Key Points
Data demonstrate comparable efficacy between VENmono and VEN + CD20 therapy in a heavily pretreated, high-risk CLL cohort.
Abstract
Venetoclax (VEN) is approved for relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) as monotherapy (VENmono) or in combination with rituximab. Whether VEN plus anti-CD20 (VENcombo) is superior to VENmono is unknown. We conducted a multicenter, retrospective cohort analysis comparing 321 CLL patients treated with VENmono vs VENcombo across the United States and the United Kingdom. We examined demographics, baseline characteristics, dosing, adverse events, response rates, and outcomes. The primary endpoints were progression-free survival (PFS) and overall survival (OS), estimated by Kaplan-Meier method, in patients treated with VENmono vs VENcombo. Univariate and bivariate analyses were performed with COX regression. Three hundred twenty-one CLL patients were included (3 median prior treatments, 78% prior ibrutinib). The overall response rates (ORRs) were similar (VENmono, 81% ORR, 34% complete remission [CR] vs VENcombo, 84% ORR, 32% CR). With a median follow-up of 13.4 months, no differences in PFS and OS were observed between the groups. In unadjusted analyses, the hazard ratios (HRs) for PFS and OS for VENmono vs VENcombo were HR 1.0 (95% confidence interval [CI], 0.6-1.8; P = .7) and HR 1.2 (95% CI, 0.6-2.3; P = .5), respectively. When adjusting for differences between the cohorts, the addition of an anti-CD20 antibody in combination with VEN did not impact PFS (HR, 1.0; 95% CI, 0.5-2.0; P = .9) or OS (HR, 1.1; 95% CI, 0.4-2.6; P = .8). We demonstrate comparable efficacy between VENmono and VENcombo in a heavily pretreated, high-risk, retrospective cohort, in terms of both response data and survival outcomes. Prospective studies are needed to validate these findings.
Introduction
Venetoclax (VEN) is approved as monotherapy (VENmono) or in combination with rituximab (R) for relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL).1 Whether VEN-R (VR) is superior to VENmono is unknown and has not been studied and is not planned to be studied in randomized trials.2-4 Answering this question is now more pressing in view of the accumulating recent data that fail to show a progression-free survival (PFS) benefit to adding R or ublituximab to ibrutinib in front-line and R/R CLL.5-8 This lack of difference contrasts with the suggested benefit when R or ublituximab and ibrutinib were studied in nonrandomized phase 2 studies.9,10 In the clinical trial that led to VR approval, patients were not heavily pretreated, with very few (2%; 8/389) exposed to ibrutinib or idelalisib. These characteristics differ from those of patients treated with VEN in other clinical trials or outside of studies.11 We aimed to compare response and survival outcomes among CLL patients treated with VEN plus an anti-CD20 monoclonal (VENcombo) antibody against patients treated with VENmono in the R/R setting. Therefore, we conducted a multicenter, international study examining the outcomes of 321 CLL patients treated with VEN.
Methods
We conducted a retrospective cohort study of R/R CLL patients treated with VEN across 24 US and 42 UK academic and community centers in partnership with the UK CLL Forum and the CLL Collaborative Study of Real World Evidence.
To define the study cohort, investigators were asked to identify and collect data on all CLL patients treated with VEN at their center/practice, including those treated on clinical studies. Utilizing a standardized case report form, investigators collected data through medical chart review of CLL patients, including demographics, disease characteristics, VEN dosing, tumor lysis syndrome (TLS) risk and prophylaxis, and adverse events (AEs). Detailed pre-VEN data were collected to confirm that VENmono vs VENcombo groups represented a balanced comparison based on potential confounders.
