Key Points
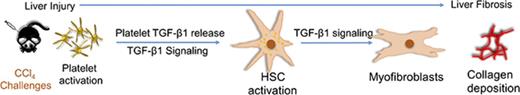
Fibrosis in the liver is a common cause of liver disease, partially mediated by platelet TGF-β1 as shown in a mouse model of liver injury.
Depleting platelet TGF-β1 results in decreased liver fibrosis suggesting that blocking platelet TGF-β1 may ameliorate or prevent fibrosis.
Abstract
Transforming growth factor-β1 (TGF-β1) signaling in hepatic stellate cells (HSCs) plays a primary role in liver fibrosis, but the source of TGF-β1 is unclear. Because platelets are rich in TGF-β1, we examined the role of platelet TGF-β1 in liver fibrosis by challenging wild-type (WT) mice and mice deficient in platelet TGF-β1 (PF4CreTgfb1f/f) with carbon tetrachloride (CCl4), an inducer of acute hepatic injury and chronic fibrosis. CCl4 elicited equivalent hepatic injury in WT and PF4CreTgfb1f/f mice based on loss of cytochrome P450 (Cyp2e1) expression, observed at 6 hours and peaking at 3 days after CCl4 challenge; PF4CreTgfb1f/f mice exhibited less liver fibrosis than control mice. Activated platelets were observed during acute liver injury (6 hours), and WT mice with transient platelet depletion (thrombocytopenia) were partially protected from developing fibrosis compared with control mice (P = .01), suggesting an association between platelet activation and fibrosis. Transient increases in TGF-β1 levels and Smad2 phosphorylation signaling were observed 6 hours and 3 days, respectively, after CCl4 challenge in WT, but not PF4CreTgfb1f/f, mice, suggesting that increased TGF-β1 levels originated from platelet-released TGF-β1 during the initial injury. Numbers of collagen-producing HSCs and myofibroblasts were higher at 3 days and 36 days, respectively, in WT vs PF4CreTgfb1f/f mice, suggesting that platelet TGF-β1 may have stimulated HSC transdifferentiation into myofibroblasts. Thus, platelet TGF-β1 partially contributes to liver fibrosis, most likely by initiating profibrotic signaling in HSCs and collagen synthesis. Further studies are required to evaluate whether blocking platelet and TGF-β1 activation during acute liver injury prevents liver fibrosis.
Introduction
Pathologic liver fibrosis, characterized by the accumulation of extracellular matrix protein (mainly collagen), is a frequent consequence of chronic injury and an initiating factor in many liver diseases.1 Transforming growth factor-β1 (TGF-β1) is a potent profibrotic cytokine that has multifunctional roles in regulating cell proliferation, cell differentiation, wound healing, angiogenesis, and immune function.2-4 TGF-β1–mediated signaling is a key stimulator of collagen production,5 and excessive collagen production causes organ fibrosis.6-8 TGF-β signaling has been shown to contribute to liver fibrosis in both mice and humans.1,7,9-12 Hepatic stellate cells (HSCs) are the major cell types that can transdifferentiate into collagen-producing myofibroblasts in the liver and lead to pathological fibrosis.1,11,13,14 TGF-β1 signaling through Smad2 phosphorylation (p-Smad2) is critical for activation of HSCs and their transdifferentiation into myofibroblasts, thus suggesting that this pathway is essential for initiating collagen production and liver fibrosis.15
There are several potential sources of TGF-β1 in the liver that may contribute to fibrosis, including HSCs themselves, macrophages, hepatocytes, cholangiocytes, and fibroblasts,16 but the cellular source(s) of TGF-β1 remain unclear. We previously demonstrated that platelet-derived TGF-β1 contributes to cardiac fibrosis in a high-pressure cardiac overload mouse model17 ; because platelets contain 40 to 100 times more TGF-β1 than other cell types and TGF-β1 is released upon platelet activation,18-20 we hypothesized that TGF-β1 released from platelets during acute liver injury initiates signaling and thereby induces liver fibrosis. However, the role of platelets in liver fibrosis is controversial because studies have shown both beneficial21-23 and detrimental24-27 effects of platelets in liver pathogenesis and fibrosis. Reconciliation of these contradictory findings has not been achieved, nor has the contribution of platelet-derived factors in various conditions been established.
