Key Points
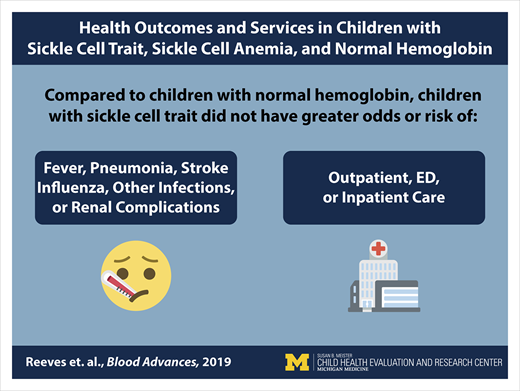
Children with sickle cell trait did not have more select health outcomes or health service encounters than children with normal hemoglobin.
Abstract
The health effects of sickle cell trait among children are unknown. We compared select health outcomes and health services utilization among children with sickle cell trait, sickle cell anemia (SCA), and normal hemoglobin. Newborn screening records were used to identify children with sickle cell trait and SCA born in Michigan (1997-2014) who were enrolled in Michigan Medicaid for ≥1 year from 2012 to 2014. Each select health outcome (acute otitis media, acute respiratory infections, fever, invasive pneumococcal disease, pneumonia and influenza, renal complications, spleen problems, stroke) was defined as ≥1 claim with a diagnosis code for the respective outcome within a study year. Health services utilization was summarized as counts of emergency department, inpatient, and outpatient encounters. The relationship between hemoglobin status and each health outcome or utilization was assessed by logistic or negative binomial regression with generalized estimating equations. The study population consisted of 18 257 children with sickle cell trait, 368 with SCA, and 74 523 with normal hemoglobin (227 188 total person-years). Compared with those with normal hemoglobin, children with sickle cell trait had lower odds of acute otitis media (odds ratio [OR], 0.88; 95% confidence interval [CI], 0.84-0.91), acute respiratory infections (OR, 0.94; 95% CI, 0.92-0.97), pneumonia and influenza (OR, 0.93; 95% CI, 0.87-0.99), and outpatient visits (incidence rate ratio, 0.95; 95% CI, 0.93-0.97). Children with SCA had higher or nonsignificant odds of all outcomes and types of health services utilization. These results indicate that children with sickle cell trait may not be at additional health risk for these outcomes. However, additional case-control studies may be necessary to identify rare events.
Introduction
Sickle cell disease is a chronic disease affecting predominantly minority populations in the United States and is associated with significant morbidity and mortality. The serious health effects of specific subtypes of sickle cell disease, particularly sickle cell anemia (SCA), are well documented.1-4 SCA affects ∼1 in 600 African American births in the United States and is associated with numerous complications, such as acute chest syndrome, pain crises, and increased risks for stroke and infection.1,3,5-10 Given the importance of early intervention in preventing mortality and morbidity associated with SCA, all children are screened at birth for all sickle cell disease variants, including SCA, as part of newborn screening (NBS) programs.11,12
In addition to all cases of sickle cell disease, NBS programs identify the carrier status (ie, sickle cell trait). Sickle cell trait affects ∼1 in 13 African American births in the United States, with a global burden of ∼300 million individuals.13,14 Sickle cell trait has often been considered a relatively benign condition; however, under extreme circumstances, such as high altitude or intense exertion, sickle cell trait is associated with rare morbidities and mortality.13,15-24 On a population level, recent studies with large cohorts of adults have identified an increased risk for chronic kidney disease and venous thromboembolism among individuals with sickle cell trait; additional cohorts have demonstrated no additional risk for certain other conditions.13,16,23,25-31 Although many SCA complications have not been linked to sickle cell trait, there are plausible physiologic pathways that could increase the incidence of these select health outcomes among those with sickle cell trait. For example, those with sickle cell trait may experience splenic infarct, which could leave them more vulnerable to infection.32,33 Very little evidence is available to characterize the potential serious health-related consequences of sickle cell trait among children. Because the spleen plays a key role in fighting infection, this may leave some children with sickle cell trait more vulnerable to infections. However, although sickle cell trait is associated with an increased risk for renal complications in adults, this has never been explored in a pediatric population.30 Further, studies report conflicting results regarding the risk of sickle cell trait for stroke in adults; no studies have examined this outcome among children.34,35
Questions regarding the elevated health risks of sickle cell trait remain unanswered because of the inherent difficulty in identifying children with sickle cell trait. This difficulty is attributed to the follow-up challenges experienced when NBS results are obtained for children with sickle cell trait.36,37 Unlike SCA cases, many individuals with sickle cell trait may not be aware of their carrier status nor are their health care providers aware of their status.38,39 The objective of this study was to assess and compare select health outcomes and health services utilization among children with sickle cell trait and normal hemoglobin by using NBS records linked with Michigan Medicaid administrative claims. Secondarily, we compared these outcomes among children with SCA with children with normal hemoglobin. We hypothesized that, at the population level, children with sickle cell trait would experience more select health outcomes and greater health services utilization than those with normal hemoglobin.