To understand patterns of VEN use, we collected data on its administration as monotherapy and, if paired, which additional agent was given. Regarding VEN dosing, we collected data on dose reductions, dose interruptions, and duration of interruptions. Regarding VEN combination therapy, investigators were asked if VEN was given as (1) monotherapy or (2) paired with another agent, and if “paired” was selected, they were then asked to select (1) R, (2) obinutuzumab, (3) ibrutinib, or (4) other agent(s). Specific data on “other” combinations were not collected. Patients treated with ibrutinib and VEN were not included in this analysis. Data regarding dose intensity and number of cycles of the paired agent were not collected. For the planned stratified analyses, patients were categorized as VENmono or VENcombo (combination group included patients who were treated with VEN plus either R or obinutuzumab).
TLS risk was defined according to guidance provided in the VEN US Food and Drug Administration package insert as low, medium, or high risk. Investigators were asked to define TLS events by Howard criteria.12 Response rates were defined by International Workshop on Chronic Lymphocytic Leukemia criteria as complete remission (CR), partial remission, stable disease, and progressive disease.13 Because of study design, response assessments and follow-up intervals were not standardized, but time to best response was collected. Because of the relative difficulty in identifying and collecting a comprehensive list of AEs retrospectively, investigators were asked to perform detailed chart reviews to accurately gather data on select grade ≥3 AEs (Common Terminology Criteria for Adverse Events criteria), including neutropenia, thrombocytopenia, diarrhea, and febrile neutropenia. Timing, frequency, causality, and duration of AEs were not collected.
The primary study endpoints were PFS and overall survival (OS) in patients treated with VENmono compared with VEN plus an anti-CD20 antibody. PFS and OS were estimated by the Kaplan-Meier method.14 To test the association between VEN pairing status and survival outcomes, we used COX regression to perform univariate and bivariate analyses to compare PFS and OS for patients stratified by VENmono vs VENcombo. We adjusted for potential confounders, including chromosome deletion 17p (del17p) status, chromosome deletion 11q (del11q) status, Bruton tyrosine kinase inhibitor exposure in a prior line of therapy, number of lines of prior therapies, patient age at start of VEN, and complex karyotype (3 or more abnormalities). For all regression analyses, hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. All other comparisons were descriptive. Statistical analyses were performed using STATA 10.1 (Stata Statistical Software: released 10, 2007; StataCorp LP, College Station, TX). The study was institutional review board approved.
Results
Baseline characteristics
A total of 321 CLL patients were included (VENmono, n = 270; VENcombo, n = 51). A similar proportion of patients was treated in the context of a clinical trial in both groups (n = 7, 16% VENcombo vs n = 32, 12% VENmono). Baseline characteristics stratified by VENmono vs VENcombo are included in Table 1. Patient characteristics were similar except that more patients on VENmono had complex karyotype (≥3 abnormalities, P = .04), and VENmono patients were more heavily pretreated (median 3 prior therapies vs 2, P = .03). Both of these differences might be expected to bias results in favor of the VENcombo group, and therefore, the planned comparison with the study hypothesis was deemed to be appropriate. Moreover, 89% were previously treated with a kinase inhibitor, and 78% received prior ibrutinib (vs 2% reported in the MURANO cohort of VEN plus R).1 The median time from most recent therapy to starting VEN was 1.1 months.
Baseline characteristics
| . | Number of patients with available data . | VEN monotherapy (n = 270) . | VEN combination (n = 51; 38 R, 13 obinutuzumab) . |
|---|---|---|---|
| Median age VEN start | 321 | 68 (37-91) | 66 (45-88) |
| Median prior therapies | 321 | 3 (0-12) | 2 (0-15) |
| Prior ibrutinib | 319 | 79% | 73% |
| Del17p | 323 | 43% | 44% |
| TP53 mutation | 154 | 37% | 25% |
| IGHV unmutated | 118 | 83% | 82% |
| Complex karyotype | 213 | 43% | 25% |
| . | Number of patients with available data . | VEN monotherapy (n = 270) . | VEN combination (n = 51; 38 R, 13 obinutuzumab) . |
|---|---|---|---|
| Median age VEN start | 321 | 68 (37-91) | 66 (45-88) |
| Median prior therapies | 321 | 3 (0-12) | 2 (0-15) |
| Prior ibrutinib | 319 | 79% | 73% |
| Del17p | 323 | 43% | 44% |
| TP53 mutation | 154 | 37% | 25% |
| IGHV unmutated | 118 | 83% | 82% |
| Complex karyotype | 213 | 43% | 25% |
AEs
Available data on AEs stratified by VENmono vs VENcombo are included in Table 2. AEs appeared to be comparable between the VENmono and VENcombo groups. Overall, TLS risk was estimated to be low in 38%, intermediate in 34%, and high in 28%. TLS occurred in 9.7% of patients (9 total clinical events). The overall discontinuation rates for VENmono vs VENcombo were similar at 41% and 39%, respectively.