To test our hypothesis, we used a well-established mouse model of carbon tetrachloride (CCl4)-induced acute and chronic liver injury with fibrosis to compare the effects of CCl4 in C57BL/6 wild-type (WT) mice and mice carrying a megakaryocyte/platelet-specific targeted conditional deletion of the TGF-β1 gene (PF4CreTgfb1f/f). We also assessed liver fibrosis in WT mice with acutely induced transient thrombocytopenia caused by a single dose of a platelet-specific anti-glycoprotein 1bα (anti-GP1bα) antibody and compared the results with the fibrosis found in control immunoglobulin G (IgG)-treated mice. Here, we show that TGF-β1 released from platelets during acute liver injury induces profibrotic TGF-β1 signaling, partially causing liver fibrosis. These results suggest a previously unknown role for platelet TGF-β1 in liver fibrosis. Finally, based on our data, we discuss the contradictory roles of platelets in liver fibrosis.
Materials and methods
Mice
C57Bl/6J mice were either obtained from The Jackson Laboratory or bred in-house, and are designated either WT or C57Bl/6 for simplicity. We obtained TGF-β1 allele “floxed” mice (Tgfb1flox/flox, designated Tgfb1f/f) from The Jackson Laboratory.28 We then inbred the Tgfb1f/f mice with C57Bl/6 mice for 10 generations to obtain Tgfb1f/f on a C57Bl/6 background. These mice were subsequently crossed with transgenic mice expressing Cre recombinase under the control of the megakaryocyte-specific platelet factor 4 (PF4) promoter (PF4Cre+/−, obtained from The Jackson Laboratory).29
To create mice homozygous for both PF4Cre+/+ and Tgfb1f/f, we crossed all litters from PF4Cre+/+Tgfb1f/f parents and genotyped these litters until all pups were homozygous for both PF4Cre+/+ and Tgfb1f/f, thus suggesting that the parents were homozygous for both alleles. To avoid false positives, we bred the same parents for 3 to 4 rounds; each time, the pups were all homozygous PF4Cre+/+Tgfb1f/f, designated PF4CreTgfb1f/f for simplicity. We also performed phenotypic characterization by measuring TGF-β1 levels in both platelets and plasma in mice heterozygous and homozygous for PF4Cre carrying the Tgfb1f/f allele. All mice were housed in a controlled environment (23°C ± 2°C; 12-hour light/dark cycles) and fed a standard diet (PicoLab Rodent Diet). All experimental procedures were approved by the Oklahoma Medical Research Foundation Animal Care and Use Committee. Each mouse was numbered, and all experiments were performed by investigators blinded to the genotype of the mice.
CCl4-induced liver fibrosis model
Liver fibrosis was induced by a well-established method using CCl4 in a murine model as previously described.30 Mice were challenged with an intraperitoneal injection of either 100 µL of mineral oil or CCl4 (1 µL/g body weight [BW] in a total of 100 µL, prepared by mixing CCl4 in mineral oil). CCl4 was injected once, and acute liver injury was monitored after 6 hours, 1 day, and 3 days. To produce chronic injury and fibrosis, CCl4 was injected repeatedly, as indicated by arrows in Figure 1B. In some cases, mice were injected with antibodies or harvested earlier, at indicated time points.
