Key Points
A single rare DNMT3A mutation and recurrent amplification of ETS1, PTPN6, and TGFBR2 are identified in iMCD and UCD.
Genetic alterations in oncogenes, tumor suppressors, and chromatin-remodeling genes are seen in FDCS.
Abstract
Castleman disease (CD) is a rare lymphoproliferative disorder subclassified as unicentric CD (UCD) or multicentric CD (MCD) based on clinical features and the distribution of enlarged lymph nodes with characteristic histopathology. MCD can be further subtyped based on human herpes virus 8 (HHV8) infection into HHV8-associated MCD, HHV8−/idiopathic MCD (iMCD), and polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin change (POEMS)–associated MCD. In a subset of cases of UCD, an associated follicular dendritic cell sarcoma (FDCS) may be seen. Although numerous reports of the clinical and histologic features of UCD, MCD, and FDCS exist, an understanding of the genetic and epigenetic landscape of these rare diseases is lacking. Given this paucity of knowledge, we analyzed 15 cases of UCD and 3 cases of iMCD by targeted next-generation sequencing (NGS; 405 genes) and 3 cases of FDCS associated with UCD hyaline vascular variant (UCD-HVV) by whole-exome sequencing. Common amplifications of ETS1, PTPN6, and TGFBR2 were seen in 1 iMCD and 1 UCD case; the iMCD case also had a somatic DNMT3A L295Q mutation. This iMCD patient also showed clinicopathologic features consistent with a specific subtype known as Castleman-Kojima disease (thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly [TAFRO] clinical subtype). Additionally, 1 case of UCD-HVV showed amplification of the cluster of histone genes on chromosome 6p. FDCS associated with UCD-HVV showed mutations and copy number changes in known oncogenes, tumor suppressors, and chromatin structural-remodeling proteins.
Introduction
Castleman disease (CD), also known as angiofollicular lymphoid hyperplasia, giant lymph node hyperplasia, and lymphoid hamartoma, is a rare lymphoproliferative disorder.1 Based on clinical features and the distribution of enlarged lymph nodes with characteristic histopathology, CD can be subclassified into localized unicentric CD (UCD) or multicentric CD (MCD). MCD is further subtyped based on human herpes virus 8 (HHV8) status to either HHV8-associated MCD or HHV8− MCD. HHV8− MCD is further subdivided into idiopathic MCD (iMCD) or polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin change (POEMS)–associated MCD. HHV8-associated MCD occurs due to uncontrolled HHV8 infection in individuals with HIV infection or other causes of immunodeficiency. Histologically, UCD can be subdivided into a hyaline vascular variant (UCD-HVV) and a plasma cell variant (UCD-PCV), as well as a mixed variant (UCD-MV), which shares morphologic features of both UCD-HVV and UCD-PCV. The pathogenesis of iMCD and UCD is still not well understood.2 In 2010, Takai et al3 described 3 patients with findings consistent with iMCD and a further characteristic constellation of clinical features including thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly (TAFRO): a clinical subtype of iMCD also known as Castleman-Kojima syndrome.4,5 Since then, numerous patients have been identified; some reportedly respond well to immunosuppressive therapy, specifically cyclosporine and prednisolone.
In recent years, cytogenetic and molecular studies have demonstrated clonal abnormalities in UCD and iMCD.6,7 Recently, studies of a rare clinically aggressive mesenchymal tumor, follicular dendritic cell sarcoma (FDCS), have been performed with next-generation sequencing (NGS) and identified somatic mutations in a subset of cases.8,9 However, the mutational landscape of MCD and UCD, and FDCS associated with UCD-HVV remains an enigma. As such, we performed targeted sequencing of lymph node tissue specimens from patients with iMCD and UCD as well as whole-exome sequencing (WES) of FDCS to delineate the underlying molecular aberrations that may have a pathogenetic role.
Materials and methods
Patient cohort
Fifteen cases of UCD, 3 cases of iMCD (2 of 3 cases showed clinicopathologic features further consistent with the TAFRO clinical subtype of iMCD), and 3 cases of FDCS associated with UCD-HVV, diagnosed between 2002 and 2015, were identified from the archives of the Department of Pathology, Stanford University Medical Center (Stanford, CA). All cases and slides were reviewed and diagnoses confirmed. Patient medical record charts were reviewed, and clinical and laboratory data as well as treatment data were reviewed by Stanford specialists in hematology and hematopathology (R.S.O., J.L.Z., T.M., and R.A.W.). This study was approved by Stanford University’s Institutional Review Board.
High-throughput sequencing and analysis
NGS with alignment, single-nucleotide variant, copy-number variant (CNV), translocation, and indel analysis of DNA data were performed as previously described; translocations were evaluated using the Fusion and Chromosomal Translocation Enumeration and Recovery Algorithm (FACTERA).10-12 DNA was isolated from lymph node tissues using the QIAamp DNA FFPE tissue kit (Qiagen). Targeted exome sequencing of 405 genes (FoundationOne [F1]) was performed for the 15 UCD and 3 iMCD cases and WES for the 3 FDCSs associated with UCD-HVV cases. Targeted hybrid-capture NGS (F1; Foundation Medicine Inc) of 405 genes was performed as previously described.12 In brief, 50 to 200 ng of DNA was fragmented before library construction by sonication and adaptor ligated libraries created. Sanger sequencing of select genes was performed at an early stage to evaluate DNA quality and also assure that NGS assays were performing as anticipated. Solution hybridization was performed using pools of biotinylated DNA baits. Libraries were pooled and sequenced on an Illumina HiSeq2500. A median exon coverage of 150 to 250 was considered qualified. A mutant allele frequency (MAF) cutoff of 1% for known somatic variants (based on COSMIC v62) and 5% for novel somatic variants was followed; for indels, the MAF cutoff was 3% for known somatic variants and 10% for novel somatic variants. One hundred base-pair paired-end WES on 3 FDCS cases was performed using the Sureselect whole-exome V5 kit (Agilent Technologies) at an average depth of coverage of 180-fold. Alignment was performed using Burrows-Wheeler Aligner –maximum exact matches (BWA-MEM) with further analysis performed using GATK (version 3), Varscan2, and SNNPET (Agilent Technologies). For all samples, callers were run with default settings in individual sample analysis modes. To call a mutation, 20× coverage of the base analyzed (as defined by the mutation caller itself) was required, as well as coverage in both the forward and reverse directions. Unequal representation of read counts (>80%/20% split) were additionally discounted. A minimum allele frequency of 0.05 was required in addition to the coverage restriction. Indel analysis was performed with GATK, SNNPET, and Varscan2. GATK was run according to the best practices guidelines and VarScan2 and SNNPET were run with default parameters. The variant filtration detailed in this section was similarly applied to indel analysis. Annotation of variants was performed using Surecall (version 3.5.1.46; Agilent Technologies) and Seattleseq (version 9.10; University of Washington, Seattle WA). Variants were additionally curated using Exome Aggregation Consortium (EXAC), Clinvar, 1000 genomes, and Exome Variant Server (EVS) and The Cancer Genome Atlas TCGA database. CNV analysis was performed with Surecall and CNVSeq, both run with default parameters, and analysis was performed on the “hits” file. WES data from 5 reactive lymph nodes and targeted sequencing data (405 genes) from an additional 73 reactive lymph nodes were additionally used as negative controls; pathogenic somatic mutations or gene amplifications at default threshold parameters were not seen.