Patients and methods
This study was approved by the Institutional Review Boards at the University of Michigan (HUM00096573) and the Michigan Department of Health and Human Services [IRB #201502-07-NR-(R1)].
Study population
Children with sickle cell trait and SCA (specifically, hemoglobin SS and hemoglobin Sβ0-thalassemia) were identified using NBS records in the state of Michigan from 1997 to 2014. At birth, all children are screened for all hemoglobinopathy variants, including sickle cell trait and SCA, using methods such as high-performance liquid chromatography and isoelectric focusing.40 After referral to a pediatric hematologist, hemoglobin electrophoresis is performed for newborns with hemoglobinopathy results suggestive of disease. Therefore, the NBS records reflective of the results of these tests are the gold standard of identification of children with SCA. Children with sickle cell trait or SCA were then linked to Michigan Medicaid records with electronic birth certificates using a previously validated method.41,42 Children continuously enrolled in Michigan Medicaid with no supplementary forms of health insurance for ≥1 year from 2012 to 2014 were eligible for the study population. Continuous enrollment was defined as enrollment ≥11 months during a calendar year, consistent with the Healthcare Effectiveness Data and Information Set definition.43 Given the beginning of the birth cohort in 1997, all children in the study population were <18 years.
Using Michigan Medicaid records, a sample of 10 unique children continuously enrolled in Michigan Medicaid for ≥1 year from 2012 to 2014 was identified for each case of sickle cell trait and SCA and were matched by birth month and year, year(s) of enrollment in Michigan Medicaid, and race. These children were then compared with NBS records using name, date of birth, and Medicaid identification number. Only children with confirmed normal hemoglobin were eligible for the study population; those with abnormal screening results or missing NBS records were excluded. Because NBS quantifies hemoglobin by type, these records can be used to identify children with normal hemoglobin. Four eligible children with normal hemoglobin were randomly chosen for each child with sickle cell trait or SCA to form the final study population. All Michigan Medicaid claims were obtained for children in the study population for each year of eligibility.
Measures
Select health outcomes.
Select health outcomes were identified for conditions more common in children with SCA than in children with normal hemoglobin.44-46 These outcomes were broadly defined as acute otitis media, acute respiratory infections, fever, invasive pneumococcal disease, pneumonia and influenza, renal complications, spleen problems, and stroke and were identified by International Classification of Diseases, Ninth Edition (ICD-9) code in Medicaid claims (Table 1). Each of the select health outcomes was considered present within the year if a child had ≥1 inpatient admission, ED visit, or outpatient visit with a diagnosis reported in any coded position for the respective outcome.
