Key Points
UCB recipients have slower T-cell reconstitution but more robust NK and B-cell recovery after allo-HCT than MSD recipients.
Delayed CD4+ total and naive T-cell reconstitution after allo-HCT increases the risk of infection, mortality, and chronic GVHD.
Abstract
Slow immune reconstitution is a major obstacle to the successful use of allogeneic hematopoietic cell transplantation (allo-HCT). As matched sibling donor (MSD) allo-HCT is regarded as the gold standard, we evaluated the pace of immune reconstitution in 157 adult recipients of reduced-intensity conditioning followed by MSD peripheral blood HCT (n = 68) and compared these to recipients of umbilical cord blood (UCB; n = 89). At day 28, UCB recipients had fewer natural killer (NK) cells than MSD recipients, but thereafter, NK cell numbers (and their subsets) were higher in UCB recipients. During the first 6 months to 1 year after transplant, UCB recipients had slower T-cell subset recovery, with lower numbers of CD3+, CD8+, CD8+ naive, CD4+ naive, CD4+ effector memory T, regulatory T, and CD3+CD56+ T cells than MSD recipients. Notably, B-cell numbers were higher in UCB recipients from day 60 to 1 year. Bacterial and viral infections were more frequent in UCB recipients, yet donor type had no influence on treatment-related mortality or survival. Considering all patients at day 28, lower numbers of total CD4+ T cells and naive CD4+ T cells were significantly associated with increased infection risk, treatment-related mortality, and chronic graft-versus-host disease (GVHD). Patients with these characteristics may benefit from enhanced or prolonged infection surveillance and prophylaxis as well as immune reconstitution–accelerating strategies.
Introduction
Delayed immune reconstitution is one of the major obstacles to successful recovery from allogeneic hematopoietic cell transplantation (allo-HCT), as it is associated with increased risk of infection-associated mortality.1-9 Allo-HCT from HLA-matched sibling donors (MSD) generally provides the best clinical outcomes and thus is regarded as the gold standard for transplantation.10-13 However, because only one-third of patients have an MSD, many patients receive alternative donor transplantation using umbilical cord blood (UCB), unrelated adult volunteers, or related haploidentical donors.14-23 The major advantages of UCB transplantation are the ready availability of UCB units, low risks of injury to the donor, and the lower rates of chronic graft-versus-host disease (GVHD).14,24-25 The major limitations of UCB transplantation are delayed hematopoietic recovery and increased risk of viral infections.3,5,7,26-27 Although the use of double-unit UCB grafts has improved the probability of neutrophil engraftment,28-30 available data on immune reconstitution after UCB transplantation are based on a few single-center reports, limited by small sample size and variability in the conditioning intensities and platforms used.3,5,7,31 Thus, measures of immune recovery after UCB transplantation and its association with infection and treatment-related mortality (TRM) remain unclear, particularly after the commonly used reduced-intensity conditioning (RIC) regimen with fludarabine (Flu), cyclophosphamide (Cy), and total body irradiation (TBI). We evaluated the kinetics of immune reconstitution in adult recipients of RIC allo-HCT for hematological malignancy using HLA 0-2/6 locus mismatched double UCB as compared with HLA MSD peripheral blood grafts.
Methods
Patient selection and treatment
This study included adult patients (≥18 years) with hematological malignancies who received MSD peripheral blood or HLA 0-2/6 locus mismatched double UCB RIC allo-HCT at the University of Minnesota from 2009 to 2014 and were enrolled into a prospective longitudinal immune reconstitution study. Our institutional review board approved all transplant treatment and immune reconstitution monitoring protocol procedures for written informed consent. Peripheral blood samples were prospectively collected at post-HCT days 28, 60, 100, 180, and 365. Patients were excluded if they had received experimental cellular therapies or a prior allo-HCT or died or relapsed before day 28 of transplant.
UCB donor selection was based on institutional guidelines requiring a minimum of 4 of 6 HLA loci matching to the patient at antigen level for HLA-A and HLA-B and at allele level for HLA-DRB1.14 In double UCB transplantation, a minimum of 4 of 6 HLA loci matching was required between 2 UCB units, but not necessarily at the same loci as with the patient.14 Minimum required total nucleated cell dose at cryopreservation was 1.5 × 107 cells/kg per UCB unit.
All study patients received the same RIC regimen consisting of Flu 30 mg/m2 daily for 5 days, Cy at a single dose of 50 mg/kg, and a single fraction of TBI 200 cGy. Equine antithymocyte globulin (ATG) at the dose of 15 mg/kg twice daily on days −6 to −2 was included in conditioning regimen, irrespective of the donor type, for patients who had not received immunosuppressive chemotherapy in the prior 3 months or had a prior autologous transplant. GVHD prophylaxis consisted of mycophenolate mofetil (MMF) administered from day −3 to minimum day +30 or 7 days after neutrophil engraftment in all patients, and cyclosporine (CSA) was administered from day −3 to day +180, but 45 of the 89 UCB recipients received sirolimus instead of CSA.27 All patients received filgrastim (5 mg/kg per day) from day +1 until recovery of absolute neutrophil count ≥2.5 × 109 cells/L for 2 consecutive days. Other than this, similar supportive care was used for UCB and MSD recipients per institutional guidelines, including antimicrobial prophylaxis consisting of fluoroquinolone for bacterial infections, trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis jiroveci, either fluconazole or voriconazole for fungal infections, and acyclovir for viral infections.26
Immunophenotyping
Flow cytometry was performed at days 28, 60, 100, 180, and 365 after transplantation. Briefly, peripheral blood mononuclear cells were isolated, processed and analyzed using standard techniques including isolation with ficoll and monoclonal antibody staining at 4°C, followed by wash with stain buffer (BD Pharmingen) and resuspension (using Cytofix for the initial step). Cells then were stored at 4°C and protected from light until acquisition on the LSRII (BD) flow cytometer within 48 hours. Flow cytometry was performed using 9-color panels of monoclonal antibodies specific for the following surface antigens: immunoglobulin G1 (IgG1), IgG2a, IgM, CD4, CD16, CD27, CD57, CCR7, NKB1 (BioLegend); IgG2a, CD3, CD8 (Invitrogen); IgG1, IgG2b, CD14, CD158a (eBioscience); IgG1, IgG2b, IgM, CD15, CD19, CD25, CD45, CD45RA, CD56, CD127, CD158b (BD Horizon); and IgG2b, NKG2A (Beckman); IgG1, NKG2A (R&D Systems). The immunophenotype data were analyzed using FlowJo software (Tree Star, Ashland, OR). Briefly, the lymphocyte gate was set by gating first on CD45+ cells and then viewing CD45+ events as forward vs side scatter plots and then setting the lymphocyte gate based on standard gating conventions.
