the risk of relapse.
Multivariate analyses for OS, NRM, and relapse
| Variable . | n (%) . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| OS | |||
| Age, y | |||
| <65 | 454 (74) | Reference | – |
| ≥65 | 158 (26) | 1.53 (1.19-1.96) | .0008 |
| Comorbidity index | |||
| HCT-CI < 3 | 403 (66) | Reference | – |
| HCT-CI ≥ 3 | 209 (34) | 1.19 (0.94-1.51) | .154 |
| Donor | |||
| HLA-MRD | 271 (44) | Reference | – |
| HLA-MUD | 250 (41) | 1.12 (0.89-1.43) | .338 |
| HLA-MMUD | 89 (15) | 1.20 (0.87-1.66) | .273 |
| DRI | |||
| Low | 140 (23) | Reference | – |
| Intermediate | 289 (47) | 1.46 (1.08-1.98) | .015 |
| High or very high | 183 (30) | 2.66 (1.94-3.66) | <.0001 |
| NRM | |||
| Age, y | |||
| <65 | 454 (74) | Reference | – |
| ≥65 | 158 (26) | 1.32 (0.80-2.16) | .270 |
| Comorbidity index | |||
| HCT-CI < 3 | 403 (66) | Reference | – |
| HCT-CI ≥ 3 | 209 (34) | 1.53 (0.99-2.35) | .056 |
| Donor | |||
| HLA-MRD | 271 (44) | Reference | – |
| HLA-MUD | 250 (41) | 1.78 (1.09-2.91) | .022 |
| HLA-MMUD | 89 (15) | 3.23 (1.88-5.57) | .0002 |
| Relapse | |||
| DRI | |||
| Low | 140 (23) | Reference | – |
| Intermediate | 289 (47) | 1.27 (0.95-1.69) | .11 |
| High or very high | 183 (30) | 1.77 (1.28-2.44) | .005 |
| Donor | |||
| HLA-MRD | 271 (44) | Reference | – |
| HLA-MUD | 250 (41) | 0.96 (0.75-1.23) | .75 |
| HLA-MMUD | 89 (15) | 0.68 (0.44-1.06) | .085 |
| Donor and recipient sex matching | |||
| Sex matched | 308 (50) | Reference | – |
| Sex mismatched | 304 (50) | 0.73 (0.58-0.92) | .0072 |
| CD3 chimerism status | |||
| Full donor chimerism | 329 (58) | Reference | – |
| Mixed chimerism | 240 (42) | 1.44 (1.13-1.84) | .004 |
| Acute GVHD | |||
| Absent | 528 (86) | Reference | – |
| Present | 84 (14) | 0.64 (0.43-0.96) | .030 |
| Chronic GVHD | |||
| Absent | 464 (76) | Reference | – |
| Present | 148 (24) | 0.61 (0.46-0.81) | .0007 |
| Variable . | n (%) . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| OS | |||
| Age, y | |||
| <65 | 454 (74) | Reference | – |
| ≥65 | 158 (26) | 1.53 (1.19-1.96) | .0008 |
| Comorbidity index | |||
| HCT-CI < 3 | 403 (66) | Reference | – |
| HCT-CI ≥ 3 | 209 (34) | 1.19 (0.94-1.51) | .154 |
| Donor | |||
| HLA-MRD | 271 (44) | Reference | – |
| HLA-MUD | 250 (41) | 1.12 (0.89-1.43) | .338 |
| HLA-MMUD | 89 (15) | 1.20 (0.87-1.66) | .273 |
| DRI | |||
| Low | 140 (23) | Reference | – |
| Intermediate | 289 (47) | 1.46 (1.08-1.98) | .015 |
| High or very high | 183 (30) | 2.66 (1.94-3.66) | <.0001 |
| NRM | |||
| Age, y | |||
| <65 | 454 (74) | Reference | – |
| ≥65 | 158 (26) | 1.32 (0.80-2.16) | .270 |
| Comorbidity index | |||
| HCT-CI < 3 | 403 (66) | Reference | – |
| HCT-CI ≥ 3 | 209 (34) | 1.53 (0.99-2.35) | .056 |
| Donor | |||
| HLA-MRD | 271 (44) | Reference | – |
| HLA-MUD | 250 (41) | 1.78 (1.09-2.91) | .022 |
| HLA-MMUD | 89 (15) | 3.23 (1.88-5.57) | .0002 |
| Relapse | |||
| DRI | |||
| Low | 140 (23) | Reference | – |
| Intermediate | 289 (47) | 1.27 (0.95-1.69) | .11 |
| High or very high | 183 (30) | 1.77 (1.28-2.44) | .005 |