Administrative claims–based select health outcome definitions
| Health outcome . | ICD-9 codes . |
|---|---|
| Acute otitis media | 381.*, 382.* |
| Acute respiratory infections | 460.*, 461.*, 462.*, 463.*, 464.*, 465.*, 466.* |
| Fever | 0879, 78060, 78061 |
| Invasive pneumococcal disease | 320.1, 038.2, 481, 320.2, 041.2 |
| Pneumonia and influenza | 480.*, 482.*, 483.*, 484.*, 485.*, 486.*, 487.*, 488.* |
| Renal complications | 580.*, 581.*, 582.*, 583.*, 584.*, 585.*, 587.* |
| Spleen problems | 28952, 44283, 90223, 9023*, 90234, 865.* |
| Stroke | 342.*, 431.*, 432.*, 433.*, 434.*, 435.*, 436.*, 437.*, 438.*, 767.* |
| Health outcome . | ICD-9 codes . |
|---|---|
| Acute otitis media | 381.*, 382.* |
| Acute respiratory infections | 460.*, 461.*, 462.*, 463.*, 464.*, 465.*, 466.* |
| Fever | 0879, 78060, 78061 |
| Invasive pneumococcal disease | 320.1, 038.2, 481, 320.2, 041.2 |
| Pneumonia and influenza | 480.*, 482.*, 483.*, 484.*, 485.*, 486.*, 487.*, 488.* |
| Renal complications | 580.*, 581.*, 582.*, 583.*, 584.*, 585.*, 587.* |
| Spleen problems | 28952, 44283, 90223, 9023*, 90234, 865.* |
| Stroke | 342.*, 431.*, 432.*, 433.*, 434.*, 435.*, 436.*, 437.*, 438.*, 767.* |
Indicates all symptoms or manifestations of the main diagnosis preceding the decimal point.
Health services utilization.
Health services utilization was summarized as separate counts of the following types of encounters: ED visits discharged home, inpatient admissions, and nonpreventive outpatient visits. Each encounter was identified using validated Healthcare Effectiveness Data and Information Set definitions, which include Current Procedural Terminology, ICD-9, and revenue code value sets.47 No previous study has assessed health services utilization among those with sickle cell trait. Given the population-based nature of this large-scale study, excess utilization of health services may serve as a proxy for experiencing any unknown select health outcomes among this population.
Statistical analysis
Frequencies and percentages were calculated for demographic characteristics of children in the study population by each study year and hemoglobin status. The proportion of person-years with each select health outcome was summarized overall and by hemoglobin status (ie, sickle cell trait, SCA, normal hemoglobin); proportions were compared across hemoglobin status using χ2 tests. The mean, standard deviation (SD), median, and interquartile range were calculated for each type of annual health service utilization, both overall and by hemoglobin status; mean counts of annual health service utilization were compared across hemoglobin groups using analysis of variance.
Logistic regression with generalized estimating equations (GEEs) was used to assess the relationship between each select health outcome (present/not present) and hemoglobin status (sickle cell trait, SCA), with normal hemoglobin as the reference category. Models were adjusted for race, Medicaid enrollment year, and birth year. Racial categories used in models were collapsed from those presented in Table 2 because of small numbers. GEEs were used to account for the correlation among children contributing multiple years of enrollment throughout the study period.48
Demographics of children enrolled in Michigan Medicaid in 2014 by hemoglobin status (N = 73 417)
| . | Sickle cell trait (n = 14 346) . | SCA (n = 336) . | Normal hemoglobin (n = 58 735) . |
|---|---|---|---|
| Race | |||
| African American | 11 794 (82.21) | 263 (78.27) | 48 239 (82.13) |
| White | 1 184 (8.25) | 8 (2.38) | 4 767 (8.12) |
| Asian | 2 (0.01) | 0 (0) | 8 (0.01) |
| American Indian/Alaska native | 44 (0.31) | 0 (0) | 176 (0.30) |
| Unknown | 957 (6.67) | 64 (19.05) | 4 081 (6.95) |
| Ethnicity | |||
| Hispanic | 365 (2.55) | 1 (0.30) | 1 464 (2.49) |
| Sex | |||
| Female | 7 002 (48.81) | 175 (52.08) | 28 963 (49.31) |
| Male | 7 344 (51.19) | 161 (47.92) | 29 772 (50.69) |
| . | Sickle cell trait (n = 14 346) . | SCA (n = 336) . | Normal hemoglobin (n = 58 735) . |
|---|---|---|---|
| Race | |||
| African American | 11 794 (82.21) | 263 (78.27) | 48 239 (82.13) |
| White | 1 184 (8.25) | 8 (2.38) | 4 767 (8.12) |
| Asian | 2 (0.01) | 0 (0) | 8 (0.01) |
| American Indian/Alaska native | 44 (0.31) | 0 (0) | 176 (0.30) |
| Unknown | 957 (6.67) | 64 (19.05) | 4 081 (6.95) |
| Ethnicity | |||
| Hispanic | 365 (2.55) | 1 (0.30) | 1 464 (2.49) |
| Sex | |||
| Female | 7 002 (48.81) | 175 (52.08) | 28 963 (49.31) |
| Male | 7 344 (51.19) | 161 (47.92) | 29 772 (50.69) |
All data are n (%). The average age (SD) for each hemoglobin status subgroup was 8 years (5).