Study end points and definitions
The primary end points of the study were to compare immune reconstitution after RIC UCB vs MSD allo-HCT and to identify parameters of immune cell subset recovery associated with infections and TRM (defined as death from any cause without relapse of hematological malignancy). Secondary end points included associations of immune reconstitution with disease-free survival (DFS; defined as being alive and in remission), overall survival (OS; defined as the time from transplant to death from any cause), relapse incidence, and acute and chronic GVHD. The frequency and density of infections where studied within days 28 to 365 post-HCT. Episodes of bacterial, viral, and fungal infections were retrospectively assessed, as previously described.26 Preemptive antiviral therapy was initiated for cytomegalovirus (CMV) reactivation, defined as peripheral blood CMV antigenemia (≥2 pp65-positive cells/50 000; prior to 2006) or DNAemia (≥500 copies by quantitative polymerase chain reaction; after 2006),32 and for peripheral blood Epstein-Barr virus DNAemia (≥1000 copies by polymerase chain reaction)33 as previously described. Neutrophil engraftment was defined as the first day of absolute neutrophil count C ≥0.5 × 109/L for 3 consecutive days. Platelet engraftment was defined as recovery of platelet count >20 × 109L, without platelet transfusion support for the 7 days prior. The grading of acute and chronic GVHD was performed as previously described.34-36 Refined disease risk index (DRI) was used to classify disease risk and status at transplant as being low risk, intermediate risk, or high/very high risk as previously reported.37 Patient comorbidities at transplant were assessed using the HCT comorbidity index.38
Statistical analysis
Baseline patient and transplant characteristics, posttransplantation complications, and outcomes were prospectively collected. Demographic data, transplant characteristics, and immune reconstitution measures were summarized by standard descriptive statistical methods. χ2 or Fisher’s exact test was used to compare categorical variables between the donor type/ATG groups, whereas continuous variables were compared by general Wilcoxon test. Incorporating multiple infections per patient, the infection density was calculated by dividing overall number of infections by the overall observed time of all the patients. We focused on post-HCT day 28 to day 365 as time interval to study the effect of immune reconstitution measures on frequency of bacterial, fungal, and viral infections. To explore association between infection and immune reconstitution, individual infection density was calculated by dividing the number of infections of a patient by the observed time period after post-HCT day 28 to death or day 365 if the patient was alive at the end of the 1-year period. A minimum P value approach was used to determine the cut points of day 28 absolute cell counts per microliter based on the individual infection density outcome adjusted by donor type.39 Multivariable linear regressions were used to compare the individual infection densities between high (at or above the cut point) and low (below the cut point) groups adjusted by donor type with the covariate effects and P values being corrected by using the twofold cross-validation method.40-41 Regressions using log-transformed continuous absolute cell counts as covariates were performed to verify the results from the binary covariate models. The effects of the binary cell count variables on the other clinical end points were not corrected. Cumulative incidence estimator was used to calculate the probabilities of first occurrence of an infection, relapse, acute and chronic GVHD considering the nonevent deaths as a competing risk. The cumulative incidence of TRM was calculated considering the relapse as a competing risk.42 Fine and Gray regression analysis was used to compare the differences between cumulative incidence curves for the end points of TRM, relapse, and GVHD.43 The Kaplan-Meier method was used to estimate the probabilities of DFS and OS through 2 years after allo-HCT, and the log-rank test was used for univariate comparisons.44 Cox proportional hazard regression model was used to estimate the adjusted survival curves.45 Factors that were clinically meaningful or with univariate P < .15 were considered in multivariable analysis. Prognostic factor models for all clinical outcomes were built by backward selection method (P < .05 was considered significant for remaining in the model). All statistical analyses were implemented using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 157 adult patients (≥18 years) received RIC allo-HCT for hematologic malignancies at the University of Minnesota from 2009 to 2014 and had immune reconstitution data available. Of these, 89 patients received UCB allo-HCT, and 68 received MSD peripheral blood allo-HCT (Table 1). Median age at transplant for all patients was 60.7 years (range, 21.2-73.3 years), and nearly one-third of the patients had multiple comorbidities (≥3 HCT comorbidity index). Leukemia (66%) was the most common indication for transplantation, followed by lymphoma (25%). DRI was high/very high risk in 23 (15%) of patients. ATG was used in the conditioning of 43 patients (27%), and ∼55% of patients were CMV seropositive. UCB and MSD donor groups had a similar distribution of patient, disease, and transplant characteristics, including DRI, CMV serostatus, and ATG use in conditioning.