Sanger sequencing
Sanger sequencing was performed on a subset of genes to confirm single-nucleotide variants identified in WES and targeted sequencing (supplemental Table 1) with concordance of 100%.
Statistical and hierarchical gene relational analysis
Student t and Fisher exact tests were performed as previously described. Unsupervised hierarchical gene cluster analysis was performed using Morpheus (Broad Institute, Cambridge, MA).
Results
Patients
Twenty-one patients were included in this study: 15 cases of UCD, 3 cases of iMCD, and 3 cases of FDCS associated with UCD-HVV.13 All cases were negative for HHV8 and HIV. Three cases of FDCSs arising in the setting of UCD were also studied; 2 male and 1 female, 27, 73, and 55 years of age. The clinical characteristics of the cases are shown in Tables 1 and 2.
UCD and MCD case demographics
| Case . | Subtype . | Sex . | Age, y . | Site of involvement/ lymphadenopathy . | Clinical sequelae . | Organomegaly . | Viral status . | Laboratory results . | Treatment . | Status (time of follow-up) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UCD-HVV | M | 26 | Interaortocaval right lower quadrant | Isolated adenopathy | None | HIV−, HHV8− | NS | Resection | Alive (10 y) |
| 2 | UCD-HVV | M | 35 | Head/right neck | Isolated adenopathy | None | HHV8− | NS | Resection | Alive (2 y) |
| 3 | iMCD-PC (2 major criteria; 8 minor criteria) | F | 70 | Axillary, inguinal, mediastinal, intraabdominal lymphadenopathy | Fatigue, edema and pleural effusions; TAFRO syndrome | None | HHV8−, HIV−, EBV− | ESR, 98 mm/h; CRP, 11.16 mg/L; Hgb, 8.1 g/dL; PLT, 49 × 109/L; IgG 2230 mg/dL; albumin, 2.6 g/dL; eGFR 36 mL/min/m2 | Resection, Rituximab-Etoposide/Siltuximab | Alive (2 y) |
| 4 | UCD-HVV | F | 40 | Left axillary and subpectoral lymphadenopathy | Localized adenopathy | None | HHV8−, HIV−, EBV− | CRP, 3.1 mg/dL; Hgb, 9.4 g/dL | Resection | Alive (4 y) |
| 5 | UCD-MV | F | 59 | Bilateral cervical lymphadenopathy/nasopharyngeal mass | Extensive multinodal adenopathy | None | HHV8−, HIV−, EBV− | Resection, tocilizumab | Alive (4 y) | |
| 6 | UCD-PCV | F | 57 | Left neck lymphadenopathy | Extensive localized adenopathy | None | HHV8−, EBV− | CRP, 2.7 mg/dL | Resection, rituximab | Alive (5 y) |
| 7 | UCD-HVV | F | 28 | Left neck lymphadenopathy | Localized adenopathy | None | HIV−, HHV8− | NS | Resection | Alive (5 y) |
| 8 | iMCD-PC (2 major criteria; 4 minor criteria) | M | 35 | Large anterior mediastinal mass, cervical lymphadenopathy | Weight loss, night sweats, fatigue | None | HHV8−, HIV−, EBV− | Hgb, 12.1 g/dL; PLT, 568 × 109/L; Hypergamma-globulinemia | Resection | Alive (3 y) |
| 9 | UCD-HVV | M | 30 | Retroperitoneal lymphadenopathy | Isolated adenopathy | None | HHV8− | NS | Resection | Alive (6 y) |
| 10 | UCD-HVV | F | 42 | Left pelvic lymphadenopathy | Isolated adenopathy | None | HHV8−, HIV− | NS | Resection, XRT | Alive (4 y) |
| 11 | iMCD-M (2 major; 7 minor criteria) | M | 21 | Diffuse retroperitoneal, inguinal lymphadenopathy | TAFRO syndrome; fatigue, fever, weight loss; hepatomegaly; edema | Hepatospleno-megaly | HHV8−, EBV−, HIV− | CRP, 16.4 mg/dL; Hgb, 7.9 g/dL; PLT, 77 K/μL; eGFR, 43 mL/min/m2 | Siltuximab, rituximab | Alive (2 y) |
| 12 | UCD-HVV | F | 17 | Neck lymphadenopathy | Localized adenopathy | N/A | HHV8− | NS | Resection, localized XRT | Alive (11 y) |
| 13 | UCD-HVV | M | 30 | Anterior mediastinal mass | Localized mass | N/A | HHV8− | NS | Resection, steroids | Alive (4 y) |
| 14 | UCD-HVV | M | 36 | Neck lymphadenopathy | Localized mass | None | HHV8− | NS | Resection, XRT | Alive (1 y) |
| 15 | UCD-HVV | F | 17 | Left adnexal mass | Localized mass | None | HHV8− | NS | Resection | Alive (4 y) |
| 16 | UCD-HVV | F | 24 | Anterior mediastinal mass | Fatigue; localized mass | N/A | HHV8− | NS | Resection | Alive (13 y) |
| 17 | UCD-HVV | F | 14 | Paratracheal mediastinal mass | Localized mass | N/A | HHV8− | NS | Resection | Alive (8 y) |
| 18 | UCD-HVV | F | 29 | Pelvic mass | Isolated adenopathy | None | HHV8− | NS | Resection | Alive (2 y) |