Adverse events
| . | Number of patients with available data . | VEN monotherapy, % . | VEN combination, % . | P . |
|---|---|---|---|---|
| TLS (overall) | 321 | 11.5 | 5.8 | .07 |
| Grade 3 neutropenia | 233 | 40.4 | 34 | .45 |
| Grade 3 thrombocytopenia | 232 | 30.8 | 23 | .27 |
| Neutropenic fever | 232 | 8.6 | 2.3 | .37 |
| Grade 3 diarrhea | 230 | 8.7 | 5.1 | .36 |
| Discontinuation rate | 321 | 41 | 39 | .77 |
| . | Number of patients with available data . | VEN monotherapy, % . | VEN combination, % . | P . |
|---|---|---|---|---|
| TLS (overall) | 321 | 11.5 | 5.8 | .07 |
| Grade 3 neutropenia | 233 | 40.4 | 34 | .45 |
| Grade 3 thrombocytopenia | 232 | 30.8 | 23 | .27 |
| Neutropenic fever | 232 | 8.6 | 2.3 | .37 |
| Grade 3 diarrhea | 230 | 8.7 | 5.1 | .36 |
| Discontinuation rate | 321 | 41 | 39 | .77 |
Following dose escalation, 70% of patients achieved a stable VEN dose of 400 mg daily; 33% required ≥1 dose interruption, and 26% required ≥1 dose reduction. VEN dose interruptions (≥1, 35% vs 29%) and dose reductions (≥1, 28% vs 22%) were comparable between VENmono and VENcombo groups, respectively.
Response and survival outcomes
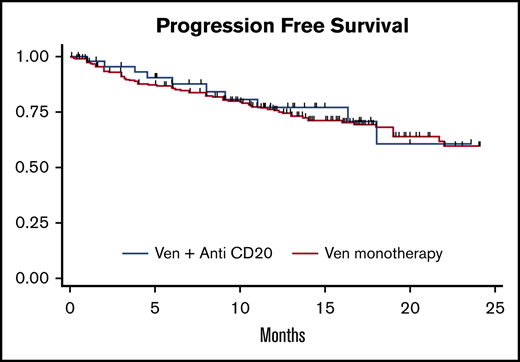
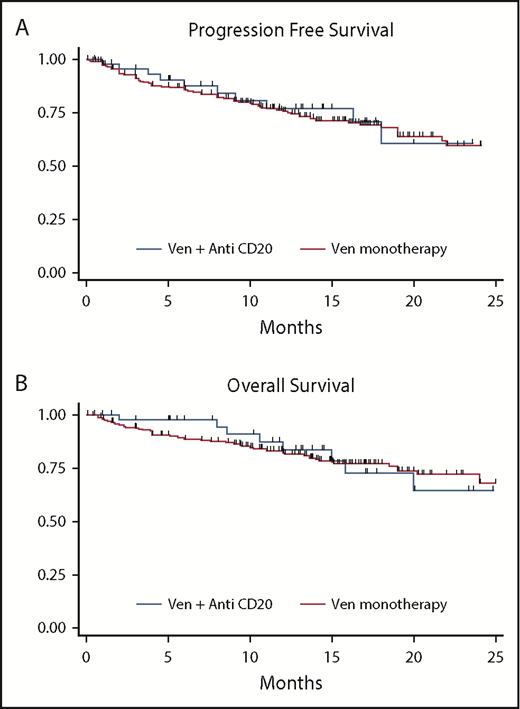
For the entire cohort, the overall response rate (ORR) to VEN was 82% (33% CR) and was similar with VENmono (81% ORR, 34% CR) vs VENcombo (84% ORR, 32% CR). The median time to best response was 2.5 months for VENmono and 2.1 months for VENcombo. With a median follow-up of 13.4 months for the entire cohort (VENmono 13.7 vs VENcombo 12.8 months), a total of 91 progression events and 71 deaths were observed. Although the estimated median PFS and OS were not reached, the estimated 12-month PFS and OS were 74% and 82%, respectively, for the entire cohort. In unadjusted analyses, the HRs for PFS and OS for VENmono vs VENcombo were HR 1.0 (95% CI, 0.6-1.8; P = .7) and HR 1.2 (95% CI, 0.6-2.3; P = .5), respectively. Figure 1 depicts comparable PFS and OS stratified VENmono vs VENcombo in the R/R setting.
PFS and OS. PFS (A) and OS (B) stratified by VEN monotherapy (VENmono) and VEN plus anti-CD20 (VENcombo) in R/R CLL.
PFS and OS. PFS (A) and OS (B) stratified by VEN monotherapy (VENmono) and VEN plus anti-CD20 (VENcombo) in R/R CLL.
Table 3 includes bivariate analyses of VEN pairing status (VENmono vs VENcombo) adjusted for pre-VEN prognostic factors and their impact on HRs for PFS and OS. When adjusting for complex karyotype, number of prior therapies, del17p, del11q, IGHV status, prior ibrutinib exposure, and prior chemoimmunotherapy exposure, PFS and OS remained comparable between the VENmono and VENcombo groups.
Adjusted bivariate analysis
| . | PFS . | OS . |
|---|---|---|
| Unadjusted VEN combo (vs mono) | HR, 1.0; CI, 0.6-1.8; P = .7 | HR, 1.2; CI, 0.6-2.3; P = .5 |