| Donor | |||
| HLA-MRD | 271 (44) | Reference | – |
| HLA-MUD | 250 (41) | 0.96 (0.75-1.23) | .75 |
| HLA-MMUD | 89 (15) | 0.68 (0.44-1.06) | .085 |
| Donor and recipient sex matching | |||
| Sex matched | 308 (50) | Reference | – |
| Sex mismatched | 304 (50) | 0.73 (0.58-0.92) | .0072 |
| CD3 chimerism status | |||
| Full donor chimerism | 329 (58) | Reference | – |
| Mixed chimerism | 240 (42) | 1.44 (1.13-1.84) | .004 |
| Acute GVHD | |||
| Absent | 528 (86) | Reference | – |
| Present | 84 (14) | 0.64 (0.43-0.96) | .030 |
| Chronic GVHD | |||
| Absent | 464 (76) | Reference | – |
| Present | 148 (24) | 0.61 (0.46-0.81) | .0007 |
Significant P values are shown in bold.
Impact of chimerism on relapse and survival
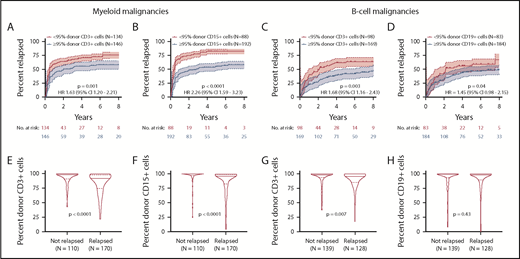
For myeloid malignancies, mixed CD15 chimerism (present in 31%) was more prognostic of disease relapse than was mixed CD3 chimerism (present in 48%) (Figure 3A-B,E-F). Patients with myeloid malignancies and mixed CD15 chimerism had a 4-year cumulative incidence of relapse of 83% (95% CI, 77%-87%) vs 54% (95% CI, 46%-60%) for patients achieving full donor CD15 chimerism (P < .0001). Mixed CD15 chimerism, but not mixed CD3 chimerism, was also associated with inferior OS for patients with myeloid malignancies (P < .0001; supplemental Figure 9). For B-cell malignancies, mixed CD3 chimerism was more prognostic of disease relapse than was mixed CD19 chimerism (Figure 3C-D,G-H). Patients with B-cell malignancies and mixed CD3 chimerism had a 4-year cumulative incidence of relapse of 62% (95% CI, 53%-69%) vs 39% (95% CI, 29%-48%) for patients achieving full donor CD3 chimerism (P = .003). However, for patients with B-cell malignancies, mixed chimerism did not adversely affect OS (P = .19), as the higher risk of relapse was offset by the lower risk of NRM, and durable remissions were still observed in 30% to 40% of patients with mixed chimerism.
Association between chimerism and relapse. Top panels show the cumulative incidence of disease relapse with 95% CI for patients with mixed chimerism (red) compared with patients with full donor chimerism (blue). Top left panels show relapse rates for patients with myeloid malignancies (n = 280) according to CD3 (A) and CD15 (B) chimerism status. Top right panels show relapse rates for patients with B-cell malignancies (n= 267) according to CD3 (C) and CD19 (D) chimerism status. Bottom panels show the distribution of chimerism values (peak chimerism by day +180) for patients who relapsed or never relapsed. Bottom left panels show CD3 (E) and CD15 (F) chimerism distributions for patients with myeloid malignancies. Bottom right panels show CD3 (G) and CD19 (H) chimerism distributions for patients with B-cell malignancies. Solid horizontal lines indicate the medians, and dashed horizontal lines indicate the interquartile range. Patients with graft failure or rejection (n = 33), missing chimerism data (n = 10, or T-cell malignancies (n = 25) were excluded.