Similarly, negative binomial regression models with GEEs were used to assess the relationship between count of health services utilization (ED, inpatient, outpatient) and hemoglobin status (sickle cell trait, SCA), with normal hemoglobin as the reference category. Models were adjusted for race, Medicaid enrollment year, and birth year. For all models, generalized model fit was tested by comparing the Akaike information criterion when using a Poisson regression or a negative binomial regression.49 For each model, negative binomial regression resulted in a lower Akaike Information Criterion and, therefore, was used for all analyses. All analyses were performed using SAS 9.4.
Results
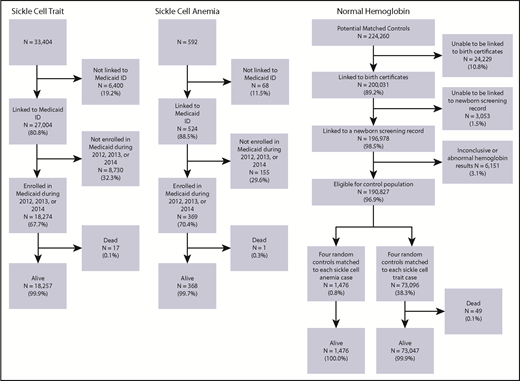
Overall, 33 404 children with sickle cell trait and 592 children with SCA were born in the state of Michigan during the 1997 to 2014 study period (Figure 1). Among these children, 18 274 (54.7%) with sickle cell trait and 369 (62.3%) with SCA were enrolled in Michigan Medicaid for ≥1 year from 2012 to 2014 and not flagged as deceased. A total of 196 978 children with potentially normal hemoglobin was identified using Michigan Medicaid records linked to an NBS record; among these children, 6151 (3.1%) were excluded because of missing or abnormal hemoglobin status in NBS records. Among the remaining 190 827 (96.9%) children, a sample of 4 children (n = 74 572) with normal hemoglobin was chosen for each sickle cell trait and SCA case; children flagged as deceased were excluded. The final study population consisted of 18 257 children with sickle cell trait, 368 children with SCA, and 74 523 children with normal hemoglobin (Figure 1). In total, these 93 148 children contributed a total of 227 188 person-years of Medicaid enrollment. The demographics of the study population in 2014, which are similar across the entire study period, are shown in Table 2.