PF4CreTgfb1f/fmice are partially protected from developing CCl4-induced liver fibrosis. (A) Homozygous PF4CreTgfb1f/f mice had >90% less TGF-β1 in their platelets than did their littermate controls, as measured with a Duo-Set enzyme-linked immunosorbent assay (ELISA) kit (n = 7; P < .0001). (B) Depiction of the experimental protocol showing CCl4 or oil challenge time points (indicated by arrows) for the duration of the 36-day experiment. (C) Picrosirius red staining was performed on liver sections of oil- or CCl4-challenged WT or PF4CreTgfb1f/f mice according to the protocol shown in panel B. Fibrotic areas in red (images taken under a normal light microscope) and a mixture of green, red, and yellow fluorescent color pictures (taken under polarized light) showed collagen accumulation (representative images are shown). (D) Quantification of fibrotic areas from images taken with a polarized light microscope showed that PF4CreTgfb1f/f mice had smaller fibrotic areas than those of C57Bl/6 (WT) or littermate control (Tgfb1f/f) mice (percentage of picrosirius red–stained fibrosis areas was 2.5% ± 0.5% in PF4CreTgfb1f/f mice, 3.3% ± 0.7% in WT mice, 3.5% ± 1.7% in Tgfb1f/f mice, and 1.0% ± 0.3% in oil-challenged WT controls; P = .002 for PF4CreTgfb1f/f mice vs WT mice; P = .05 for PF4CreTgfb1f/f vs Tgfb1f/f mice; P < .0001 for CCl4-challenged vs oil-challenged WT mice). (E) Total collagen content in liver tissue was measured by the hydroxyproline assay, with lower collagen levels found in PF4CreTgfb1f/f mice (n = 11) than in WT (n = 9) and Tgfb1f/f (n=-5) mice after CCl4 challenge (P = .1 WT vs PF4CreTgfb1f/f mice; P = .06 Tgfb1f/f vs PF4CreTgfb1f/f mice).
PF4CreTgfb1f/fmice are partially protected from developing CCl4-induced liver fibrosis. (A) Homozygous PF4CreTgfb1f/f mice had >90% less TGF-β1 in their platelets than did their littermate controls, as measured with a Duo-Set enzyme-linked immunosorbent assay (ELISA) kit (n = 7; P < .0001). (B) Depiction of the experimental protocol showing CCl4 or oil challenge time points (indicated by arrows) for the duration of the 36-day experiment. (C) Picrosirius red staining was performed on liver sections of oil- or CCl4-challenged WT or PF4CreTgfb1f/f mice according to the protocol shown in panel B. Fibrotic areas in red (images taken under a normal light microscope) and a mixture of green, red, and yellow fluorescent color pictures (taken under polarized light) showed collagen accumulation (representative images are shown). (D) Quantification of fibrotic areas from images taken with a polarized light microscope showed that PF4CreTgfb1f/f mice had smaller fibrotic areas than those of C57Bl/6 (WT) or littermate control (Tgfb1f/f) mice (percentage of picrosirius red–stained fibrosis areas was 2.5% ± 0.5% in PF4CreTgfb1f/f mice, 3.3% ± 0.7% in WT mice, 3.5% ± 1.7% in Tgfb1f/f mice, and 1.0% ± 0.3% in oil-challenged WT controls; P = .002 for PF4CreTgfb1f/f mice vs WT mice; P = .05 for PF4CreTgfb1f/f vs Tgfb1f/f mice; P < .0001 for CCl4-challenged vs oil-challenged WT mice). (E) Total collagen content in liver tissue was measured by the hydroxyproline assay, with lower collagen levels found in PF4CreTgfb1f/f mice (n = 11) than in WT (n = 9) and Tgfb1f/f (n=-5) mice after CCl4 challenge (P = .1 WT vs PF4CreTgfb1f/f mice; P = .06 Tgfb1f/f vs PF4CreTgfb1f/f mice).
Tissue preparation and quantification of fibrosis
Detailed methods are presented in the supplemental Methods. Briefly, animals were sacrificed and their livers were fixed in 4% paraformaldehyde and then sectioned. Fibrosis was evaluated histologically by picrosirius red staining. Fibrotic areas were determined by quantifying polarized light images using NIH ImageJ software (supplemental Figure 1B).
Immunohistochemistry
Dual immunohistochemical staining was performed by incubating tissue sections with specific primary antibodies (listed in supplemental Table 1), followed by secondary antibodies tagged with either Alexa 488 or Alexa 594. Fluorescence images were obtained with a Zeiss 710 confocal or Nikon Eclipse 80i fluorescence microscope.
Hydroxyproline assay
Hydroxyproline content in liver tissues was measured according to the manufacturer’s instructions using a commercial hydroxyproline assay kit (Sigma-Aldrich).