Patient characteristics
| Variable . | Total (n = 157) . | MSD (n = 68) . | UCB (n = 89) . | P . |
|---|---|---|---|---|
| Age, y | ||||
| Median (range) | 61 (21-73) | 60 (21-73) | 61 (22-73) | .35 |
| <60 | 74 (47) | 35 (52) | 39 (44) | .34 |
| ≥60 | 83 (53) | 33 (49) | 50 (56) | — |
| Sex | .64 | |||
| Male | 91 (58) | 38 (56) | 53 (60) | |
| Female | 66 (42) | 30 (44) | 36 (40) | |
| HCT-CI | .58 | |||
| 0 | 49 (31) | 24 (35) | 25 (28) | |
| 1-2 | 59 (38) | 23 (34) | 36 (40) | |
| ≥3 | 48 (31) | 21 (31) | 27 (30) | |
| Missing | 1 (1) | — | 1 (1) | |
| Disease risk index | .27 | |||
| Low | 24 (15) | 14 (21) | 10 (11) | |
| Intermediate | 110 (70) | 45 (66) | 65 (73) | |
| High/very high | 23 (15) | 9 (13) | 14 (16) | |
| Diagnosis | .77 | |||
| Leukemia | 103 (66) | 43 (63) | 60 (67) | |
| Lymphoma | 39 (25) | 19 (28) | 20 (23) | |
| Other | 4 (3) | 1 (2) | 3 (3) | |
| ATG in conditioning | .82 | |||
| Yes | 43 (27) | 18 (27) | 25 (28) | |
| No | 114 (73) | 50 (74) | 64 (72) | |
| CMV seropositive | .92 | |||
| Yes | 87 (55) | 38 (56) | 49 (55) | |
| No | 70 (45) | 30 (44) | 40 (45) | |
| Total infused cell count, median (range) | — | |||
| TNC, ×108/kg | — | 7.8 (4.3-31.6) | 0.4 (0.2-0.9) | |
| CD3, ×108/kg | — | 2.7 (1.2-6.9) | 0.2 (0.1-0.8) | |
| CD34, ×106/kg | — | 5.5 (2.0-18.2) | 0.5 (0.2-3.5) | |
| Follow-up time for survivors, median (range), mo | 35 (12-65) | 35 (12-61) | 35 (12-65) | .51 |
| Variable . | Total (n = 157) . | MSD (n = 68) . | UCB (n = 89) . | P . |
|---|---|---|---|---|
| Age, y | ||||
| Median (range) | 61 (21-73) | 60 (21-73) | 61 (22-73) | .35 |
| <60 | 74 (47) | 35 (52) | 39 (44) | .34 |
| ≥60 | 83 (53) | 33 (49) | 50 (56) | — |
| Sex | .64 | |||
| Male | 91 (58) | 38 (56) | 53 (60) | |
| Female | 66 (42) | 30 (44) | 36 (40) | |
| HCT-CI | .58 | |||
| 0 | 49 (31) | 24 (35) | 25 (28) | |
| 1-2 | 59 (38) | 23 (34) | 36 (40) | |
| ≥3 | 48 (31) | 21 (31) | 27 (30) | |
| Missing | 1 (1) | — | 1 (1) | |
| Disease risk index | .27 | |||
| Low | 24 (15) | 14 (21) | 10 (11) | |
| Intermediate | 110 (70) | 45 (66) | 65 (73) | |
| High/very high | 23 (15) | 9 (13) | 14 (16) | |
| Diagnosis | .77 | |||
| Leukemia | 103 (66) | 43 (63) | 60 (67) | |
| Lymphoma | 39 (25) | 19 (28) | 20 (23) | |
| Other | 4 (3) | 1 (2) | 3 (3) | |
| ATG in conditioning | .82 | |||
| Yes | 43 (27) | 18 (27) | 25 (28) | |
| No | 114 (73) | 50 (74) | 64 (72) | |
| CMV seropositive | .92 | |||
| Yes | 87 (55) | 38 (56) | 49 (55) | |
| No | 70 (45) | 30 (44) | 40 (45) | |
| Total infused cell count, median (range) | — | |||
| TNC, ×108/kg | — | 7.8 (4.3-31.6) | 0.4 (0.2-0.9) | |
| CD3, ×108/kg | — | 2.7 (1.2-6.9) | 0.2 (0.1-0.8) | |
| CD34, ×106/kg | — | 5.5 (2.0-18.2) | 0.5 (0.2-3.5) | |
| Follow-up time for survivors, median (range), mo | 35 (12-65) | 35 (12-61) | 35 (12-65) | .51 |
Values are reported as n (%) of patients, unless indicated otherwise.
HCT-CI, hematopoietic cell transplant comorbidity index; TNC, total nucleated cell.
Immune reconstitution
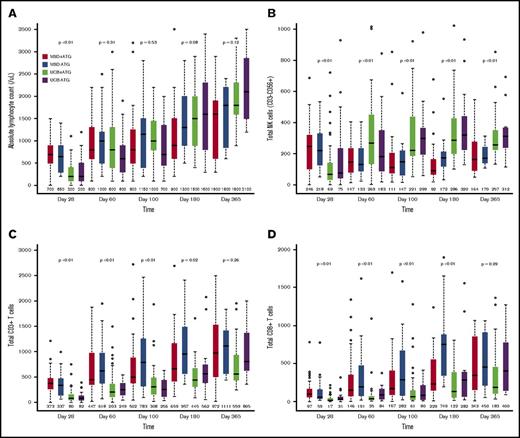
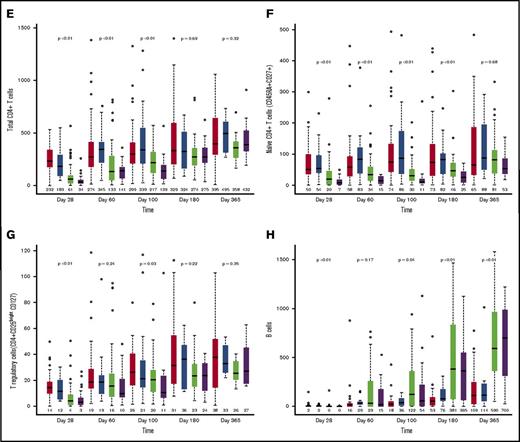
Median absolute lymphocyte count on posttransplant day 28 was significantly lower after UCB allograft, with no significant differences in absolute lymphocyte count thereafter (Figure 1A). Compared with patients receiving MSD, UCB recipients had lower absolute numbers of total natural killer (NK) cells (CD3−CD56+) at day 28, but thereafter, NK cell numbers were significantly higher for UCB recipients at all time points (Figure 1B; all P < .01). In addition, UCB recipients had significantly more rapid recovery of the immature CD56bright NK cells and the more mature CD56dim NK cells (supplemental Figure 1). Regarding CD56dim subpopulations, the numbers of killer cell immunoglobulin-like receptor–expressing NK cells and adaptive NK cells (NKG2C+CD57+) were all significantly higher in UCB recipients at all times after day 28 (all P < .01).