The proportion of children in the study population who had ≥1 diagnosis code for each of the following health outcomes in a year were as follows: 13.6% acute otitis media, 32.6% acute respiratory infections, 14.2% fever, 0.03% invasive pneumococcal disease, 3.5% pneumonia and influenza, 0.2% renal complications, 0.03% spleen problems, and 0.4% stroke. The frequencies differed significantly by hemoglobin status for each of the select health outcomes (P < .0001) (Table 3). Given the low proportion of person-years with a diagnosis code of spleen problems, these negative health outcomes were excluded from further analysis. Children with sickle cell trait had lower odds of acute otitis media (odds ratio [OR], 0.88; 95% confidence interval [CI], 0.84-0.91), acute respiratory infections (OR, 0.94; 95% CI, 0.92-0.97), and pneumonia and influenza (OR, 0.93; 95% CI, 0.87-0.99) compared with children with normal hemoglobin (Table 4). In contrast, children with SCA had higher rates of select health outcomes compared with children with normal hemoglobin for acute respiratory infections (OR, 1.29; 95% CI, 1.10-1.52), fever (OR, 8.25; 95% CI, 6.85-9.94), invasive pneumococcal disease (OR, 15.55, 95% CI, 5.66-42.72), pneumonia and influenza (OR, 7.38; 95% CI, 6.04-9.03), renal complications (OR, 4.31; 95% CI, 1.78-10.41), and stroke (OR, 12.71; 95% CI, 8.00-20.23) (Table 4).
Annual health services utilization and select health outcomes by hemoglobin status
| . | Sickle cell trait . | SCA . | Normal hemoglobin . | P . |
|---|---|---|---|---|
| Select health outcome, n (%) | ||||
| Acute otitis media | 5 546 (12.48) | 125 (12.63) | 25 225 (13.88) | <.0001 |
| Acute respiratory infections | 14 000 (31.50) | 378 (38.18) | 59 566 (32.77) | <.0001 |
| Fever | 6 212 (13.98) | 489 (49.39) | 25 440 (14.00) | <.0001 |
| Invasive pneumococcal disease | 9 (0.02) | 4 (0.40) | 52 (0.03) | <.0001 |
| Pneumonia and influenza | 1 445 (3.25) | 201 (20.30) | 6 363 (3.50) | <.0001 |
| Renal complications | 73 (0.16) | 7 (0.71) | 276 (0.15) | <.0001 |
| Spleen problems | 6 (0.01) | 48 (4.85) | 7 (0) | <.0001 |
| Stroke | 169 (0.38) | 47 (4.75) | 750 (0.41) | <.0001 |
| Health service utilization, mean encounters (SD) | ||||
| ED visit | 0.75 (1.23) | 1.05 (1.58) | 0.74 (1.24) | <.0001 |
| Inpatient visit | 0.09 (0.46) | 1.32 (1.93) | 0.09 (0.41) | <.0001 |
| Nonpreventive outpatient visit | 1.76 (2.74) | 6.41 (5.77) | 1.87 (2.89) | <.0001 |
| . | Sickle cell trait . | SCA . | Normal hemoglobin . | P . |
|---|---|---|---|---|
| Select health outcome, n (%) | ||||
| Acute otitis media | 5 546 (12.48) | 125 (12.63) | 25 225 (13.88) | <.0001 |
| Acute respiratory infections | 14 000 (31.50) | 378 (38.18) | 59 566 (32.77) | <.0001 |
| Fever | 6 212 (13.98) | 489 (49.39) | 25 440 (14.00) | <.0001 |
| Invasive pneumococcal disease | 9 (0.02) | 4 (0.40) | 52 (0.03) | <.0001 |
| Pneumonia and influenza | 1 445 (3.25) | 201 (20.30) | 6 363 (3.50) | <.0001 |
| Renal complications | 73 (0.16) | 7 (0.71) | 276 (0.15) | <.0001 |
| Spleen problems | 6 (0.01) | 48 (4.85) | 7 (0) | <.0001 |
| Stroke | 169 (0.38) | 47 (4.75) | 750 (0.41) | <.0001 |
| Health service utilization, mean encounters (SD) | ||||
| ED visit | 0.75 (1.23) | 1.05 (1.58) | 0.74 (1.24) | <.0001 |
| Inpatient visit | 0.09 (0.46) | 1.32 (1.93) | 0.09 (0.41) | <.0001 |