Statistical analysis
All data are expressed as mean ± standard deviation or standard error of the mean. Statistical calculations were performed using GraphPad Prism, and the Student t test was used to determine whether differences were statistically significant. P < .05 was considered statistically significant.
Results
Generation of homozygous mice carrying megakaryocyte/platelet-specific deletion of TGF-β1 (PF4CreTgfb1f/f)
Mice homozygous for both PF4Cre+/+ and Tgfb1f/f alleles (designated as PF4CreTgfb1f/f) were generated by crossing heterozygous PF4Cre+/− and homozygous Tgfb1f/f mice on the C57Bl/6J background for at least 10 generations. Homozygous PF4CreTgfb1f/f mice were born in a normal Mendelian ratio and did not display any gross phenotype. They had lower platelet (by ∼94%) and plasma (by ∼50%) TGF-β1 levels than the WT controls. The TGF-β1 levels in 109 platelets per mL were 73.0 ± 10.0 ng in WT mice and 4.0 ± 1.0 ng in PF4CreTgfb1f/f mice (Figure 1A; P < .0001, n = 7), and in plasma were 2.5 ± 0.7 ng/mL in WT mice and 1.3 ± 0.2 ng/mL in PF4CreTgfb1f/f mice (P < .001, n = 8).
PF4CreTgfb1f/f mice are partially protected from developing CCl4-induced liver fibrosis
To study the role of platelet-derived TGF-β1 in liver fibrosis, PF4CreTgfb1f/f and WT control mice were challenged with repeated CCl4 or oil injection as shown in Figure 1B. Two mice (1 in each group) that died before the 36-day experiment end point were excluded from the analysis; there was no difference in death rates between genotypes. Thirty-six days after the initial CCl4 challenge, liver weight-to-BW ratios were similar in WT and PF4CreTgfb1f/f mice, but all WT and Tgfb1f/f littermate control mice had severe fibrosis with increased collagen staining (shown in red under normal light and in fluorescent color under polarized light), whereas PF4CreTgfb1f/f mice had relatively less fibrosis (Figure 1C). Control WT mice challenged with oil had very little or no fibrosis. Picrosirius red–stained collagen fibers in the fibrotic areas, quantified from whole-liver lobe images taken under polarized light, showed significantly lower levels of fibrosis in PF4CreTgfb1f/f mice compared with WT and Tgfb1f/f mice at 36 days after the initial CCl4 challenge (Figure 1D).
The total collagen content in the liver tissue was measured using hydroxyproline assays. Hydroxyproline content was higher after CCl4 challenge than after oil challenge in WT and Tgfb1f/f mice. The mean hydroxyproline content was ∼20% lower in PF4CreTgfb1f/f mice than in WT and Tgfb1f/f mice after CCl4 challenge (Figure 1E), although the differences were not statistically significant (P = .1 vs WT, and P = .06 vs Tgfb1f/f).
CCl4 challenge induces similar acute liver injury in both PF4CreTgfb1f/f and WT mice
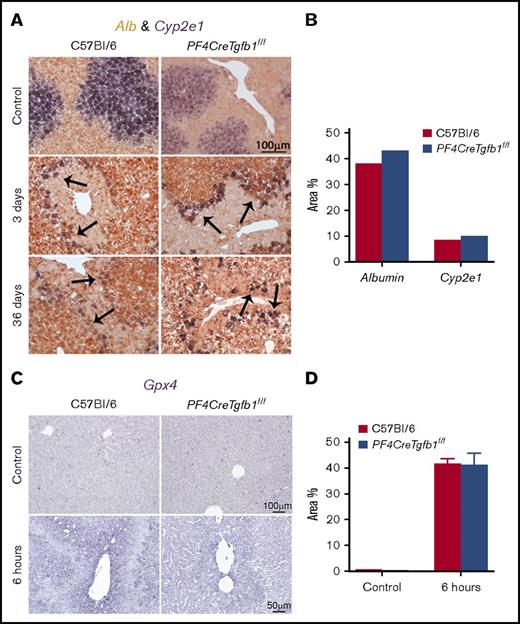
CCl4 induces liver injury, and the cytochrome P450 isozyme (Cyp2e1) is the key metabolic enzyme in the liver that breaks down CCl4.30,31 Normally, Cyp2e1 is primarily expressed in hepatocytes located close to the pericentral veins, whereas albumin (Alb) is mostly produced by hepatocytes in the periportal areas; CCl4 changes the expression patterns of these genes during CCl4 injury in a time-dependent manner.31 To evaluate acute liver injury, in situ hybridization was performed before and after CCl4 challenge, and the expression of Cyp2e1 and Alb genes was evaluated using dual- and single-color in situ hybridization staining. Expression of Cyp2e1 in the area close to the pericentral vein was uniform in both WT and PF4CreTgfb1f/f mice before CCl4 challenge (Figure 2A; supplemental Figure 2). A time-dependent loss of Cyp2e1-expressing cells was observed as early as 6 hours, and maximum loss of these cells was observed 3 days after CCl4 challenge in both WT and PF4CreTgfb1f/f mice (Figure 2A; supplemental Figure 2). Alb gene expression was unaffected at all time points (Figure 2A; supplemental Figure 2).