Immune reconstitution after MSD vs UCB RIC allogeneic transplant. (A) Absolute lymphocyte count; (B) total NK cells (CD3−CD56+); (C) total CD3+ T cells; (D) total CD8+ T cells; (E) total CD4+ T cells; (F) naïve CD4+ T cells (CD45RA+CD27+); (G) T regulatory cells (CD4+CD25bright CD127); and (H) B cells. The boxes show the interquartile range of absolute cell count (/μL) for each immune cell type. The bold horizontal lines inside the boxes indicate the median absolute cell count (/μL); the whiskers represent 1.5× the height of the box (or minimum/maximum values if there is no value in that range); and the circles indicate the outliers.
Immune reconstitution after MSD vs UCB RIC allogeneic transplant. (A) Absolute lymphocyte count; (B) total NK cells (CD3−CD56+); (C) total CD3+ T cells; (D) total CD8+ T cells; (E) total CD4+ T cells; (F) naïve CD4+ T cells (CD45RA+CD27+); (G) T regulatory cells (CD4+CD25bright CD127); and (H) B cells. The boxes show the interquartile range of absolute cell count (/μL) for each immune cell type. The bold horizontal lines inside the boxes indicate the median absolute cell count (/μL); the whiskers represent 1.5× the height of the box (or minimum/maximum values if there is no value in that range); and the circles indicate the outliers.
In contrast to NK cells, delayed T-cell recovery was observed in UCB recipients as compared with MSD recipients, independent of ATG use. There were lower CD3+ T-cell counts within 6 months (Figure 1C; all P ≤ .02) and CD3+CD56+ T-cell counts at all time points in the first year of transplant (all P < .01). These differences were mainly driven by significantly lower numbers of CD8+ T cells in UCB recipients over the first 6 months after transplant (Figure 1D; all P < .01). In contrast, UCB recipients had significantly fewer CD4+ T cells for the first 100 days after transplant (Figure 1E; all P < .01) but were similar to MSD recipients thereafter.
Because an UCB graft contains mostly naive T cells, we evaluated CD8+ and CD4+ T-cell subsets over time. Overall, the differences between UCB and MSD recipients decreased over time after transplantation. Examination of CD8+ T-cell subsets in UCB recipients showed that the absolute numbers of CD8+ naive (CD45RA+CD27+) and effector memory (CD45RA−CD27−) T cells remained lower at all times during the 6-month period after transplant (all P ≤ .01). In contrast, the number of CD8+ central memory (CD45RA−CD27+) T cells was significantly lower in UCB recipients than in MSD recipients only during the first 100 days. The absolute numbers of CD4+ naive T cells were lower in UCB recipients than in MSD recipients at all time points during the first 180 days of transplant (all P < .01), whereas the numbers of CD4+ central memory, effector memory, and regulatory T cells (CD25brightCD127) were lower in UCB recipients only during the first 100 days (all P < .03). Notably, the number of B cells (CD19+) was significantly higher in UCB recipients starting at day 100 and remained higher than those in MSD recipients thereafter (Figure 1F; all P < .05). However, the level of immunoglobulin G (IgG) after transplant was not different between the donor types at all time points examined. Strikingly, ATG use in the conditioning regimen had little effect on recovery of immune cell subsets after RIC transplantation, regardless of donor type. We also examined immune cell recovery parameters among UCB recipients treated with CSA/MMF vs sirolimus/MMF GVHD prophylaxis and found similar immune reconstitution patterns in these two GVHD prophylaxis groups (data not shown).
Infection density after transplantation
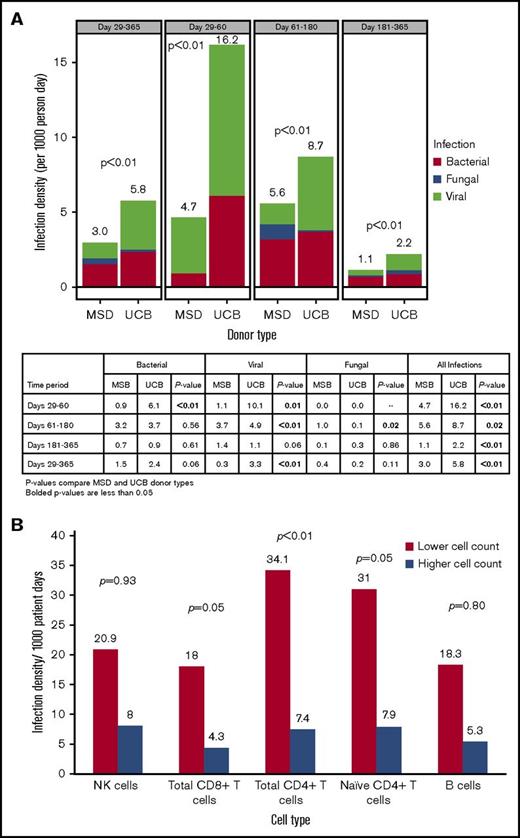
Infection density analysis showed a significantly higher frequency of viral (3.3 vs 0.3; P < .01) infections (per 1000 patient-days) in UCB vs MSD recipients from days 29 to 365 after transplant, whereas the frequency of bacterial (2.4 vs 1.5; P = .06) and fungal infections (0.2 vs 0.4; P = .11) was not significantly different (Figure 2A). Total infection risk (bacterial, viral, and fungal) during this same time period was higher for the UCB group than for the MSD group (5.8 vs 3.0; P < .01). Although total infections remained significantly higher for UCB recipients at all time points within 1 year of transplantation, the higher risk of infection for UCB recipients was driven by viruses and bacteria between days 29 and 60 and by viruses only between days 61 and 180, whereas fungal infection risk was significantly higher for MSD recipients during this later time period after transplant.