| Nonpreventive outpatient visit | 1.76 (2.74) | 6.41 (5.77) | 1.87 (2.89) | <.0001 |
Measures of association and 95% CIs for types of health service utilization and select health outcomes
| . | Sickle cell trait . | SCA . |
|---|---|---|
| Select health outcomes | ORs (95% CI) | |
| Acute otitis media (n = 30 896) | 0.88 (0.84-0.91) | 0.88 (0.70-1.10) |
| Acute respiratory infections (n = 73 944) | 0.94 (0.92-0.97) | 1.29 (1.10-1.52) |
| Fever (n = 32 141) | 1.00 (0.97-1.03) | 8.25 (6.85-9.94) |
| Invasive pneumococcal disease (n = 65) | 0.71 (0.35-1.44) | 15.55 (5.66-42.72) |
| Pneumonia and influenza (n = 8 009) | 0.93 (0.87-0.99) | 7.38 (6.04-9.03) |
| Renal complications (n = 356) | 1.08 (0.77-1.53) | 4.31 (1.78-10.41) |
| Stroke (n = 966) | 0.92 (0.76-1.10) | 12.72 (8.00-20.23) |
| Health services utilization | IRRs (95% CI) | |
| ED discharged home | 1.02 (0.99-1.04) | 1.46 (1.28-1.66) |
| Inpatient visit | 1.03 (0.98-1.09) | 33.31 (1.42-38.95) |
| Nonpreventive outpatient visit | 0.95 (0.93-0.97) | 3.78 (3.43-4.16) |
| . | Sickle cell trait . | SCA . |
|---|---|---|
| Select health outcomes | ORs (95% CI) | |
| Acute otitis media (n = 30 896) | 0.88 (0.84-0.91) | 0.88 (0.70-1.10) |
| Acute respiratory infections (n = 73 944) | 0.94 (0.92-0.97) | 1.29 (1.10-1.52) |
| Fever (n = 32 141) | 1.00 (0.97-1.03) | 8.25 (6.85-9.94) |
| Invasive pneumococcal disease (n = 65) | 0.71 (0.35-1.44) | 15.55 (5.66-42.72) |
| Pneumonia and influenza (n = 8 009) | 0.93 (0.87-0.99) | 7.38 (6.04-9.03) |
| Renal complications (n = 356) | 1.08 (0.77-1.53) | 4.31 (1.78-10.41) |
| Stroke (n = 966) | 0.92 (0.76-1.10) | 12.72 (8.00-20.23) |
| Health services utilization | IRRs (95% CI) | |
| ED discharged home | 1.02 (0.99-1.04) | 1.46 (1.28-1.66) |
| Inpatient visit | 1.03 (0.98-1.09) | 33.31 (1.42-38.95) |
| Nonpreventive outpatient visit | 0.95 (0.93-0.97) | 3.78 (3.43-4.16) |
Reference: normal hemoglobin.
Across the entire study period, the average annual number of ED visits per child was 0.74 (SD, 1.24), the average annual number of inpatient admissions per child was 0.09 (SD, 0.45), and the average annual number of outpatient visits per child was 1.87 (SD, 2.89); annual means differed across hemoglobin status (all P < .0001) (Table 3). Children with sickle cell trait did not have significantly different rates of ED visits or inpatient admissions than children with normal hemoglobin, but they did have slightly lower rates of nonpreventive outpatient visits (incidence rate ratio [IRR], 0.95; 95% CI: 0.93-0.97) (Table 4). For all categories of health services utilization, children with SCA had higher annual mean counts. The negative binomial regression models with GEEs indicated that children with SCA are more likely to have ED visits (IRR, 1.46; 95% CI, 1.28-1.66), inpatient admissions (IRR, 33.31; 95% CI, 1.42-38.95), and nonpreventive outpatient visits (IRR, 3.78; 95% CI, 3.43-4.16) than children with normal hemoglobin (Table 4).
Discussion
This is the first study to evaluate select health outcomes and health services utilization among a large population of children with sickle cell trait. Given the robust design of this study, our results indicate that children with sickle cell trait may not be at additional risk for the select health outcomes or additional health services utilization at a population level compared with children with normal hemoglobin.