| VEN combo (vs mono) + number prior therapies | HR, 1.0; CI, 0.6-1.7; P = .9 | HR, 1.2; CI, 0.6-2.2; P = .5 |
| VEN combo (vs mono) + del17p | HR, 1.0; CI, 0.6-1.8; P = .12 | HR, 1.3; CI, 0.7-2.7; P = .4 |
| VEN combo (vs mono) + del11p | HR, 1.4; CI, 0.5-1.5; P = .3 | HR, 1.2; CI, 0.5-2.7; P = .4 |
| VEN combo (vs mono) + complex karyotype | HR, 1.0; CI, 0.5-2.1; P = .9 | HR, 1.1; CI, 0.4-2.6; P = .18 |
| VEN combo (vs mono) + IGHV unmutated | HR, 1.1; CI, 0.5-2.3; P = .8 | HR, 1.0; CI, 0.4-2.5; P = .9 |
| VEN combo (vs mono) + prior ibrutinib | HR, 0.9; CI, 0.5-1.8; P = .9 | HR, 1.1; CI, 0.6-2.2; P = .8 |
| VEN combo (vs mono) + prior chemoimmunotherapy | HR, 1.2; CI, 0.6-2.4; P = .7 | HR, 1.1; CI, 0.5-2.4; P = .8 |
| . | PFS . | OS . |
|---|---|---|
| Unadjusted VEN combo (vs mono) | HR, 1.0; CI, 0.6-1.8; P = .7 | HR, 1.2; CI, 0.6-2.3; P = .5 |
| VEN combo (vs mono) + number prior therapies | HR, 1.0; CI, 0.6-1.7; P = .9 | HR, 1.2; CI, 0.6-2.2; P = .5 |
| VEN combo (vs mono) + del17p | HR, 1.0; CI, 0.6-1.8; P = .12 | HR, 1.3; CI, 0.7-2.7; P = .4 |
| VEN combo (vs mono) + del11p | HR, 1.4; CI, 0.5-1.5; P = .3 | HR, 1.2; CI, 0.5-2.7; P = .4 |
| VEN combo (vs mono) + complex karyotype | HR, 1.0; CI, 0.5-2.1; P = .9 | HR, 1.1; CI, 0.4-2.6; P = .18 |
| VEN combo (vs mono) + IGHV unmutated | HR, 1.1; CI, 0.5-2.3; P = .8 | HR, 1.0; CI, 0.4-2.5; P = .9 |
| VEN combo (vs mono) + prior ibrutinib | HR, 0.9; CI, 0.5-1.8; P = .9 | HR, 1.1; CI, 0.6-2.2; P = .8 |
| VEN combo (vs mono) + prior chemoimmunotherapy | HR, 1.2; CI, 0.6-2.4; P = .7 | HR, 1.1; CI, 0.5-2.4; P = .8 |
When adjusting for observed differences between the cohorts, including both complex karyotype and the number of prior therapies, the addition of an anti-CD20 antibody in combination with VEN did not impact PFS (HR, 1.0; 95% CI, 0.5-2.0; P = .9) or OS (HR, 1.1; 95% CI, 0.4-2.6; P = .8) as compared with VENmono.
Discussion
Given the recent broadening of the approval for VEN in R/R CLL, it is important to study whether a clinical benefit exists for VEN plus anti-CD20 over VENmono.2-4 With both options available, clinicians may seek guidance in the appropriate selection of these regimens. In the absence of randomized studies, we sought to answer this question by retrospectively analyzing data on patients treated across various centers in the United States and the United Kingdom.
We demonstrate comparable efficacy between VENmono and VEN plus CD20-directed therapy in a heavily pretreated, high-risk cohort, in terms of both response data and survival outcomes. In adjusted and unadjusted models, there were no differences in PFS and OS between these cohorts. PFS and OS were similar between the groups when controlling for complex karyotype, prior ibrutinib exposure, and adjusting for the number of prior therapies. Our findings were particularly surprising because we could not identify a patient population who benefited from the addition of an anti-CD20 antibody to VEN in terms of response and survival outcomes.