| Case . | Subtype . | Sex . | Age, y . | Site of involvement/ lymphadenopathy . | Clinical sequelae . | Organomegaly . | Viral status . | Laboratory results . | Treatment . | Status (time of follow-up) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UCD-HVV | M | 26 | Interaortocaval right lower quadrant | Isolated adenopathy | None | HIV−, HHV8− | NS | Resection | Alive (10 y) |
| 2 | UCD-HVV | M | 35 | Head/right neck | Isolated adenopathy | None | HHV8− | NS | Resection | Alive (2 y) |
| 3 | iMCD-PC (2 major criteria; 8 minor criteria) | F | 70 | Axillary, inguinal, mediastinal, intraabdominal lymphadenopathy | Fatigue, edema and pleural effusions; TAFRO syndrome | None | HHV8−, HIV−, EBV− | ESR, 98 mm/h; CRP, 11.16 mg/L; Hgb, 8.1 g/dL; PLT, 49 × 109/L; IgG 2230 mg/dL; albumin, 2.6 g/dL; eGFR 36 mL/min/m2 | Resection, Rituximab-Etoposide/Siltuximab | Alive (2 y) |
| 4 | UCD-HVV | F | 40 | Left axillary and subpectoral lymphadenopathy | Localized adenopathy | None | HHV8−, HIV−, EBV− | CRP, 3.1 mg/dL; Hgb, 9.4 g/dL | Resection | Alive (4 y) |
| 5 | UCD-MV | F | 59 | Bilateral cervical lymphadenopathy/nasopharyngeal mass | Extensive multinodal adenopathy | None | HHV8−, HIV−, EBV− | Resection, tocilizumab | Alive (4 y) | |
| 6 | UCD-PCV | F | 57 | Left neck lymphadenopathy | Extensive localized adenopathy | None | HHV8−, EBV− | CRP, 2.7 mg/dL | Resection, rituximab | Alive (5 y) |
| 7 | UCD-HVV | F | 28 | Left neck lymphadenopathy | Localized adenopathy | None | HIV−, HHV8− | NS | Resection | Alive (5 y) |
| 8 | iMCD-PC (2 major criteria; 4 minor criteria) | M | 35 | Large anterior mediastinal mass, cervical lymphadenopathy | Weight loss, night sweats, fatigue | None | HHV8−, HIV−, EBV− | Hgb, 12.1 g/dL; PLT, 568 × 109/L; Hypergamma-globulinemia | Resection | Alive (3 y) |
| 9 | UCD-HVV | M | 30 | Retroperitoneal lymphadenopathy | Isolated adenopathy | None | HHV8− | NS | Resection | Alive (6 y) |
| 10 | UCD-HVV | F | 42 | Left pelvic lymphadenopathy | Isolated adenopathy | None | HHV8−, HIV− | NS | Resection, XRT | Alive (4 y) |
| 11 | iMCD-M (2 major; 7 minor criteria) | M | 21 | Diffuse retroperitoneal, inguinal lymphadenopathy | TAFRO syndrome; fatigue, fever, weight loss; hepatomegaly; edema | Hepatospleno-megaly | HHV8−, EBV−, HIV− | CRP, 16.4 mg/dL; Hgb, 7.9 g/dL; PLT, 77 K/μL; eGFR, 43 mL/min/m2 | Siltuximab, rituximab | Alive (2 y) |
| 12 | UCD-HVV | F | 17 | Neck lymphadenopathy | Localized adenopathy | N/A | HHV8− | NS | Resection, localized XRT | Alive (11 y) |
| 13 | UCD-HVV | M | 30 | Anterior mediastinal mass | Localized mass | N/A | HHV8− | NS | Resection, steroids | Alive (4 y) |
| 14 | UCD-HVV | M | 36 | Neck lymphadenopathy | Localized mass | None | HHV8− | NS | Resection, XRT | Alive (1 y) |
| 15 | UCD-HVV | F | 17 | Left adnexal mass | Localized mass | None | HHV8− | NS | Resection | Alive (4 y) |
| 16 | UCD-HVV | F | 24 | Anterior mediastinal mass | Fatigue; localized mass | N/A | HHV8− | NS | Resection | Alive (13 y) |
| 17 | UCD-HVV | F | 14 | Paratracheal mediastinal mass | Localized mass | N/A | HHV8− | NS | Resection | Alive (8 y) |
| 18 | UCD-HVV | F | 29 | Pelvic mass | Isolated adenopathy | None | HHV8− | NS | Resection | Alive (2 y) |
CRP, C-reactive protein; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; F, female; Hgb, hemoglobin; IgG, immunoglobulin G; iMCD-M, HHV8−/iMCD with mixed pathology; iMCD-PC, HHV8−/iMCD with plasmacytic pathology; M, male; N/A, not available; NS, not significant; PLT, platelet; XRT, radiotherapy.
FDCS case demographics
| Case . | Sex . | Age, y . | Site of involvement . | Viral status . | Treatment/Clinical course . | Status . |
|---|---|---|---|---|---|---|
| 19 | M | 27 | Right inguinal lymphadenopathy | HHV8− | Excision | Alive (3 y) |
| 20 | F | 73 | Left neck mass | HHV8−; EBV− | N/A | N/A |
| 21 | M | 55 | Epigastric lymph nodes and liver masses | HHV8− | Excision and CHOP | Alive (2 y) |
| Case . | Sex . | Age, y . | Site of involvement . | Viral status . | Treatment/Clinical course . | Status . |
|---|---|---|---|---|---|---|
| 19 | M | 27 | Right inguinal lymphadenopathy | HHV8− | Excision | Alive (3 y) |
| 20 | F | 73 | Left neck mass | HHV8−; EBV− | N/A | N/A |
| 21 | M | 55 | Epigastric lymph nodes and liver masses | HHV8− | Excision and CHOP | Alive (2 y) |
CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone.