Infection density. (A) Infection density by donor type and post-HCT time period. (B) Infection density at days 29 to 365 by lower and higher absolute cell counts.
Infection density. (A) Infection density by donor type and post-HCT time period. (B) Infection density at days 29 to 365 by lower and higher absolute cell counts.
A minimum P value approach was used to identify the association between the absolute cell count cutoffs for CD8+ total T cells (34.8 cells/μL), CD4+ total T cells (8.9 cells/μL), and naive T cells (1.3 cells/μL) at day 28 and total infection risk between days 29 to 365, but the association with NK cells (43.8 cells/μL) and B cells (0.4 cells/μL) did not achieve statistical significance. The frequency of total infections was significantly higher in patients with lower cell counts (below the median absolute counts) of CD8+ total (18.0 vs 4.3 per 1000 patient-days, twofold cross validated P = .05), CD4+ total (34.1 vs 7.4 per 1000 patient-days, twofold cross validated P < .01), and naive (31.0 vs 7.9 per 1000 patient-days, twofold cross validated, P = .05) T cells than in those with higher cell counts (at or above the median absolute counts) (Figure 2B). Infection-related mortality at 1 year after transplant was 4% (n = 4) in the UCB group and 6% (n = 4) in MSD group (P = .70).
TRM
The probability of TRM for the entire cohort was 8% (range, 4% to 13%) at day 100 (11% for UCB vs 4% for MSD; P = .15). When considering both the UCB and MSD recipients, the cumulative incidence of TRM at day 100 for patients with lower vs higher NK cell (16% vs 6%, P = .09), CD8+ total T-cell (11% vs 6%, P = .28), and B-cell counts (11% vs 6%, P = .41) at day+28 was not significantly different. In contrast, significantly higher TRM at day 100 was observed in patients with lower CD4+ cell (25% vs 6%, P < .01) and naive CD4+ T-cell counts (27% vs 6%, P < .01) at day 28. In multivariable analysis adjusted for donor type and DRI, NK cell, CD8+ total T-cell, and B-cell counts were not significantly associated with TRM (Table 2). In contrast, lower cell counts for CD4+ total T cells and naive T cells were independently associated with an increased risk of TRM (4.3-fold) at day 100 after transplantation.
Survival and relapse by immune cell type
| Variable . | . | Multivariable . | ||
|---|---|---|---|---|
| Total no. of patients . | RR/HR . | 95% CI . | P . | |
| TRM at day 100 | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.66-7.77 | .20 |
| Lower (<43.8) | 31 | 2.26 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.33-6.28 | .63 |
| Lower (<34.8) | 66 | 1.44 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 1.27-14.39 | .02 |
| Lower (<8.9) | 16 | 4.27 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 1.26-14.39 | .02 |
| Lower (<1.3) | 15 | 4.26 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.60-5.06 | .31 |
| Lower (<0.4) | 56 | 1.74 | ||
| DFS at 1 y | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.83-2.66 | .18 |
| Lower (<43.8) | 31 | 1.49 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.45-1.35 | .36 |
| Lower (<34.8) | 66 | 0.77 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.77-3.22 | .21 |
| Lower (<8.9) | 16 | 1.58 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.58-2.59 | .60 |
| Lower (<1.3) | 15 | 1.22 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4 cells/μL) | 97 | 1.0 | 0.64-1.74 | .84 |
| Lower (<0.4 cells/μL) | 56 | 1.05 | ||
| OS at 1 y | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.76-2.97 | .24 |
| Lower (<43.8) | 31 | 1.51 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.55-2.06 | .84 |
| Lower (<34.8) | 66 | 1.07 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.74-3.83 | .22 |
| Lower (<8.9) | 16 | 1.68 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.80-4.31 | .15 |
| Lower (<1.3) | 15 | 1.86 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.81-2.70 | .20 |
| Lower (<0.4) | 56 | 1.48 | ||
| Relapse at 1 y | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.62-2.60 | .52 |
| Lower (<43.8) | 31 | 1.27 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.40-1.60 | .53 |
| Lower (<34.8) | 66 | 0.80 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.28-2.29 | .67 |
| Lower (<8.9) | 16 | 0.80 | ||
| CD4+ naive T cells (CD45RA+ CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.15-1.73 | .27 |
| Lower (<1.3) | 15 | 0.50 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.33-1.28 | .21 |
| Lower (<0.4) | 56 | 0.65 | ||
| Variable . | . | Multivariable . | ||
|---|---|---|---|---|
