Key Points
By treating active sites of disease, low-dose radiation can help achieve remission in relapsed multiply refractory MCL.
Low-dose radiation (4 Gy) is safe to deliver with concurrent chemotherapy, to multiple sites, and repeatedly as needed.
Abstract
Mantle cell lymphoma (MCL) generally exhibits an aggressive disease course with poor outcomes. Despite inherent radiosensitivity, radiation therapy (RT) is not commonly used for MCL. This study assesses the role of low-dose RT (LDRT) with concurrent chemotherapy in relapsed, multiply refractory MCL. From 2014 through 2018, 19 patients with relapsed, refractory MCL had 98 sites treated with 4 Gy. Median follow-up from initial LDRT was 15.4 months. Patients had received a median 7 courses of chemotherapy since diagnosis, and 58% were ibrutinib-refractory. Of the 98 sites, 76% were refractory to ongoing chemotherapy, and LDRT was delivered with concurrent chemotherapy for 76%. The complete response (CR) rate was 81% at a median 2.7 months post-LDRT. There were no differences in CR despite ibrutinib-refractory disease, prior chemotherapy courses (>5), or tumor size (>3 cm). There were no RT-related toxicities. Overall survival at 1 year following initial LDRT was 90%, and 1-year progression-free survival following last course was 55%. In summary, LDRT is effective for relapsed, multiply refractory MCL, and may be safely delivered with chemotherapy, to multiple sites, and repeatedly without issue. By treating active sites of disease, LDRT can provide durable local control, help achieve remission, and potentially bridge patients to subsequent novel therapies.
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma (NHL) frequently involving extranodal sites. Although accounting for only 6% of NHL, MCL is a challenge given its aggressive course with poor outcomes. Patients respond to first-line therapy but relapse frequently and develop chemorefractory disease.1
Novel agents have been introduced for the management of relapsed, refractory MCL. Bortezomib, lenalidomide, ibrutinib, and acalabrutinib achieve overall response and complete response (CR) rates of 28% to 81% and 8% to 27% as single therapies2-8 ; and in combination with rituximab and/or dexamethasone, these rise to 52% to 88% and 24% to 44%, respectively.9,10 Yet, patients who fail ibrutinib are resistant to further therapies and have poor outcomes, with median survival of 3 to 10 months.11-13
Although effective, radiation therapy (RT) is not commonly used for MCL, except for early-stage disease or palliation.14 Reports indicate local control (LC) and CR rates of 91% to 100% and 64% to 69%, respectively, with traditional doses of 30 Gy.15-17 However, long-course RT is inconvenient for patients and is accompanied by chemotherapy breaks due to toxicity concerns.
Alternatively, the utility of low-dose RT (LDRT; ≤8 Gy) has been demonstrated in the management of NHL.18,19 A report from Stanford showed excellent response with LDRT for 25 sites of relapsed, refractory MCL, demonstrating LC and CR rates of 88% and 68%, respectively, with minimal toxicity.20 Hence, MCL remains radiosensitive even in heavily pretreated disease.
Given its safety and effectiveness, we hypothesized a beneficial role for concurrent LDRT with chemotherapy for the definitive management of relapsed, refractory MCL. By treating active disease, LDRT (4 Gy) could provide durable local control, help achieve remission, and potentially bridge refractory patients to subsequent therapies.
Methods
Patients and sites
This study was conducted under approval of the University of Texas MD Anderson Cancer Center Institutional Review Board (protocol #PA15-1064). Nineteen patients, with a total of 98 sites of relapsed, refractory MCL, were treated from 2014 to 2018 with LDRT. Median follow-up was 51.3 months (range, 18.9-246.1 months) from initial diagnosis and 15.4 months (range, 3.8-43.7 months) from initial LDRT. Median age at initial LDRT was 69 years (range, 52-86 years). Fifteen (79%) had classical histology, and 4 (11%) had blastoid variant. Of the 98 sites, median tumor size was 2.8 cm (range, 0.9-11.5 cm), and 74 (76%) were refractory to ongoing chemotherapy at time of LDRT (Table 1). “Refractory” was defined as disease that was either unresponsive or progressing despite ongoing systemic therapy.