Mutational analysis
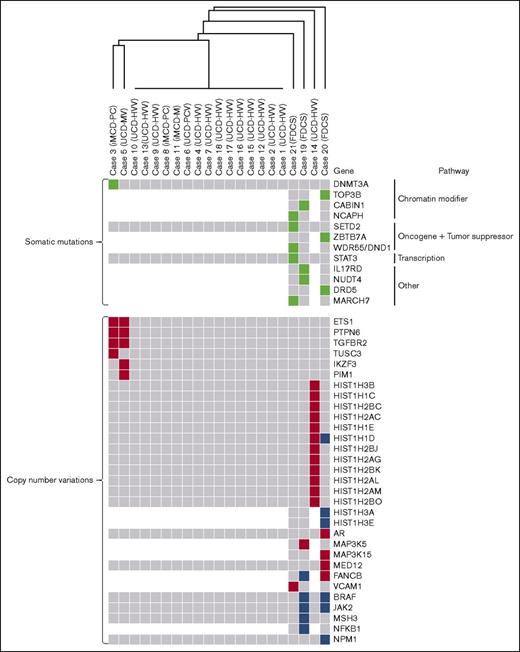
We performed F1 NGS of 405 genes on 15 UCD and 3 iMCD cases to an average depth of 800-fold coverage and observed a somatic mutation in 1 patient with iMCD-PC (case 3), who also met criteria for the TAFRO clinical subtype, in DNMT3a (L295Q) at a variant allele frequency (VAF) of 9%, which was reverified by repeating targeted sequencing (Table 3; Figure 1). This mutation was not seen in paired normal control bone marrow tissue, peripheral blood, nor in uninvolved adipose tissue directly adjacent to the lymph node by Sanger sequencing or targeted NGS. The mutation was not listed as a germ line variant in any servers (EXAC, Clinvar, 1000 genomes, and EVS). Our analyses did not reveal somatic mutations in the remaining 17 of 18 UCD and iMCD cases. We additionally performed WES to an average depth of 180-fold coverage on 3 FDCSs associated with UCD-HVV. These 3 cases showed 3 to 5 somatic mutations per case in known oncogenes, tumor suppressors, and chromatin-remodeling proteins (Table 3; Figure 1). Notable mutations included those in known oncogenes and tumor suppressors such as SETD2 (A1896E) and WDR55/DND1 (E334K), and chromatin modifiers such as NCAPH (E287K) and TOP3B (E623K). Mutations in genes frequently mutated in subsets of hematopoietic neoplasms, such as ZBTB7a (K424N), also mutated in acute myeloid leukemia,14 and STAT3 (D170N), a gene frequently mutated in T-cell large granular lymphocytic leukemia and chronic natural killer (NK) lymphoproliferative disorders, was also present in 1 FDCS case (Table 3).15-17 No somatic mutations were recurrently seen among UCD and iMCD cases or with FDCS cases.
Somatic mutations identified in a case of HHV8−/iMCD (case 3) and FDCSs arising from UCD
| Case . | Gene . | Position . | VAF, % . | Nucleotide change . | Type of mutation . | AA change . | CADD . | Pathway . |
|---|---|---|---|---|---|---|---|---|
| CD | ||||||||
| 3 | DNMT3A | Chr2:25470590 | 9 | T>A | Missense | L295Q | Unknown | DNA methylation |
| FDCSs | ||||||||
| 19 | IL17RD | Chr3:57135385 | 38 | T>C | Missense | I329T | 22.2 | FGFR pathway |
| 19 | NUDT4 | Chr12:93772182 | 38 | C>G | Missense | S28R | 13.7 | Intracellular trafficking |
| 19 | CABIN1 | Chr22:24479275 | 39 | A>G | Missense | Y898C | 19.25 | Chromatin structure, T-cell receptor mediated signaling |
| 20 | ZBTB7A | Chr19:4048233 | 14 | C>G | Missense | K424N | 17.6 | Oncogene; regulation of glycolysis |
| 20 | DRD5 | Chr4:9783892 | 40 | T>A | Missense | I80N | 19.7 | Dopamine; Other |
| 20 | TOP3B | Chr22:22311800 | 40 | C>T | Missense | E623K | 16.2 | Topoisomerase; DNA structure |
| 21 | STAT3 | Chr17:40490791 | 26 | C>T | Missense | D170N | 25 | Transcription |
| 21 | WDR55/DND1 | Chr5:140050940 | 15 | C>T | Missense | E334K | 12.6 | Oncogene; translational regulation |
| 21 | NCAPH | Chr2:97017707 | 20 | A>G | Missense | E287K | 13.2 | Chromatin structure |
| 21 | SETD2 | Chr3:47125583 | 24 | G>T | Missense | A1896E | 16.2 | Tumor suppressor; histone methyltransferase |
| 21 | MARCH7 | Chr2:160605297 | 20 | A>G | Missense | N443S | 16.3 | Ubiquitin ligase |
| Case . | Gene . | Position . | VAF, % . | Nucleotide change . | Type of mutation . | AA change . | CADD . | Pathway . |
|---|---|---|---|---|---|---|---|---|
| CD | ||||||||
| 3 | DNMT3A | Chr2:25470590 | 9 | T>A | Missense | L295Q | Unknown | DNA methylation |
| FDCSs | ||||||||
| 19 | IL17RD | Chr3:57135385 | 38 | T>C | Missense | I329T | 22.2 | FGFR pathway |
| 19 | NUDT4 | Chr12:93772182 | 38 | C>G | Missense | S28R | 13.7 | Intracellular trafficking |
| 19 | CABIN1 | Chr22:24479275 | 39 | A>G | Missense | Y898C | 19.25 | Chromatin structure, T-cell receptor mediated signaling |
| 20 | ZBTB7A | Chr19:4048233 | 14 | C>G | Missense | K424N | 17.6 | Oncogene; regulation of glycolysis |
| 20 | DRD5 | Chr4:9783892 | 40 | T>A | Missense | I80N | 19.7 | Dopamine; Other |
| 20 | TOP3B | Chr22:22311800 | 40 | C>T | Missense | E623K | 16.2 | Topoisomerase; DNA structure |
| 21 | STAT3 | Chr17:40490791 | 26 | C>T | Missense | D170N | 25 | Transcription |
| 21 | WDR55/DND1 | Chr5:140050940 | 15 | C>T | Missense | E334K | 12.6 | Oncogene; translational regulation |
| 21 | NCAPH | Chr2:97017707 | 20 | A>G | Missense | E287K | 13.2 | Chromatin structure |
| 21 | SETD2 | Chr3:47125583 | 24 | G>T | Missense | A1896E | 16.2 | Tumor suppressor; histone methyltransferase |
| 21 | MARCH7 | Chr2:160605297 | 20 | A>G | Missense | N443S | 16.3 | Ubiquitin ligase |
AA, amino acid; CADD, Combined Annotation Dependent Depletion; FGFR, fibroblast growth factor receptor.