| Total no. of patients . | RR/HR . | 95% CI . | P . | |
| TRM at day 100 | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.66-7.77 | .20 |
| Lower (<43.8) | 31 | 2.26 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.33-6.28 | .63 |
| Lower (<34.8) | 66 | 1.44 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 1.27-14.39 | .02 |
| Lower (<8.9) | 16 | 4.27 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 1.26-14.39 | .02 |
| Lower (<1.3) | 15 | 4.26 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.60-5.06 | .31 |
| Lower (<0.4) | 56 | 1.74 | ||
| DFS at 1 y | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.83-2.66 | .18 |
| Lower (<43.8) | 31 | 1.49 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.45-1.35 | .36 |
| Lower (<34.8) | 66 | 0.77 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.77-3.22 | .21 |
| Lower (<8.9) | 16 | 1.58 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.58-2.59 | .60 |
| Lower (<1.3) | 15 | 1.22 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4 cells/μL) | 97 | 1.0 | 0.64-1.74 | .84 |
| Lower (<0.4 cells/μL) | 56 | 1.05 | ||
| OS at 1 y | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.76-2.97 | .24 |
| Lower (<43.8) | 31 | 1.51 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.55-2.06 | .84 |
| Lower (<34.8) | 66 | 1.07 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.74-3.83 | .22 |
| Lower (<8.9) | 16 | 1.68 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.80-4.31 | .15 |
| Lower (<1.3) | 15 | 1.86 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.81-2.70 | .20 |
| Lower (<0.4) | 56 | 1.48 | ||
| Relapse at 1 y | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.62-2.60 | .52 |
| Lower (<43.8) | 31 | 1.27 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.40-1.60 | .53 |
| Lower (<34.8) | 66 | 0.80 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.28-2.29 | .67 |
| Lower (<8.9) | 16 | 0.80 | ||
| CD4+ naive T cells (CD45RA+ CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.15-1.73 | .27 |
| Lower (<1.3) | 15 | 0.50 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.33-1.28 | .21 |
| Lower (<0.4) | 56 | 0.65 | ||
HR was adjusted for age, donor type, and DRI and used for disease-free and overall survival analysis. HR denotes an increased risk of mortality. Relative risk (RR) was adjusted for donor type and DRI and used for TRM and relapse analysis.
Survival and relapse
Considering the whole cohort of patients, the probability of OS was 64% (range, 56% to 71%) and the probability of DFS was 54% (range, 45% to 61%) 1 year after transplant. When comparing UCB and MSD, the probabilities of OS (65% vs 64%, P = .82) and DFS (53% vs 54%, P = .98) at 1 year were not different. We found no association between any of the immune cell subsets examined at day 28 and either OS or DFS. In multivariable analysis adjusted for donor type, patient age, and DRI, lower cell count of any of the immune cell subsets examined did not independently influence the risk of overall mortality or treatment failure (inverse of DFS).
Cumulative incidence of relapse 1 year after transplant was 29% (range, 22% to 33%) for all patients, and it was similar in UCB (27%) and MSD (31%) groups (P = .58). We also examined the effect of the absolute number of NK cells, CD8+ total T cells, CD4+ total and naive T cells, and B cells at day 28 on relapse and observed no difference in risk of relapse 1 year after transplant in patients with lower vs higher cell counts.
Hematopoietic engraftment and GVHD
Hematopoietic engraftment was successful in the majority of study participants, with >90% of the patients (n = 144) achieving neutrophil engraftment by day 28 after transplant (91% for UCB and 99% for MSD peripheral blood patient groups; P = .04) (Table 3). The risk of primary graft failure was only 3% (n = 4) for the entire cohort, with cumulative incidence of neutrophil recovery by day 42 of 96% for patients receiving UCB and 100% for those receiving MSD (P < .01). Median time of neutrophil engraftment was 14 days for the UCB group and 10 days for the MSD group (P = .12). Platelet engraftment by day 180 was 87% for the entire cohort (81% for patients receiving UCB and 96% for those receiving MSD; P < .01). In multivariable analysis after adjusting for donor type, lower NK cell count at day 28 was independently associated with worse neutrophil engraftment by day 42 (hazard ratio [HR] = 0.5; 95% confidence interval [CI], 0.3-0.8; P < .01). Lower NK cell counts (HR = 0.6; 95% CI, 0.4-0.9; P < .01) also predicted worse platelet engraftment by day 180.