The results of this study emphasize the current understanding of the state of sickle cell trait. Although events such as renal medullary carcinoma and extreme exertion-related sudden death have been conclusively linked to sickle cell trait, these events are rare and often associated with extreme conditions, such as severe dehydration.27,50 It would be uncommon for children to experience these situations; therefore, our results are not surprising. However, our findings indicate that children with sickle cell trait may be less likely to experience acute otitis media, acute respiratory infections, and pneumonia and influenza compared with children with normal hemoglobin. It is unlikely that children with sickle cell trait would have fewer complications compared with children with normal hemoglobin. Therefore, a potential explanation for our results may be the lower rate of nonpreventive outpatient visits among children with sickle cell trait. If a child does not have as many interactions with the health care system, there are fewer opportunities for diagnoses of select health outcomes. Therefore, select health outcomes may be systematically underestimated for children with sickle cell trait, and alternative methods for ascertaining these health outcomes may be necessary to make a true comparison between the study population groups. Further, our study is consistent with other findings that children with SCA have higher rates of health services utilization, particularly inpatient stays and ED visits, compared with children with normal hemoglobin.8
Additional evidence to support our findings lies in the robust study design of this research. Our identification of children with SCA, sickle cell trait, and normal hemoglobin is based on NBS records. Therefore, all cases of SCA were confirmed through multiple methods, including hemoglobin electrophoresis. Although sickle cell trait cases were not subject to additional confirmatory screening beyond high-performance liquid chromatography and isoelectric focusing, misclassification of hemoglobin status within our population is nearly nonexistent.41 Therefore, these data sources are the best available resource to perform this type of population-based cohort study. However, because our cohort study was designed to assess population-level patterns in health services utilization and select health outcomes, a case-control study may be an appropriate next step for further assessment of any risk that sickle cell trait confers to an individual. A case-control study would match cases and controls based on the outcome status and is particularly efficient for identifying differences in exposure status for rare outcomes.
There are several limitations to this study. Because the study population includes only children enrolled in Michigan Medicaid, this may not be representative of the entire population of children with sickle cell trait and SCA in the United States. This study is also subject to any coding or missing data concerns within administrative claims.41,42,51 For example, a plausible explanation for our null results regarding sickle cell trait is that there are missing claims; however, given that the health services utilization and select health outcomes for SCA follow the results in numerous other studies, we do not feel that this limitation has a significant impact on our findings. In addition, the assessment of health services utilization and select health outcomes is cross-sectional. Because complications of sickle cell trait may occur across the lifespan, this may limit this study’s ability to identify these outcomes.52,53 Although the use of NBS records indicates that all SCA cases have been subject to confirmatory testing, additional hemoglobin electrophoresis was not conducted among cases of sickle cell trait.40 Because our study is focused on children in the state of Michigan, these results may not be generalizable to children with sickle cell trait that live at higher altitudes.54-59 Finally, our characterization of select health outcomes using ICD-9 Clinical Modification coding was broad and may mask some differences within specific outcomes.
In conclusion, this study is the first large-scale investigation of health services utilization and select health outcomes among children with sickle cell trait. Our results suggest that sickle cell trait may not confer an additional risk for utilization or the specific outcomes that we assessed; however, additional case-control studies may be necessary to identify rare events.
Acknowledgments
The authors thank Acham Gebremariam for invaluable insight into the statistical models and Shiming Dong for assistance with data acquisition.
This work was supported by the Blue Cross Blue Shield of Michigan Foundation (grant 2149.ii).
Authorship
Contribution: The study was designed by S.L.R. and K.J.D.; data were collected, statistical analyses were conducted, and the manuscript was drafted by S.L.R., H.K.J., and J.P.G.; NBS program results were provided by M.K.; and all authors contributed to the interpretation of results and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah L. Reeves, University of Michigan, 300 North Ingalls, Room 6D19, Ann Arbor, MI 48109-5456; e-mail: sleasure@umich.edu.