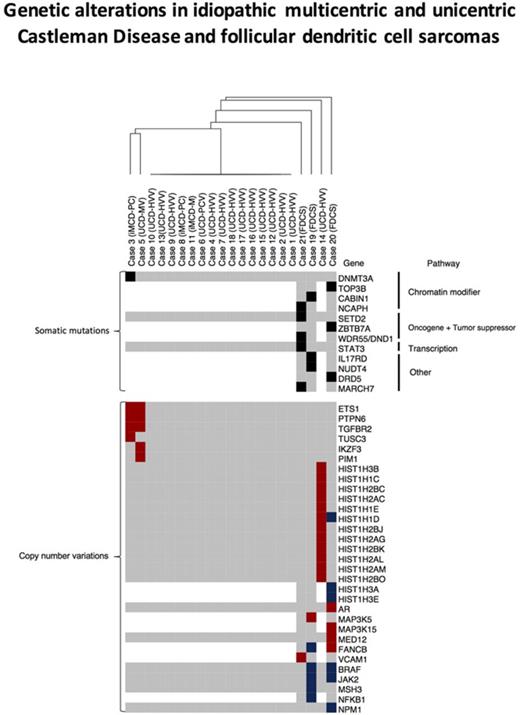
Somatic gene mutations and CNVs in cases of UCD and HHV8−/iMCD and FDCS associated with UCD-HVV. Depicted is an unsupervised hierarchical cluster analysis of genetic alterations in UCD, iMCD, and FDCS associated with UCD-HVV. The top portion depicts somatic mutations in all UCD, iMCD, and FDCS cases (green boxes indicate genes mutated in individual cases); the bottom portion depicts CNVs in all UCD, iMCD, and FDCS cases. CNV gains are in red, whereas CNV losses are in blue. Gray boxes indicate genes without abnormalities. White areas/boxes indicate genes not evaluated by targeted sequencing. iMCD-M, HHV8−/iMCD with mixed histology; iMCD-PC, HHV8−/iMCD with plasma cell histology.
Somatic gene mutations and CNVs in cases of UCD and HHV8−/iMCD and FDCS associated with UCD-HVV. Depicted is an unsupervised hierarchical cluster analysis of genetic alterations in UCD, iMCD, and FDCS associated with UCD-HVV. The top portion depicts somatic mutations in all UCD, iMCD, and FDCS cases (green boxes indicate genes mutated in individual cases); the bottom portion depicts CNVs in all UCD, iMCD, and FDCS cases. CNV gains are in red, whereas CNV losses are in blue. Gray boxes indicate genes without abnormalities. White areas/boxes indicate genes not evaluated by targeted sequencing. iMCD-M, HHV8−/iMCD with mixed histology; iMCD-PC, HHV8−/iMCD with plasma cell histology.
We identified CNV changes that met our threshold parameters in 3 of our UCD and iMCD cases; their histology is also shown in Figure 2. Case 14 (UCD-HVV) had copy-number gains in the HIST1H genes. Interestingly, 2 cases, case 3 (HHV8−/iMCD with plasma cell histology [iMCD-PC]), and case 5 (UCD-MV), showed common copy-number gains in the genes PTPN6, ETS1, and TGFBR2 (Figure 1). We also identified multiple CNV changes in our FDCS cases (Table 4). Of our FDCS cases, case 19 displayed mainly losses, whereas case 20 showed both copy-number gains and losses; case 21 presented with mainly copy-number gains. Overlapping CNVs were seen between case 19 and case 20, which both showed losses in BRAF; additionally, FANCB was altered in both case 19 and case 20 though 1 was loss (case 19) and the other gain (case 20). CNVs in genes previously described as altered in FDCS, such as NFKBI and JAK2, were additionally present.9 Finally, loss of several HISTH genes was seen in case 20. Translocations were not identified in cases by NGS.
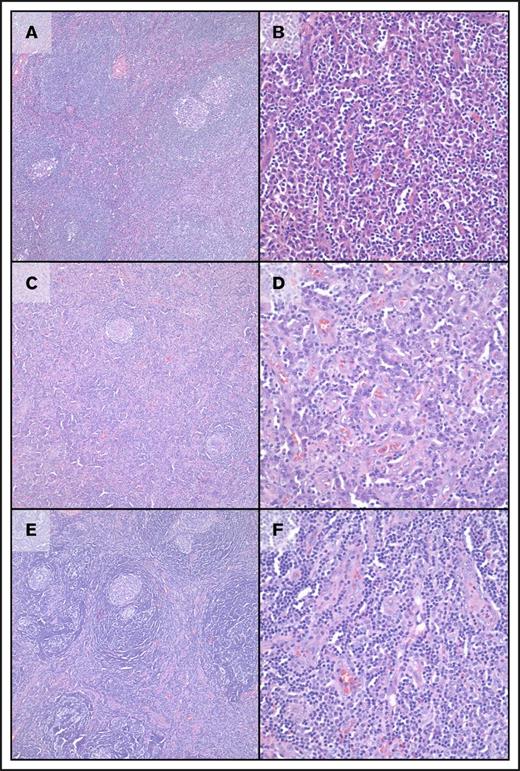
Morphologic features of cases of UCD and MCD with genetic abnormalities. (A-B) Case 3, HHV8−/iMCD with plasmacytic histology. At low-power magnification, regressed follicles are seen with onion skinning of mantle zones and “twinning” of follicles; increased plasma cells are clearly seen in interfollicular regions with mild vascularity. (C-D) Case 5, UCD-MV. At low-power magnification, regressed follicles are seen with onion skinning of mantle zones along with increased vascularity in interfollicular regions. At high-power magnification, increased plasma cells are clearly seen along with increased vascularity. (E-F) Case 14, UCD-HVV. At low-power magnification, regressed follicles are seen with prominent onion skinning of mantle zones and “twinning” of follicles. At high-power magnification, increased vascularity is present without increased plasma cells. In all panels, images on cases are taken in sequence at ×100 (left) and ×400 (right) magnification; all images are from hematoxylin and eosin–stained slides.