Hematopoietic engraftment and GVHD by immune cell type
| Variable . | . | Multivariable . | ||
|---|---|---|---|---|
| Total no. of patients . | RR . | 95% CI . | P . | |
| Neutrophil engraftment at day 42 | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.31-0.82 | <.01 |
| Lower (<43.8) | 31 | 0.51 | ||
| Total CD8+ T cells (CD3+CD4-CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 1.02-2.13 | .04 |
| Lower (<34.8) | 66 | 1.47 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.42-1.27 | .27 |
| Lower (<8.9) | 16 | 0.73 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.36-1.13 | .12 |
| Lower (<1.3) | 15 | 0.64 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.56-1.15 | .24 |
| Lower (<0.4) | 56 | 0.80 | ||
| Platelet engraftment at day 180 | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.36-0.86 | .01 |
| Lower (<43.8) | 31 | 0.56 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.56-1.32 | .48 |
| Lower (<34.8) | 66 | 0.86 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.47-1.45 | .51 |
| Lower (<8.9) | 16 | 0.83 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.38-1.36 | .31 |
| Lower (<1.3) | 15 | 0.72 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.50-1.02 | .06 |
| Lower (<0.4) | 56 | 0.71 | ||
| Grade II-IV acute GVHD at day 180 | ||||
| Total NK cells (CD3-CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.52-2.23 | .85 |
| Lower (<43.8) | 31 | 1.08 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.44-2.05 | .90 |
| Lower (<34.8) | 66 | 0.95 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.46-2.66 | .81 |
| Lower (<8.9) | 16 | 1.11 | ||
| CD4+ naive T cells (CD45RA+ CD27+) | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.27-1.99 | .54 |
| Lower (<1.3) | 15 | 0.73 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.59-2.19 | .70 |
| Lower (<0.4) | 56 | 1.14 | ||
| Chronic GVHD at 1 y | ||||
| Total NK cells (CD3-CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.67-4.45 | .26 |
| Lower (<43.8) | 31 | 1.72 | ||
| Total CD8+ T cells (CD3+CD4-CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.61-3.02 | .45 |
| Lower (<34.8) | 66 | 1.36 | ||
| Total CD4+ T cells (CD3+CD4+CD8-), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 1.02-5.79 | .04 |
| Lower (<8.9) | 16 | 2.43 | ||
| CD4+ naive T cells (CD45RA+ CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 1.63-7.98 | <.01 |
| Lower (<1.3) | 15 | 3.60 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.92-3.96 | .08 |
| Lower (<0.4) | 56 | 1.91 | ||
| Variable . | . | Multivariable . | ||
|---|---|---|---|---|
| Total no. of patients . | RR . | 95% CI . | P . | |
| Neutrophil engraftment at day 42 | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.31-0.82 | <.01 |
| Lower (<43.8) | 31 | 0.51 | ||
| Total CD8+ T cells (CD3+CD4-CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 1.02-2.13 | .04 |
| Lower (<34.8) | 66 | 1.47 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.42-1.27 | .27 |
| Lower (<8.9) | 16 | 0.73 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.36-1.13 | .12 |
| Lower (<1.3) | 15 | 0.64 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.56-1.15 | .24 |
| Lower (<0.4) | 56 | 0.80 | ||
| Platelet engraftment at day 180 | ||||
| Total NK cells (CD3−CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.36-0.86 | .01 |
| Lower (<43.8) | 31 | 0.56 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.56-1.32 | .48 |
| Lower (<34.8) | 66 | 0.86 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.47-1.45 | .51 |
| Lower (<8.9) | 16 | 0.83 | ||
| CD4+ naive T cells (CD45RA+CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.38-1.36 | .31 |
| Lower (<1.3) | 15 | 0.72 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.50-1.02 | .06 |
| Lower (<0.4) | 56 | 0.71 | ||
| Grade II-IV acute GVHD at day 180 | ||||
| Total NK cells (CD3-CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.52-2.23 | .85 |
| Lower (<43.8) | 31 | 1.08 | ||
| Total CD8+ T cells (CD3+CD4−CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.44-2.05 | .90 |
| Lower (<34.8) | 66 | 0.95 | ||
| Total CD4+ T cells (CD3+CD4+CD8−), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 0.46-2.66 | .81 |
| Lower (<8.9) | 16 | 1.11 | ||
| CD4+ naive T cells (CD45RA+ CD27+) | ||||
| Higher (≥1.3) | 139 | 1.0 | 0.27-1.99 | .54 |
| Lower (<1.3) | 15 | 0.73 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.59-2.19 | .70 |
| Lower (<0.4) | 56 | 1.14 | ||
| Chronic GVHD at 1 y | ||||
| Total NK cells (CD3-CD56+), cells/μL | ||||
| Higher (≥43.8) | 126 | 1.0 | 0.67-4.45 | .26 |
| Lower (<43.8) | 31 | 1.72 | ||
| Total CD8+ T cells (CD3+CD4-CD8+), cells/μL | ||||
| Higher (≥34.8) | 88 | 1.0 | 0.61-3.02 | .45 |
| Lower (<34.8) | 66 | 1.36 | ||
| Total CD4+ T cells (CD3+CD4+CD8-), cells/μL | ||||
| Higher (≥8.9) | 138 | 1.0 | 1.02-5.79 | .04 |
| Lower (<8.9) | 16 | 2.43 | ||
| CD4+ naive T cells (CD45RA+ CD27+), cells/μL | ||||
| Higher (≥1.3) | 139 | 1.0 | 1.63-7.98 | <.01 |
| Lower (<1.3) | 15 | 3.60 | ||
| Total B cells (CD19+), cells/μL | ||||
| Higher (≥0.4) | 97 | 1.0 | 0.92-3.96 | .08 |
| Lower (<0.4) | 56 | 1.91 | ||
RR was adjusted for donor type.
Cumulative incidence of grade II to IV acute GVHD at day 100 was similar in the 2 donor groups (34% in the UCB group and 31% in the MSD group; P = .51). However, the rate of chronic GVHD 1 year after transplant was significantly lower in those receiving UCB (9% vs 35%; P < .01). In multivariable analysis, after adjusting for donor type, lower counts of CD4+ total T cells (HR = 2.4; 95% CI, 1.0-5.8; P = .04) and naive T cells (HR = 3.6; 95% CI, 1.6-8.0; P < .01) at day 28 independently increased the risk of chronic GVHD.
Discussion
In this study, we prospectively analyzed immune reconstitution after UCB and MSD peripheral blood RIC allo-HCT with or without ATG in adults with hematological malignancies. We identified that reconstitution of naive and total CD4+ T cells at day 28 after transplant is associated with fewer infections. The pace of recovery of these cells at day 28 was also an independent predictor of TRM in our analysis. Reconstitution of CD4+ T cells within 90 to 100 days of allo-HCT has been previously shown to predict OS in pediatric patients undergoing myeloablation.31,46 In our study, despite the increase in TRM with lower CD4+ T-cell counts, OS was not significantly affected by cell count recovery at day 28. If confirmed in an independent cohort, our results may pave the way for early identification of patients at the highest risk for infections and TRM who might benefit from intensified infection surveillance and prophylaxis and who would be candidates for the testing of novel interventions to accelerate immune recovery (eg, vaccines, homeostatic cytokine therapies, or virus-specific mature T-cell adoptive transfer).47-53
This comparative analysis between UCB and MSD recipients used a homogeneous RIC regimen (Flu/Cy/TBI) and a similar GVHD prophylaxis in the majority of patients and allowed us to assess differences in the immune reconstitution between the 2 donor types. A similar pattern of more rapid NK and B-cell recovery after UCB HCT has been reported in a study comparing UCB and matched unrelated donor RIC allo-HCT, but due to variation in conditioning/GVHD prophylaxis based on donor type, it was unclear what lead to the differences in immune reconstution.5 However, considering our findings in the context of the above study, UCB has distinct differences in immune recovery compared with adult donor sources.