Clinical and treatment characteristics of all 98 sites of relapsed, refractory MCL treated with consolidative LDRT and associations with CR
| Variable . | No. of sites (%) . | CR, univariate . | CR, multivariable . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P* . | HR (95% CI) . | P* . | ||
| Site | |||||
| Soft tissue | 63 (64) | 1.80 (1.12-2.90) | .015 | 1.79 (1.11-2.89) | .018 |
| Non–soft tissue | |||||
| Nodal | 22 (22) | ||||
| GI | 7 (7) | ||||
| Orbit | 3 (3) | ||||
| Mucosal | 2 (2) | ||||
| Bone | 1 (1) | ||||
| No. of fractions | |||||
| 2, ×2 Gy | 72 (73) | 1.17 (0.68-2.02) | .566 | ||
| 1, ×4 Gy | 26 (27) | ||||
| RT modality | |||||
| Photons | 65 (66) | 0.68 (0.43-1.09) | .109 | ||
| Nonphotons | |||||
| Electrons | 32 (33) | ||||
| Orthovoltage | 1 (1) | ||||
| Diagnosis to LDRT | |||||
| ≥36 mo | 36 (37) | 0.73 (0.46-1.18) | .201 | ||
| <36 mo | 62 (63) | ||||
| Lesion size | |||||
| ≥3 cm | 47 (48) | 0.76 (0.48–1.21) | .245 | 0.69 (0.43-1.10) | .120 |
| <3 cm | 51 (52) | ||||
| Histology | |||||
| Blastoid | 27 (28) | 0.78 (0.45-1.35) | .374 | ||
| Nonblastoid | 71 (72) | ||||
| Chemorefractory | |||||
| Yes, to ongoing | 74 (76) | 1.41 (0.86-2.32) | .175 | 1.40 (0.84-2.32) | .194 |
| No, to ongoing | 24 (24) | ||||
| Ibrutinib-refractory | |||||
| Yes | 80 (82) | 1.10 (0.65-1.88) | .716 | ||
| No | 18 (18) | ||||
| Prior chemo | |||||
| >5 courses | 33 (34) | 0.97 (0.61-1.55) | .906 | ||
| ≤5 courses | 65 (66) | ||||
| Prior SCT | |||||
| Yes | 42 (43) | 0.92 (0.58-1.44) | .706 | ||
| No | 56 (57) | ||||
| Concurrent chemo | |||||
| Yes | 74 (76) | 1.26 (0.75-2.11) | .379 | ||
| No | 24 (24) | ||||
| Post-LDRT chemo | |||||
| Yes | 79 (81) | 0.99 (0.58-1.68) | .963 | ||
| No | 19 (19) | ||||
| Variable . | No. of sites (%) . | CR, univariate . | CR, multivariable . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P* . | HR (95% CI) . | P* . | ||
| Site | |||||
| Soft tissue | 63 (64) | 1.80 (1.12-2.90) | .015 | 1.79 (1.11-2.89) | .018 |
| Non–soft tissue | |||||
| Nodal | 22 (22) | ||||
| GI | 7 (7) | ||||
| Orbit | 3 (3) | ||||
| Mucosal | 2 (2) | ||||
| Bone | 1 (1) | ||||
| No. of fractions | |||||
| 2, ×2 Gy | 72 (73) | 1.17 (0.68-2.02) | .566 | ||
| 1, ×4 Gy | 26 (27) | ||||
| RT modality | |||||
| Photons | 65 (66) | 0.68 (0.43-1.09) | .109 | ||
| Nonphotons | |||||
| Electrons | 32 (33) | ||||
| Orthovoltage | 1 (1) | ||||
| Diagnosis to LDRT | |||||
| ≥36 mo | 36 (37) | 0.73 (0.46-1.18) | .201 | ||
| <36 mo | 62 (63) | ||||
| Lesion size | |||||
| ≥3 cm | 47 (48) | 0.76 (0.48–1.21) | .245 | 0.69 (0.43-1.10) | .120 |
| <3 cm | 51 (52) | ||||
| Histology | |||||
| Blastoid | 27 (28) | 0.78 (0.45-1.35) | .374 | ||
| Nonblastoid | 71 (72) | ||||
| Chemorefractory | |||||
| Yes, to ongoing | 74 (76) | 1.41 (0.86-2.32) | .175 | 1.40 (0.84-2.32) | .194 |
| No, to ongoing | 24 (24) | ||||
| Ibrutinib-refractory | |||||
| Yes | 80 (82) | 1.10 (0.65-1.88) | .716 | ||
| No | 18 (18) | ||||
| Prior chemo | |||||
| >5 courses | 33 (34) | 0.97 (0.61-1.55) | .906 | ||
| ≤5 courses | 65 (66) | ||||
| Prior SCT | |||||
| Yes | 42 (43) | 0.92 (0.58-1.44) | .706 | ||
| No | 56 (57) | ||||
| Concurrent chemo | |||||