Morphologic features of cases of UCD and MCD with genetic abnormalities. (A-B) Case 3, HHV8−/iMCD with plasmacytic histology. At low-power magnification, regressed follicles are seen with onion skinning of mantle zones and “twinning” of follicles; increased plasma cells are clearly seen in interfollicular regions with mild vascularity. (C-D) Case 5, UCD-MV. At low-power magnification, regressed follicles are seen with onion skinning of mantle zones along with increased vascularity in interfollicular regions. At high-power magnification, increased plasma cells are clearly seen along with increased vascularity. (E-F) Case 14, UCD-HVV. At low-power magnification, regressed follicles are seen with prominent onion skinning of mantle zones and “twinning” of follicles. At high-power magnification, increased vascularity is present without increased plasma cells. In all panels, images on cases are taken in sequence at ×100 (left) and ×400 (right) magnification; all images are from hematoxylin and eosin–stained slides.
CNVs seen in UCD and HHV8−/iMCD and FDSCs arising from UCD
| Genes . | Cases . | CNV type . | CNV exons . | CNV ratio . |
|---|---|---|---|---|
| UCD and MCD | ||||
| ETS1 | 3, 5 | Gain | 10 of 10 | 1.43 |
| PTPN6 | 3, 5 | Gain | 16 of 17 | 1.39 |
| TGFBR2 | 3, 5 | Gain | 8 of 8 | 1.45 |
| TUSC3 | 3 | Gain | 9 of 11 | 1.4 |
| IKZF3 | 5 | Gain | 8 of 9 | 1.47 |
| PIM1 | 5 | Gain | 7 of 7 | 1.42 |
| HIST genes | 14 | Gain | 1 of 1 | 1.35-1.93 |
| FDCSs | ||||
| BRAF | 19, 20 | Deletion | 5 of 18; 8 of 18 | −1.15 |
| FANCB | 19 | Deletion | 3 of 10 | −1.17 |
| FANCB | 20 | Gain | 6 of 10 | 0.67 |
| JAK2 | 19, 20 | Deletion | 4 of 25, 6 of 25 | −1.15, −1.02 |
| MAP3K5 | 19 | Gain | 3 of 30 | 1.22 |
| MSH3 | 19 | Deletion | 2 of 24 | −1.71 |
| NFKB1 | 19 | Deletion | 4 of 24 | −1.59 |
| AR | 20 | Gain | 8 of 8 | 1.2 |
| HIST1H1D | 20 | Deletion | 1 of 1 | −0.69 |
| HIST1H3A | 20 | Deletion | 1 of 1 | −0.68 |
| HIST1H3E | 20 | Deletion | 1 of 1 | −0.68 |
| MAP3K15 | 20 | Gain | 29 of 29 | 0.94 |
| MED12 | 20 | Gain | 45 of 45 | 0.81 |
| NPM1 | 20 | Deletion | 2 of 10 | −0.75 |
| VCAM1 | 21 | Gain | 5 of 9 | 0.86 |
| Genes . | Cases . | CNV type . | CNV exons . | CNV ratio . |
|---|---|---|---|---|
| UCD and MCD | ||||
| ETS1 | 3, 5 | Gain | 10 of 10 | 1.43 |
| PTPN6 | 3, 5 | Gain | 16 of 17 | 1.39 |
| TGFBR2 | 3, 5 | Gain | 8 of 8 | 1.45 |
| TUSC3 | 3 | Gain | 9 of 11 | 1.4 |
| IKZF3 | 5 | Gain | 8 of 9 | 1.47 |
| PIM1 | 5 | Gain | 7 of 7 | 1.42 |
| HIST genes | 14 | Gain | 1 of 1 | 1.35-1.93 |
| FDCSs | ||||
| BRAF | 19, 20 | Deletion | 5 of 18; 8 of 18 | −1.15 |
| FANCB | 19 | Deletion | 3 of 10 | −1.17 |
| FANCB | 20 | Gain | 6 of 10 | 0.67 |
| JAK2 | 19, 20 | Deletion | 4 of 25, 6 of 25 | −1.15, −1.02 |
| MAP3K5 | 19 | Gain | 3 of 30 | 1.22 |
| MSH3 | 19 | Deletion | 2 of 24 | −1.71 |
| NFKB1 | 19 | Deletion | 4 of 24 | −1.59 |
| AR | 20 | Gain | 8 of 8 | 1.2 |
| HIST1H1D | 20 | Deletion | 1 of 1 | −0.69 |
| HIST1H3A | 20 | Deletion | 1 of 1 | −0.68 |
| HIST1H3E | 20 | Deletion | 1 of 1 | −0.68 |
| MAP3K15 | 20 | Gain | 29 of 29 | 0.94 |
| MED12 | 20 | Gain | 45 of 45 | 0.81 |
| NPM1 | 20 | Deletion | 2 of 10 | −0.75 |
| VCAM1 | 21 | Gain | 5 of 9 | 0.86 |
Discussion
Our study of UCD and iMCD, and FDCS associated with UCD-HVV identified a DNMT3a L295Q mutation in a single case of iMCD-PC, which additionally met criteria for the TAFRO clinical subtype of iMCD, and recurrent copy-number alterations in 1 case of iMCD and 1 case of UCD, as well as numerous nonrecurrent mutations and copy-number alterations in FDCS associated with UCD-HVV. Although the majority of cases of UCD and iMCD did not show somatic mutations in the 405 genes sequenced, one 70-year-old patient with iMCD-PC (case 3), and the TAFRO clinical subtype, showed a mutation in DNMT3A, a DNA methyltransferase commonly mutated in myeloid neoplasms as well as nonhematopoietic neoplasms.18,19 This mutation was not seen in matched normal control tissue (bone marrow, peripheral blood, or adjacent uninvolved adipose tissue), suggesting it is an acquired mutation. This specific DNMT3A L295Q mutation has also been described in a case of acute myeloid leukemia (AML) and the mutation is located in the Proline-Tryptophan-Tryptophan-Proline (PWWP) domain, a critical region for DNA binding.20 Mutations in the PWWP domain have been described to result in defects in the ability of DNMT3A to methylate DNA due to loss of chromatin targeting by DNMT3A.21-24 In vivo studies in mice, assessing the loss of DNMT3A function, have shown global defects in hypomethylation and its critical role in impairing hematopoietic stem cell differentiation although resulting in expansion of hematopoietic stem cell numbers.23 Data support DNMT3A functioning as a tumor suppressor in many cancer systems19 and such may be true here; however, further in vivo studies are necessary to understand how this specific mutation may affect the survival and/or proliferation of affected cells, and cytokine production observed in iMCD.18,19 Based on our understanding of iMCD and the function of DNMT3A as discussed in this paragraph, one possible hypothesis is that the L295Q DNMT3A mutation is present in an undefined population of clonal cells, possibly a stromal cell population, and results in increased proliferation of this population of cells. We speculate that a paraneoplastic syndrome then ensues potentially from primary production of cytokines (interleukin-6 [IL-6], vascular endothelial growth factor) by this cellular population. Indeed, in a recent paper by Yang et al,25 the authors demonstrate that DNMT3A overexpression in human fibroblasts results in suppression of IL-6 expression; that deletion of DNMT3A conversely might increase IL-6 expression was not addressed, but certainly the results from Yang et al are consistent with this possibility. Subsequently IL-6 can then perhaps drive the polyclonal plasmacytosis seen in iMCD.26 Alternatively, clonal proliferation of this undefined population of cells may directly stimulate an autoimmune system response, and the overall sequelae of iMCD. Yet another possibility is that this DNMT3A mutation is a passenger mutation and not a true driver mutation (though present in the key neoplastic population), and the true driver mutation was not identified by our targeted sequencing approach. A final possibility is that this mutation is actually present in the polyclonal lymphoid population, though this is unlikely because adjacent uninvolved fat, where lymphoid and plasma cells were present as well as marrow and peripheral blood, did not show a DNMT3A mutation.23