Expression of Cyp2e1 and Alb mRNA in livers after CCl4 challenge by in situ hybridization. Liver sections were subjected to in situ hybridization with specific probes for Cyp2e1 and Alb genes. (A) Double in situ hybridization showed staining of Cyp2e1-expressing cells as a deep blue color stained with NBT/BCIP-substrate bordering the injured areas (indicated by the arrows), mainly in the pericentral vein area. Alb expression, shown in deep brown stained with INT/BCIP-substrate, was observed throughout the liver, except in the pericentral vein area, at 3 days and 36 days after CCl4 challenges in both WT and PF4CreTgfb1f/f mice. (B) Quantification of Cyp2e1 and Alb expression showed cells expressing the Cyp2e1 gene in the injured areas around the pericentral veins in both WT and PF4CreTgfb1f/f mice at 3 days after CCl4 challenge. (C) Gpx4 expression in the liver was assessed by in situ hybridization using NBT/BCIP-substrate and showed similar expression levels in both WT and PF4CreTgfb1f/f mice at 6 hours after CCl4 challenge. (D) Quantification of Gpx4 6 hours after CCl4 challenge in WT and PF4CreTgfb1f/fmice.
Expression of Cyp2e1 and Alb mRNA in livers after CCl4 challenge by in situ hybridization. Liver sections were subjected to in situ hybridization with specific probes for Cyp2e1 and Alb genes. (A) Double in situ hybridization showed staining of Cyp2e1-expressing cells as a deep blue color stained with NBT/BCIP-substrate bordering the injured areas (indicated by the arrows), mainly in the pericentral vein area. Alb expression, shown in deep brown stained with INT/BCIP-substrate, was observed throughout the liver, except in the pericentral vein area, at 3 days and 36 days after CCl4 challenges in both WT and PF4CreTgfb1f/f mice. (B) Quantification of Cyp2e1 and Alb expression showed cells expressing the Cyp2e1 gene in the injured areas around the pericentral veins in both WT and PF4CreTgfb1f/f mice at 3 days after CCl4 challenge. (C) Gpx4 expression in the liver was assessed by in situ hybridization using NBT/BCIP-substrate and showed similar expression levels in both WT and PF4CreTgfb1f/f mice at 6 hours after CCl4 challenge. (D) Quantification of Gpx4 6 hours after CCl4 challenge in WT and PF4CreTgfb1f/fmice.
Quantification of both Cyp2e1- and Alb-expressing cells revealed a similar injury in both PF4CreTgfb1f/f and WT mice at 3 days after CCl4 challenge (Figure 2B). Cyp2e1-expressing cells remained on the peripheral borders of the injured areas at 3 days and remained associated with the fibrotic areas bordering the outer layer, whereas Alb gene expression was unaffected at 3 days and 36 days after CCl4 challenge (Figure 2A). These data showed that the first CCl4 challenge caused maximum injury at 3 days; however, a decrease in injury was observed after subsequent repeated chronic challenges of CCl4 for 36 days in both genotypes, potentially because of the barrier formed by the Cyp2e1-expressing cells along the border of the injured areas, which protected the liver from further injury (Figure 2A; supplemental Figure 2). Quantitative reverse transcription polymerase chain reaction also revealed similar Alb and Cyp2e1 gene expression patterns,31 with high expression before and loss of expression 3 days after CCl4 challenge (data not shown). In addition to Cyp2e1 expression, we also performed hematoxylin-and-eosin and periodic acid–Schiff staining to visualize damaged areas. These areas were nearly equivalent in WT and PF4CreTgfb1f/f mice at 6 hours, 1 day, and 3 days, but they were substantially reduced in PF4CreTgfb1f/f mice at 36 days compared with WT mice (supplemental Figure 3), consistent with less fibrosis in PF4CreTgfb1f/f mice.