Consistent with other reports demonstrating delayed CD8+ and CD4+ T-cell immune reconstitution after UCB allo-HCT,1-5,7 we also found delayed T-cell recovery in UCB recipients as compared with MSD recipients. De novo naive T-cell reconstitution is largely dependent on thymic function, and delays in T-cell recovery have been attributed to suppressed thymic function in adult patients.3 Interestingly, there was robust recovery of naive T cells after MSD HCT in our study of only adult patients, whereas naive T cells recovery was delayed in UCB recipients. Because this study used surface phenotype as a measure of naive T cells, it is limited by the lack of data on T-cell receptor excision circle analysis, which would more definitively distinguish true postthymic T cells from homeostatic expanded naive T cells. Thus, it is unclear whether these results point to differences between the 2 donor types in the propensity to give rise to thymic seeding cells, but further studies should address this point. In our study, CD4+ T-cell reconstitution after UCB transplant appears to be slower than that reported in pediatric UCB recipients,54-55 which is likely explained by lower infused total nucleated cell dose per kilogram in adult UCB recipients and a functionally intact thymus in pediatric patients.
We also observed a higher risk of chronic GVHD with delayed recovery of CD4+ total and naive T cells. A similar association between low naive CD4+ T-cell counts and a higher risk of GVHD has recently been reported in animal model.56
Profound T-cell lymphopenia after UCB allo-HCT likely explains the robust recovery of NK cells early after transplant, followed by recovery of B cells in UCB recipients compared with other adult donor types, as previously described.4 In contrast to a recent report of rabbit ATG (thymoglobulin) negatively affecting the immune reconstitution after pediatric allo-HCT,46 the use of equine ATG in the conditioning regimen in our study had no significant effect on immune reconstitution after RIC allo-HCT, even though close to one-third of our patients received ATG. This can likely be explained by the differences in ATG source as well as by timing of ATG use in these studies as previously reported.55,57
Similar to other reports, we also observed an increased risk of infections after UCB transplantation as compared with MSD transplant.58-60 Increased viral infections in particular have been one of the major complications reported in UCB recipients and have been attributed to the lack of antigen-specific cells and the relative immaturity of cord blood immune cells.61 Infection-related mortality was low overall after RIC allo-HCT and was similar after UCB and MSD allo-HCT. This is likely explained by improved supportive care and availability of better antimicrobial prophylaxis after allo-HCT in recent years.62-65 Similarly, there was a low incidence of invasive fungal infections in our study, likely due to the use of potent antifungal prophylaxis.62,64-65 In addition, more robust NK cell recovery after day 60 and B-cell recovery after day 100 of UCB transplantation can likely explain relative protection of UCB recipients from serious infections.
When examining immune cell subset recovery parameters after RIC allo-HCT, we identified an association between NK cell count recovery and hematopoietic engraftment in our analysis, which is likely explained by granulocyte-macrophage colony-stimulating factor production by NK cells, as previously reported.66 In addition, we found that CD4+ naive and total T-cell counts as early as day 28 after transplant can potentially be used to identify patients who are at highest risk of serious infections and TRM due to delayed immune reconstitution after RIC allo-HCT. The sample size of our cohort limited our ability to detect associations between certain immune cell subtypes and specific infections. Despite delayed T-cell immune reconstitution and a higher risk of infection after UCB HCT compared with MSD HCT, long-term clinical outcomes of UCB and MSD RIC allo-HCT were similar in our study. We observed no influence of the donor type on TRM, relapse, DFS, or OS in this study. In contrast, higher CD4+ naive and total T cells are protective for infections, TRM, and chronic GVHD after RIC allo-HCT with UCB or MSD peripheral blood. Therapeutic strategies to accelerate immune reconstitution in UCB allo-HCT in order to overcome the risk of viral infection could expand the use of this readily available alternative donor type to more adults with high-risk hematological malignancies. Ongoing clinical trials testing multivirus-specific cytotoxic T-lymphocyte infusions for prevention of viral infections in allo-HCT recipients will provide further insight.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Michael Franklin for assistance in editing this manuscript. The University of Minnesota Oncology Medical Informatics & Services prepared the dataset and Translational Therapy Laboratory prepared the cytometric data.
This work was supported in part by the National Institutes of Health, National Cancer Institute grants P30 CA77598 (utilizing the Oncology Medical Informatics & Services and Translational Therapy Laboratory Shared Resources of the Masonic Cancer Center, University of Minnesota) and P01 CA65493 (J.E.W., C.G.B., M.R.V., J.S.M., and B.R.B.).
Authorship
Contribution: N.B. and M.R.V. conceived the study; Q.C. and X.L. analyzed and interpreted data; N.B. wrote the manuscript; N.B., C.G.B., Q.C., A.L., X.L., J.C., R.S.M., E.W., S.A.C., B.R.B., J.S.M., D.W., J.E.W., and M.R.V. interpreted and edited the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nelli Bejanyan, Division of Hematology, Oncology and Transplantation, University of Minnesota, 420 Delaware St SE, Mayo Mail Code 480, Minneapolis, MN 55455; e-mail: nelli.bejanyan@moffitt.org.