| Yes | 74 (76) | 1.26 (0.75-2.11) | .379 | ||
| No | 24 (24) | ||||
| Post-LDRT chemo | |||||
| Yes | 79 (81) | 0.99 (0.58-1.68) | .963 | ||
| No | 19 (19) | ||||
chemo, chemotherapy; CI, confidence interval; GI, gastrointestinal; HR, hazard ratio; SCT, stem cell transplantation.
Cox proportional hazards for univariate and multivariable analyses of associations with outcomes.
Treatment
Patients had received a median 7 courses (range, 1-12 courses) of chemotherapy since diagnosis. Common agents included carfilzomib, ibrutinib, bortezomib, anthracycline, and rituximab, among others, and 8 patients (42%) previously underwent autologous stem cell transplant. Eleven (58%) were ibrutinib-refractory by initial LDRT.
Of the 19 patients, 14 received initial LDRT with concurrent systemic therapy: in 4 cases, these agents were initiated 3 to 6 months prior and continued through LDRT; and in 10 cases, concurrent chemotherapy had been initiated <1 month prior to LDRT. For the latter 10 cases, systemic therapies prior to this occurred at a median 4 months before LDRT (range, 2-48 months), consisting of ibrutinib-based regimens in half (n = 5). Finally, for the remaining 5 patients treated without concurrent chemotherapy, last systemic therapy preceded LDRT by a median of 24 months (range, 1-48 months).
Regarding LDRT, each patient had a median of 2 sites treated (range, 1-27 sites; interquartile range, 1-4.5 sites) over 1 to 8 courses for a total 98 treated sites. The first course of LDRT occurred at a median 42.7 months (range, 7.2-233.5 months) following diagnosis. For patients receiving multiple treatment courses (n = 7), median time from initial LDRT to last LDRT was 1.3 years (range, 0.8-2.1 years).
Sites were treated with 4 Gy in either 1 (27%) or 2 (73%) daily fractions via 3-dimensional conformal RT or electron beam. Dose was prescribed to tumor plus margin (eg, 5-10 mm) to account for set-up variability, consistent with involved-site RT guidelines by the International Lymphoma Radiation Oncology Group (ILROG).21,22
Of 98 sites, 74 (76%) were treated with concurrent chemotherapy (initiated prior to LDRT). Common regimens in either setting consisted of rituximab and lenalidomide (frequently with bortezomib and dexamethasone), followed by venetoclax and obinutuzumab in combination (frequently with acalabrutinib) or alone.
In 79 sites (81%), chemotherapy was continued or restarted after LDRT (but prior to new disease progression). Twenty of these lesions were initiated on a novel regimen after LDRT to which the patients had not been previously exposed, whereas the majority (n = 59) were continued on the chemotherapy they were treated with concurrently. In 39 of these sites, the concurrent agents were initiated <2 weeks prior to LDRT, most commonly consisting of rituximab and lenalidomide (frequently with bortezomib and dexamethasone), or venetoclax and obinutuzumab in combination (frequently with acalabrutinib) or alone. For the remaining 20 lesions, the concurrent agent had been initiated >1 month prior to LDRT and continued post-LDRT. The most common agents in this refractory setting were venetoclax and obinutuzumab in combination or alone.