Although our analysis is limited by the retrospective design, relatively short follow-up, and small sample size in the VENcombo group, the length of follow-up presented here is comparable to previously published prospective data that led to the approval of VEN.2-4,15 Longer follow-up of patients receiving VEN may be necessary to understand the depth and durability of response as well as the full impact of VENcombo therapy. There were important differences in our treatment cohorts, with the VENmono group including patients who were more heavily pretreated and more likely to have a complex karyotype. Despite these poor prognostic features in VENmono-treated patients,16-18 the observed survival outcomes were similar in the VENmono and VENcombo groups. Although we collected detailed information on VEN dose reductions and interruptions, we did not have detailed data on the administration schedule and rationale for the anti-CD20 antibody component of the regimen. Thus, we cannot confirm adherence to published combination fixed duration approaches nor have available data on prognostic factors for all patients. Importantly, however, these cohorts do reflect real-world practice across a large number of centers, which addresses an important evidence gap because clinical trial participants are not often representative of routine clinical practice. Regarding response data and survival outcomes, the inclusion of clinical trial participants could potentially introduce bias if imbalanced across comparator groups; however, the proportion of trial participants was similar and were included to capture the entire VEN experience across centers. Although follow-up and response assessments were not standardized due to study design, times to best response were comparable, suggesting these patients were assessed similarly. In addition, we did not confirm all patients with reported CRs had confirmatory bone marrow and computed tomography assessments. With regard to AEs, the retrospective design may make it difficult to capture all events (particularly grade 1 to 2) and therefore underestimates the true VEN toxicity profile. To address this issue, we limited the number of AEs to those of highest clinical importance and note that our AE data are comparable to the incidence of these events reported in clinical trials. Finally, although a few patients treated with VEN plus obinutuzumab were included, we note this comparison did not include enough patients to make conclusions about specific anti-CD20 antibodies or use of VEN plus obinutuzumab in the front-line setting.