Association between chimerism and relapse. Top panels show the cumulative incidence of disease relapse with 95% CI for patients with mixed chimerism (red) compared with patients with full donor chimerism (blue). Top left panels show relapse rates for patients with myeloid malignancies (n = 280) according to CD3 (A) and CD15 (B) chimerism status. Top right panels show relapse rates for patients with B-cell malignancies (n= 267) according to CD3 (C) and CD19 (D) chimerism status. Bottom panels show the distribution of chimerism values (peak chimerism by day +180) for patients who relapsed or never relapsed. Bottom left panels show CD3 (E) and CD15 (F) chimerism distributions for patients with myeloid malignancies. Bottom right panels show CD3 (G) and CD19 (H) chimerism distributions for patients with B-cell malignancies. Solid horizontal lines indicate the medians, and dashed horizontal lines indicate the interquartile range. Patients with graft failure or rejection (n = 33), missing chimerism data (n = 10, or T-cell malignancies (n = 25) were excluded.
Discussion
We present our 15-year, single-center experience using TLI-ATG conditioning in a large cohort of patients (N = 612) with hematologic malignancies, allowing for a comprehensive assessment of the advantages and limitations of this regimen. These data provide important insights on which subgroups may derive the greatest benefit from this regimen. Overall, TLI-ATG conditioning was well tolerated, with a low risk of GVHD and NRM in this high-risk cohort with a median age of 60 years, one third of patients with HCT-CI ≥ 3 and over half of patients receiving unrelated donor grafts, including 15% with MMUDs. Outpatient allogeneic HCT was feasible, with the majority of patients never hospitalized from days 0 to +100. This compares favorably to other nonmyeloablative regimens such as low-dose TBI and fludarabine, where 83% of patients were hospitalized within 3 months of transplantation in 1 series.34 It is particularly notable that only 3% of patients were hospitalized for acute GVHD.
Regarding efficacy, long-term OS and PFS were seen across a wide range of high-risk and refractory hematologic malignancies. Outcomes were particularly favorable among patients with heavily pretreated lymphoid malignancies with 1-year GRFS of 50% to 54%. For our NHL cohort, the median OS was >10 years after allogeneic HCT. For our heavily pretreated HL cohort, long-term OS was excellent at 78%, and 69% of patients were alive in CR at last follow-up.24 The majority of patients with CLL or lymphomas with residual disease converted to CR after transplant, and remissions were durable with long-term PFS of 40% in this subgroup. It is unlikely that 8 to 12 Gy TLI alone provided meaningful long-term antitumor activity against these lymphoid malignancies as prior studies indicate that significantly higher doses of 24 to 30 and 30 to 40 Gy are required for durable control of indolent and aggressive lymphomas, respectively.35,36 Disease responses were also observed beyond the radiation fields. Rather, we hypothesize that the irradiated lymphoid tissue may expose tumor-specific antigens and facilitate subsequent GVT reactions in lymphoid malignancies.37-39
For AML and MDS, this conditioning regimen, which lacks any direct antitumor activity against myeloid malignancies, allows for a relatively pure assessment of the GVT effect. It is notable that our AML and MDS cohorts were enriched for older patients (median age, 62-64 years) with significant comorbidities (median HCT-CI, 2-3) and aggressive disease biology (52% with high/very high DRI). Despite these high-risk features, long-term PFS was observed in 20% to 30% of patients with MDS and AML, with the majority of relapses occurring early and inferior outcomes among patients with residual disease (including MRD) at the time of transplant. These findings underscore the importance of optimizing remission status before considering a nonmyeloablative regimen such as TLI-ATG for AML and MDS. When possible, we recommend that AML patients achieve an MRD-negative CR and that MDS patients achieve <5% blasts before nonmyeloablative transplantation with TLI-ATG.