We also considered that this specific DNMT3A L295Q mutation might be related to clonal hematopoiesis of indeterminant potential (CHIP), or one seen in idiopathic cytopenias of undetermined significance, but this mutation was not seen in patient-matched control bone marrow biopsy tissue or peripheral blood. Additionally, this patient did not demonstrate any pathologic evidence for a myeloid neoplasm in a concurrent bone marrow biopsy, and has not developed a myeloid neoplasm over a 2-year period of follow-up. Although DNMT3A mutations are overall one of the more frequent mutations in CHIP, the frequency of non-R882 somatic mutations in patients without hematologic malignancies is <2% in 70-year-olds and this L295Q mutation has not been described in any CHIP studies evaluating DNMT3A in >30 000 individuals.27,28 The only instance of this mutation identified to date, to our knowledge, has been in a case of AML as mentioned in the previous paragraph.20 That a mutation in DNMT3A might underlie at least a subset of cases of iMCD is intriguing, though we did not identify it recurrently in the other iMCD or UCD cases.
To understand the possible association between UCD-HVV and FDCS, we analyzed the mutational landscape of FDCS associated with UCD-HVV by WES. Although no recurrent somatic mutations were seen between cases of FDCS, not surprisingly, mutations in oncogenes and genes known to be critical in tumorigenesis were identified: specifically, mutations in tumor suppressors such as SETD2 (A1896E) and WDR55/DND1 (E334K), and chromatin modifiers such as NCAPH (E287K) and TOP3B (E623K). Additionally, among our FDCS cases, mutations in ZBTB7A (K424N) and STAT3 (D170N) were seen. A known oncogene, ZBTB7A, plays a key role in the development from lymphoid progenitors into B-cell lineage and is recurrently mutated in AML,14,29 and STAT3 is a transcription factor mutated in NK, T-cell, and B-cell disorders.30-32 We also evaluated for previously published mutations seen in FDCS not associated with UCD-HVV, but recurrent mutations were not seen between those prior studies and ours.9 In some sense, this is not entirely surprising as the histology and specific immunophenotypic expression patterns of FDCS can be somewhat diverse.33 One gene mutation of major interest was BRAF for which mutations in this gene have been described in a single research study of FDCSs by Go et al.8 In our study, no BRAF mutations were seen. However, we identified copy-number aberrations (losses) in the BRAF gene in 2 of our FDCS cases.
We additionally evaluated for other genetic alterations such as copy-number changes and translocations in UCD, iMCD, and FDCS associated with UCD-HVV. Copy-number alterations were additionally seen in 3 cases of CD. Two cases, 1 of iMCD-PC (case 3) and an unusual UCD-MV (case 5) with multinodal involvement localized to the bilateral cervical chain, showed common amplification of PTPN6, ETS1, and TGFBR2. Although these genes do not share a common pathway, each is known to interface in oncogenesis or critical biologic processes relevant to UCD and iMCD in multiple systems. In mouse models, PTPN6 plays a critical role in subsets of immune system cells and deletion of PTPN6 results in alteration of dendritic cell function and features of autoimmunity; mice with null mutations in PTPN6 present with splenomegaly, lymphadenopathy, and increased anti-nuclear antibodies. Both MCD and UCD can show abnormalities in follicular dendritic cells and clinical features that overlap with autoimmune disorders, though, in our case, amplification of PTPN6 was seen rather than deletion.7,34,35 Overexpression of PTPN6 is seen in other diseases such as ovarian carcinoma and gliomas, leading to enhanced oncogenesis and more aggressive pathologic behavior.36 It is interesting to note that the 1 case of UCD (case 5) with amplification of PTPN6 showed unusual multinodal involvement, localized to the bilateral cervical chain, with continued recurrence despite systemic treatment with anti–IL-6 therapies (tocilizumab); similarly, the patient with iMCD (case 3) and amplification of PTPN6 failed to fully respond to targeted (anti-IL-6) and systemic (rituximab and etoposide) therapies. Amplification of ETS1 was also present. ETS1 is a known oncogene, and higher stromal and endothelial expression of this gene in carcinomas affects invasive properties.37,38 That it is amplified in UCD (case 5) and iMCD (case 3) again is at least consistent with the more aggressive clinical course for these 2 patients. Finally, ETS1 is further known to regulate angiogenesis; again, increased vascularization is a feature commonly seen in cases of iMCD and UCD.39,40 Amplification of TGFBR2 was also seen; a gene ubiquitously expressed, it is increased in relative quantity in stromal/vascular tissues and high expression is associated with poor prognosis in some cancer types.41 Again, given the clinical course of the case of iMCD (case 3) and UCD (case 5), the amplification and tissue expression patterns are at least consistent with what might be expected based on our understanding of this gene.42 One overarching hypothesis regarding how the amplification of PTPN6, ETS1, and TGFBR2 might relate specifically to the pathogenesis of iMCD (case 3) and the more aggressive/relapsing case of UCD (case 5) may be that amplification of these genes in an unidentified neoplastic/clonal cell results in increased cellular proliferation (all of these genes affect cellular proliferation), which ultimately results in a paraneoplastic condition with increased cytokine production (ie, IL-6) from this clonal population and resultant polyclonal plasma cell proliferation from the expression of IL-6; we note that both cases showed a polyclonal plasma cell proliferation. Furthermore, the amplification of TGFBR2 may specifically result in increases in IL-6 expression; activation of the TGFB2 pathway increases IL-6 expression in fibroblast cell lines in vitro and in in vivo mouse model systems.43,44 Alternatively, individually, each gene amplification may play different roles in the pathogenesis of iMCD and UCD aside from what is noted here; again, further research is truly needed. However, we also note that CNV changes in specific genes, as other somatic mutations, may be passenger genetic abnormalities and not driver genetic abnormalities responsible for the disease; alternatively, these CNVs may be inherited and not de novo.