Of late, clinical trials in CLL have focused primarily on biologic doublets and triplets.5,19-26 As multiple studies have demonstrated superior outcomes with the addition of CD20 antibodies to chemotherapy in both CLL and B-cell lymphomas, one would assume the same for small-molecule inhibitors. To date, this finding is not observed with ibrutinib in randomized comparisons. Burger et al demonstrated the lack of improved survival outcomes with the addition of R to ibrutinib in a 2-arm study of treatment-naive and relapsed CLL.6,9 These results were confirmed in the front-line ALLIANCE study comparing ibrutinib, R plus ibrutinib, and bendamustine plus R, which demonstrated a nearly identical 2-year PFS in the 2 ibrutinib-containing arms.7,8 Prior to reporting these randomized data, single-arm studies of ibrutinib plus anti CD20 strongly supported the idea that anti-CD20 therapy would improve depth of response and survival outcomes, particularly in high-risk patients.9 Although we note different mechanisms of action between ibrutinib and VEN, these data highlight that hypotheses generated from nonrandomized phase 2 data are not always confirmed in randomized studies.10,27
Unlike patients included in the MURANO trial (BR vs VR in R/R CLL) with 1 median prior therapy, essentially ibrutinib naive (2.6% B-cell receptor inhibitor exposure) and 27% del17p, our cohort was more heavily pretreated (median 3 prior therapies; 78% previously treated with ibrutinib) and higher risk (43% del17p).1,28 We hypothesize that our cohort, which may reflect current VEN use in clinical practice, likely contains a higher proportion of anti-CD20 refractory patients. This may explain the observed lack of benefit for the anti-CD20–containing combination over VENmono. This analysis provides a hypothesis that adding anti-CD20 antibodies to VEN in heavily pretreated patients may not improve clinically relevant outcomes.
These findings need to be confirmed and validated in future randomized studies examining (1) VEN monotherapy vs combinations and (2) treat to progression vs fixed duration/retreatment schedules. Unfortunately, we were not able to identify an ongoing or planned clinical study that compares a VEN-based combination to VEN monotherapy. Given the increasing financial burden of novel therapies, the lack of data on how to sequence after novel-novel combinations fail, and the potential for increased toxicity, our results demonstrate that it is imperative to include monotherapy control arms as we continue to investigate the incremental clinical benefit of novel agent combinations for patients with CLL.29
Authorship
Contribution: A.R.M., L.E.R., T.A.E., C.S.U., and C.P.F. contributed to writing the paper, the analysis, the data collection, and the study design; C.N., S.J.S., A.H.G., and A.A.K. contributed to writing the paper and the study design; N.L., B.T.H., D.M.B., P.M.B., F.L., B.D.C., M.S.Y., N.N.S., J.N.A., J.R., A. M. Williams, A.P.S., C.C.C., R.J., A. M. Winter, J.M.P., and A.Z. contributed to writing the paper and the data collection; A.K.S., E.B.B., K.K., J.M.G., C.D., H.T., N.B., A. Schuh, A. Sitlinger, H.W., and S.M. contributed to the data collection; and M.S. contributed to writing the paper, the data collection, and the study design.