As with other nonmyeloablative regimens, the main limitation of TLI-ATG conditioning is the relatively high risk of relapse, particularly for patients with AML/MDS. The high relapse rate likely reflects multiple factors, including (1) the lack of direct antitumor activity from the conditioning regimen, particularly for myeloid malignancies; (2) the more aggressive disease biology in older patients unfit for more intensive regimens; and (3) the higher incidence of mixed chimerism. Although mixed chimerism strongly correlated with the risk of relapse, the impact of mixed chimerism on OS varied by disease in our cohort. In patients with lymphoid malignancies, mixed chimerism did not adversely affect OS, as the higher risk of relapse was offset by the lower risk of NRM. It is notable that durable remissions were still observed in 30% to 40% of CLL and lymphoma patients with mixed chimerism, indicating that meaningful GVT reactions still occur in these patients. In contrast, mixed chimerism (particularly, mixed CD15 chimerism) was rarely sufficient for durable disease control of myeloid malignancies, in which the more aggressive disease kinetics are likely to outpace GVT reactions. Of note, mixed CD15 chimerism, in some cases, may represent residual CD15+ AML cells, although full donor CD15 chimerism was still achieved in some MRD-positive AML patients in our cohort.
Several efforts are currently underway to improve upon TLI-ATG conditioning by augmenting donor chimerism to reduce relapse rates. An ongoing trial is evaluating a hybrid TLI-ATG-TBI regimen wherein the last dose of TLI is replaced with a single very low dose of TBI (80 cGy) in an effort to improve engraftment and chimerism (clinicaltrials.gov NCT03734601). Another phase II trial is evaluating a preemptive CD8+ memory T-cell DLI to convert patients with mixed chimerism to full donor chimerism (NCT02424968). We previously showed that a DLI of purified, phenotypic CD8+ memory T cells revealed evidence of GVT activity in treating relapsed hematologic malignancies with a lower incidence of GVHD than has been reported with unmanipulated DLI.40 The preliminary results of our phase II trial of preemptive CD8+ memory T-cell DLI demonstrated conversion from mixed chimerism to full donor chimerism in 6 of 9 patients, while inciting grade II acute GVHD in only 2 of 9 patients.41 Developing methods to immunize the donor immune cell inoculum to tumor antigens, such as WT1 in AML/MDS or complementarity-determining region-3 in B-cell malignancies, may further boost GVT activity.42-44 Finally, our group recently showed that an anti-CD117 (c-KIT) antibody can deplete MDS hematopoietic stem cells and restore normal donor hematopoiesis in xenografted mice.45 Combining this c-KIT antibody with TLI-ATG conditioning for patients with MDS and AML may deplete stem cell niches, thereby improving donor cell engraftment and chimerism.
In conclusion, TLI-ATG conditioning allowed for outpatient allogeneic HCT with a low risk of GVHD and NRM, even among the highest risk subgroups including septuagenarians, patients with multiple comorbidities, and recipients of MMUDs. Despite the low risk of GVHD, potent GVT reactions were evident and may produce durable remissions for a variety of high-risk and refractory hematologic malignancies. Outcomes were particularly favorable for heavily pretreated lymphoid malignancies, which may reflect synergy between lymphoid irradiation exposing tumor-specific antigens and facilitating subsequent GVT reactions. Based on the balance of safety and efficacy, we encourage the transplant community to consider TLI-ATG conditioning for patients with heavily pretreated lymphoid malignancies, as well as for older and frailer patients with AML and MDS who are ineligible for more intensive regimens. Remission status should be optimized in the latter group to reduce the risk of relapse. Several efforts are underway to augment donor chimerism and enhance GVT activity while maintaining the excellent safety and tolerability profile of this regimen.
Presented in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 3 December 2018.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Ranjana Advani, Peter Greenberg, and Martin Tallman for helpful comments and review of the manuscript.
This study was funded by the National Institutes of Health, National Cancer Institute grant P01 CA049605.
Authorship
Contribution: M.A.S and R.L. wrote the manuscript; V.E.K., J.S.T., and P.W.L. conducted the statistical analyses; R.L., J.A.S., S.S., and R.T.H. developed the conditioning regimen; and all authors participated in data collection and analysis, revised the manuscript, and approved the final manuscript.
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Correspondence: Robert Lowsky, Division of Blood and Marrow Transplantation, Stanford University, 300 Pasteur Dr, Room H3249, Stanford, CA 94305; e-mail: rlowsky@stanford.edu.