Amplification of the cluster of HIST1H genes on chromosome 6p was seen in an additional case of UCD-HVV (case 14). Amplification of HIST1H genes is a feature seen in uterine and ovarian carcinosarcomas, which have undergone gene expression toward a more mesenchymal state.45 This case showed an increased stromal component as typical of UCD-HVV. As such, the amplification of HIST1H genes seen in the case of UCD-HVV may reflect transition of a clonal/neoplastic cell to a more mesenchymal phenotype necessarily resulting in either increased cellular proliferation or a readout of that proliferation.
Overall, genetic alterations in UCD and iMCD are not completely surprising given prior findings that at least a subset of UCD and iMCD cases show clonal origins by cytogenetic or other genetic assays.6 However, most of our UCD-HVV cases did not show genetic alterations, which could be due to the sensitivity of our assays, or the targeted nature of our sequencing panel (405 of 20 000 genes), or could point to a different nonneoplastic etiology for a subset of cases, a supposition also proposed by Fajgenbaum et al and others.3,7 It is interesting to note that the cases of UCD with CNV changes showed more aggressive behaviors and a relapsing/refractory clinical pattern. Lastly, TdT+ T cells have been identified as increased in cases of UCD, MCD, and FDCS46-48 ; however, no association between genetic abnormalities and populations of TdT+ T cells was seen in this study.
The cell of origin, resulting in UCD and iMCD disease, remains elusive. Currently based on limited studies, one hypothesis is that underlying stromal cells such as follicular dendritic cells are the clonal neoplastic cells, though this has not been fully confirmed.6,7 In this study, we attempted to manually microdissect out individual cells, but attempts to manually isolate rare cells were nonproductive as amplification of DNA from old (>7 years old) tissues was an obstacle from very small populations of cells and our limited tissue was exhausted precluding further analysis of these cases. However, further prospective research using cutting-edge immunophenotypic and genetic technologies such as multiplexed ion beam imaging coupled with single-cell genetic analysis and laser-capture microdissection could be a source for determining and further evaluating the cell of origin.49
Finally, we also identified CNV changes in our FDCS cases. Among those, CNVs in genes previously described as altered in FDCS were noted, such as NFKBI and JAK2.9 Cases 19 and 20 showed gene losses involving JAK2. We also saw copy-number deletions in the HIST1H genes in case 20, which also showed significant copy-number amplifications in MAP3K15 and in MED12. Overall, a comparison of UCD and iMCD cases, with cases of FDCS associated with UCD-HVV, demonstrated rare genetic changes in 2 cases of UCD and 1 of iMCD (3 with CNVs and 1 iMCD with an additional DNMT3A somatic mutation), whereas all FDCS cases had significant somatic mutations (3-5 per case) and numerous CNVs. Our findings also suggest that additional genetic alterations are necessary for FDCS to arise in the setting of UCD. However, it is important to note that a targeted panel of 405 genes was evaluated for the UCD and iMCD cases whereas WES was performed for the FDCS cases.
There are several limitations of this study. First, it is largely observational, and we did not perform in vivo functional studies to address how specifically these genetic alterations result in the phenotypic manifestations of UCD, iMCD, and FDCS. Additionally, with respect to UCD and iMCD, though we postulate that these genetic abnormalities reside in a clonal/neoplastic population of cells, we and others have not identified this specific cell, though evidence from other studies points to a stromal population of cells. Finally, although we did perform a broad DNA mutational analysis of 405 genes in UCD and iMCD cases, we did not interrogate all genes, intronic regions, nor translocations fully.
To the best of our knowledge, our study is the first to analyze the genetic and epigenetic features of UCD and iMCD, and FDCS associated with UCD-HVV. Our findings are compelling and clearly point to a future direction of needed work to decipher the molecular pathogenesis of these diseases.
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by research awards (R.S.O.) from Foundation Medicine and Agilent Technologies.
Authorship
Contribution: R.S.O. conceived of and designed the study, analyzed data, and wrote the manuscript; S.A. conceived of and designed the study, analyzed data, and edited the manuscript; A.N. performed research, designed experiments, analyzed data, and wrote the manuscript; A.B., N.S., J.S., J.L.Z., T.M., and R.A.W. analyzed data and edited the manuscript; and all authors reviewed the drafts and the final version of the manuscript.
Conflict-of-interest disclosure: T.M. and S.A. are employees of Foundation Medicine and have equity interest. The remaining authors declare no competing financial interests.
Correspondence: Robert S. Ohgami, Department of Pathology, Stanford University, 300 Pasteur Dr, Room L235, Stanford, CA 94305; e-mail: rohgami@stanford.edu.