End points and analysis
After completion of LDRT, patients were followed every 2 to 3 months. In-field response per site was assessed via imaging (computed tomography and/or positron emission tomography/computed tomography) in the majority of cases (90%) and/or clinical examination for a minority of soft tissue sites (10%). In-field response was categorized as CR, defined as complete clinical and/or radiographic disappearance of disease, or partial response (PR), defined as ≥50% decrease in tumor diameter, as specified by International Working Group criteria.23 Local recurrence (LR) was defined as progressive disease with increase in tumoral axis within the treatment field. Disease progression was defined as either LR or systemic progression with new sites of disease (marked as the date of axial imaging study indicating such).
Primary end points were in-field CR and freedom from LR (FFLR), both computed per site from end of LDRT; overall survival (OS), computed per patient from end of initial LDRT; and progression-free survival (PFS), computed per patient from the end of last LDRT. For CR, sites were censored at LR or at last follow-up evaluation; for PFS, death and disease progression were events; and for FFLR, LR was an event, with sites otherwise censored at last follow-up evaluation.
Actuarial rates of FFLR, OS, and PFS were calculated via Kaplan-Meier method. Timing of subsequent systemic therapy was not a factor in calculating PFS. Patient and treatment factors were assessed for associations with CR via Cox proportional hazards modeling (Table 1). Acute RT-related toxicity was graded according to the Common Terminology Criteria for Adverse Events v4.0.
Results and discussion
This is the first study to evaluate LDRT with chemotherapy as a treatment modality outside of palliative intent for relapsed, multiply refractory MCL, with the goals of treating active disease and potentially achieving remission. Our findings indicate that LDRT imparts excellent LC, minimal toxicity, and favorable outcomes in this setting.
Of the 98 sites, CR was achieved for 79 total (81%), with median time to CR of 2.7 months (95% CI, 2.1-3.2) post-LDRT. Upon removing the 1 outlier patient who had 27 sites treated, the CR rate remained high at 76% (54 of 71), supporting the robustness of these findings. An additional 5 sites achieved PR, contributing to an overall response in 86% (n = 84) of all treated sites.
These data provide practical guidance for clinicians regarding the expected timing of response following treatment, which may be delayed by several months in some cases. Only 11% of CRs were noted in <1 month, increasing to 44% by 2 months; whereas 36% of CRs were achieved after 3 months (including 18% after 4 months).
CR was associated with soft tissue site (HR, 1.80; P = .02) (Table 1); however, there were no associations with chemorefractory status (P = .18), ibrutinib-refractory status (P = .72), prior chemotherapy courses (>5; P = .61), receipt of concurrent (P = .38) or post-LDRT chemotherapy (P = .96), tumor size (>3 cm; P = .13), number of fractions (P = .57), lesions treated per course (>2; P = .12), or blastoid variant (P = .37).