Conflict-of-interest disclosure: A.R.M. has received research funding from TG Therapeutics, AbbVie, Sunesis, LOXO, Pharmaacyclics/J&J, Regeneron, and DTRM BioPharma and has received consulting fees from TG Therapeutics, AbbVie, Acerta, Sunesis, LOXO, Pharmaacyclics/J&J, Regeneron, Celgene, and Prime Oncology. L.E.R. has received a travel grant from AbbVie. T.A.E. has received honorariums from Roche, Gilead, Janssen, and AbbVie; research and travel support to scientific conferences from Janssen, and travel support to scientific conferences from AbbVie. C.N. is employed by Aptitude Health. N.L. participated in advisory boards for AbbVie, AstraZeneca, Genentech, Gilead, Janssen, Juno, Pharmacyclics, is a member of a scientific advisory board for Celgene, and had research funding to the institution from AbbVie, Acerta, AstraZeneca, Beigene, Genentech, Gilead, Juno, Oncternal, TG Therapeutics, and Verastem. B.T.H. has served on advisory boards for and received research funding from Genentech/AbbVie.D.M.B. serves as consultant for, is a member of the scientific advisory board of, and institution is the site of a PI clinical trial (grant paid to the institution) from AbbVie and Genentech. P.M.B. has received consulting fees from AbbVie/PCYC, Gilead, Verastem, Genentech. B.D.C. has received consulting fees from AbbVie, TG Therapeutics, Roche-Genentech, Pharmacyclics, Gilead, Epizyme, Celgene, Morhphosys, Astellas, AstraZeneca, Bayer, Karyopharm, receives research funding to the institution from AbbVie, TG Therapeutics, Roche-Genentech, Pharmacyclics, Gilead, Epizyme, Celgene, Trillium, AstraZeneca. M.S.Y. is a Speaker for Bayer and is a consultant for AbbVie and Octapharma. J.N.A. has received honoraria from Janssen, AstraZeneca and provides advisory/consulting for Pharmacyclics, Janssen, AbbVie, Genentech, Verastem, Sunesis, and Bayer. K.K. has received consulting fees from AbbVie. S.J.S. has received research support and honoraria from AbbVie, Genentech, Pharmacyclics, Janssen, TG Therapeutics, DTRM, and Gilead. A.P.S. has received research funding from BMS, has received consulting fees from Pharmacyclics, Janssen, AbbVie, Genentech, and serves on the Speakers Bureau for Pharmacyclics, Janssen, AbbVie, Genentech, Jazz Pharmaceuticals, Gilead, Kite Seattle, Genetics, and Verastem. C.C.C. has received honoraria from Pharmacyclics and AbbVie and has received consulting fees from AbbVie. C.S.U. has received consulting fees from Genentech, Pharmacyclics, Research AbbVie, Octapharma, and Loxo. R.J. has received honoraria from Genentech and AbbVie. J.M.P. has received consulting fees from AbbVie, Pharmacyclics, Genentech, Jazz Pharmaceuticals and serves on advisory boards for Pharmacyclics, AbbVie, and Genentech, speakers bureau for AbbVie, Pharmacyclics, Genentech, Jazz Pharmaceuticals, Gilead Sciences, Verastem, Kite, and Seattle Genetics. M.S. serves in a consultation or advisory role for AbbVie, Genentech, and Sound Biologics, Verastem, ADC Therapeutics, and Atara Biotherapeutics, and has received research funding from Mustang Bio, Celgene, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, Beigene, Acerta Pharma, and Merck. A. Schuh receives honoraria for attending advisory boards and for educational meetings from Gilead, Janssen, AbbVie, and Roche and also receives education grants from Gilead and Janssen. A.Z. has consulted and/or recieved research support from Genentech/Roche, Gilead, Juno/Celgene, AstraZeneca, MEI Pharma, Verastem, Sandoz, Beigene, Janssen, and Pharmacyclics. C.P.F. has received consulting fees from AbbVie, and performs speaker work, has received travel support and research support, and has received consulting fees from Roche. The remaining authors declare no competing financial interests.
Correspondence: Anthony Mato, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 1065; e-mail: matoa@mskcc.org.
References
Author notes
A.R.M. and L.E.R. are joint first authors.