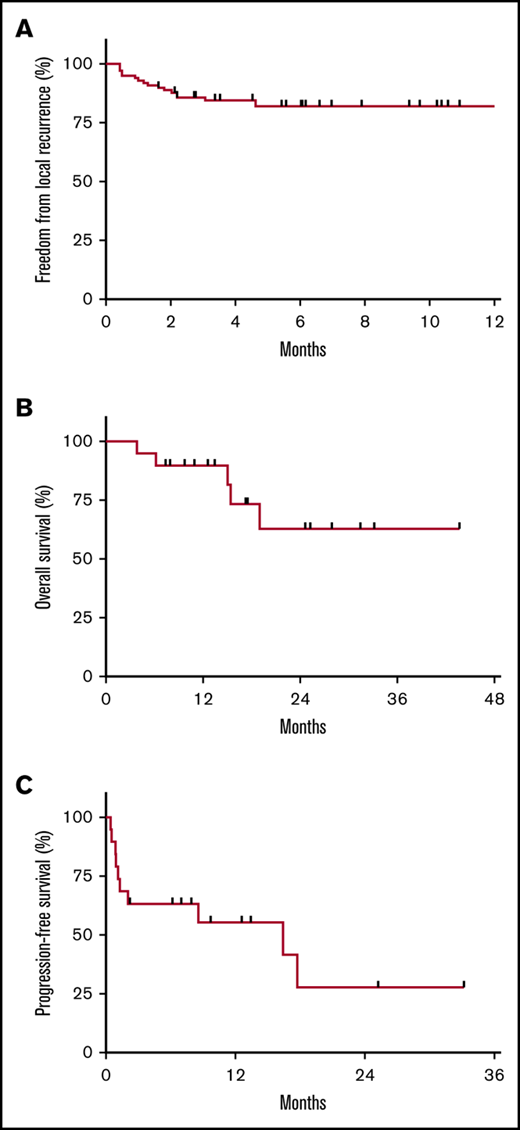
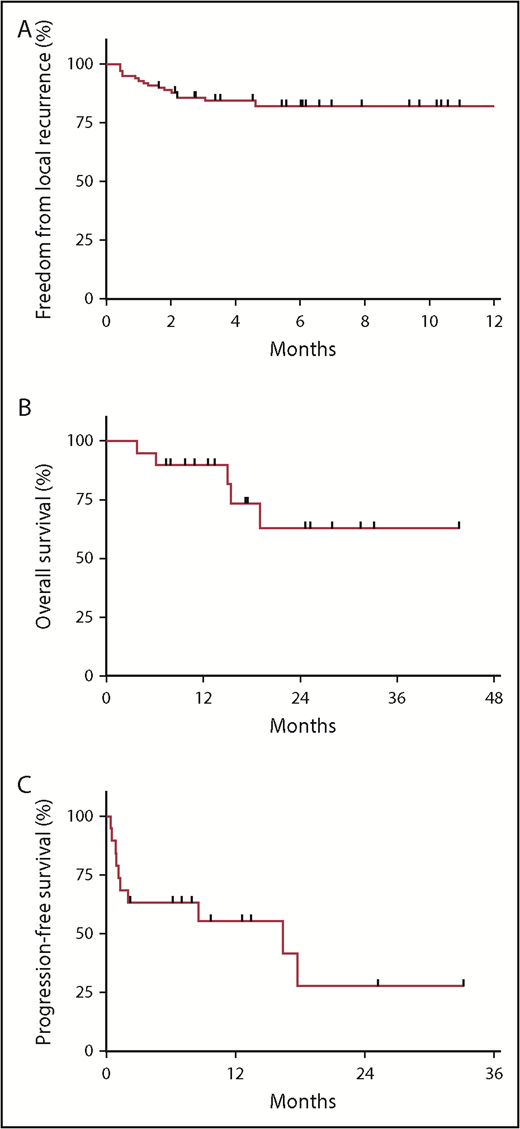
A total of 17 sites (17%) experienced LR at a median 1.3 months (range, 0.4-4.6 months) post-LDRT: 3 following PR and 14 after stable disease. Actuarial FFLR at 2 and 4 months was 88.8% and 84.5%, respectively (Figure 1A). Four recurrences were salvaged with further RT (including 3 with LDRT). Of note, the 19 sites without CR were observed among 7 patients, 3 of whom were simultaneously and subsequently treated to other sites with response, indicating that resistance at 1 site does not entail resistance at others.
Outcomes following LDRT (4 Gy). (A) FFLR at 2 and 4 months was 88.8% and 84.5%, respectively. (B) OS at 1 year following initial LDRT was 90% among the 19 patients. (C) PFS at 1 year following the last course of LDRT was 55%. Nine patients were progression-free at the time of analysis (with a median follow-up of 9.7 months), and 3 patients were completely off systemic therapy since last LDRT course.
Outcomes following LDRT (4 Gy). (A) FFLR at 2 and 4 months was 88.8% and 84.5%, respectively. (B) OS at 1 year following initial LDRT was 90% among the 19 patients. (C) PFS at 1 year following the last course of LDRT was 55%. Nine patients were progression-free at the time of analysis (with a median follow-up of 9.7 months), and 3 patients were completely off systemic therapy since last LDRT course.
Furthermore, there were no RT-related toxicities, even among 76 sites treated with concurrent chemotherapy or 26 sites treated in single fractions. Two patients had 7 sites treated simultaneously with concurrent chemotherapy, and 1 patient had 27 treated in total, without any RT-related toxicities. Many patients had sites overlapping sensitive organs treated, including bowel (n = 22), oropharynx (n = 4), orbit (n = 3), and spinal cord (n = 1), without issue.
Regarding outcomes, OS at 1 year following initial LDRT was 90% (Figure 1B), and 1-year PFS following last LDRT was 55% (Figure 1C). All deaths (n = 5) occurred among ibrutinib-refractory patients (n = 11), who had median OS and PFS of 5.6 (range, 2.8-15.4) and 1.2 months (range, 0.5-2.1 months), respectively. However, 9 patients were progression-free at analysis (with median follow-up of 9.7 months post-LDRT).
Whether this PFS benefit is attributable to LDRT, chemotherapy, or the combination remains unknown. Recall that for 10 patients, concurrent chemotherapy was initiated <1 month prior to initial LDRT, arguably too short of an interval to appreciate the full benefit of newly initiated agents. In 6 of these 10 patients, 1 or more sites were left unirradiated (outside of those treated with LDRT), resulting in mixed systemic responses: 4 cases only responded at irradiated sites, with progression of unirradiated lesions (despite recently initiated chemotherapy); whereas the other 2 had response at both irradiated and unirradiated sites, indicating some benefit attributable to the new systemic agents.
On the other hand, chemotherapy was not immediately restarted following 11 LDRT courses among a total of 6 patients. For 2 of these patients, repeated courses of LDRT were delivered back-to-back at disease progression in the absence of systemic therapy. Progression ultimately ensued for most of these cases, but they benefited from a median treatment break of 7.9 months (range, 0.4-16.4 months) prior to restarting chemotherapy, and 3 patients were still off treatment following last LDRT.
Taken together, these outcomes imply that LDRT is an effective treatment of relapsed, multiply refractory MCL, with a highly favorable therapeutic ratio. By treating active sites without toxicity, LDRT can provide durable LC, help achieve remission, and potentially bridge refractory patients to subsequent therapies. Furthermore, LDRT is safe to deliver to multiple sites repeatedly and with concurrent chemotherapy. Alternatively, LDRT can provide treatment breaks for patients recovering from toxicities.
The major limitation of this study is its single-institution, retrospective nature. As such, the study population is small and heterogeneous, including a mix of clinically indolent as well as aggressive forms of MCL. In addition, these patients represent a highly selected cohort, chosen out of a total 67 MCL patients treated with RT by the Department of Radiation Oncology at the University of Texas MD Anderson Cancer Center over the same time frame (2014-2018).
Although LDRT is clearly effective with respect to in-field LC (regardless of chemorefractory status, concurrent chemotherapy, or the addition of chemotherapy post-LDRT), subsequent studies are needed to confirm that this approach improves PFS over chemotherapy alone because roughly one-half of patients (n = 10) started new chemotherapy at the time of initial LDRT with mixed systemic results. Furthermore, specific patient selection criteria are difficult to define, given the novelty of this treatment approach. Based on these promising findings, however, we are evaluating the utility of LDRT with concurrent chemotherapy in a single-arm prospective phase 2 study of ibrutinib-refractory patients.
Acknowledgment
This work was supported in part by Cancer Center Support (Core) grant CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Authorship
Contribution: M.S.N. and B.S.D. designed the research; M.S.N. performed the research; M.S.N. and B.V.C. analyzed the data; and all authors contributed significantly to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bouthaina S. Dabaja, Department of Radiation Oncology, Unit 0097, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: bdabaja@mdanderson.org.