To assess the redox environment in the liver during acute injury, in situ hybridization was performed of glutathione peroxidase 4 (Gpx4), also known as oxidoreductase, which catalyzes the breakdown of free radicals to glutathione after toxic injury by CCl4. Gpx4 messenger RNA (mRNA) expression was upregulated in the injured areas 6 hours after CCl4 challenge in both WT and PF4CreTgfb1f/f mice (Figure 2C-D), thus indicating the occurrence of redox reactions during the initial injury caused by acute CCl4 challenge. We found a transient increase in lipid peroxidation in the liver 6 hours after CCl4 challenge in both WT and PF4CreTgfb1f/f mice (malondialdehyde assay increased from 0.04 ± 0.002 nM/μL to 0.09 ± 0.02 nM/μL in WT [P = .02] and 0.04 ± 0.002 nM/μL to 0.06 ± 0.008 nM/μL in PF4CreTgfb1f/f mice [P = .004]), indicating Gpx4 upregulation inducing redox reactions in the damaged area of the liver.
Activated platelets are present in the liver tissue 6 hours after CCl4 challenge
To investigate whether platelets are activated in the liver after a CCl4 challenge, cells from liver tissue were isolated and stained with anti-CD41 and Jon/A, an antibody that specifically detects activated platelets, prior to flow cytometry analysis. We observed an increase in CD41 and Jon/A double-positive platelets 6 hours after the CCl4 challenge compared with untreated liver in WT mice (supplemental Figure 4A). Similarly, platelets double-stained for CD41 and P-selectin, another marker expressed in activated platelets, were also elevated 6 hours after CCl4 challenge (supplemental Figure 4B), thus indicating their activated state. Activated platelet numbers decreased 1 day after CCl4 challenge in both WT and PF4CreTgfb1f/f liver, indicating transient activation of platelets 6 hours after CCl4 injection.
Induction of transient thrombocytopenia in WT mice partially protects against CCl4-induced liver fibrosis
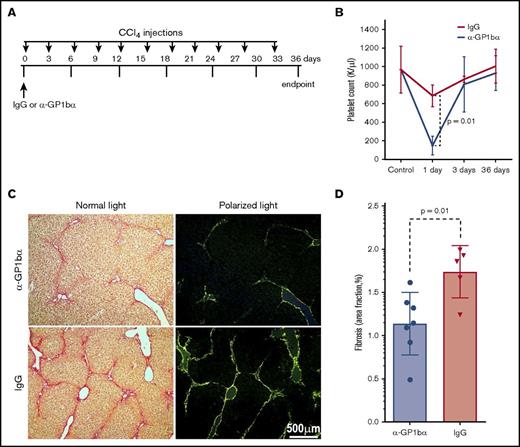
To assess whether transiently decreasing platelets in the circulation after CCl4 injection would affect liver fibrosis, WT mice were injected with a single dose of platelet-specific monoclonal antibody (mAb) anti-GP1bα (0.25 mg/kg BW) to induce thrombocytopenia 6 hours before the first CCl4 challenge (Figure 3A). Control mice were injected with an isotype-matched IgG (0.25 mg/kg BW). Pretreatment with anti-GP1bα transiently reduced circulating platelet counts by ∼75% compared with mice pretreated with IgG within 1 day after injection (Figure 3B). Platelet counts returned to normal levels within 3 days and were similar at 36 days after 11 CCl4 injections (Figure 3B). No evidence of hemorrhage or changes in other blood counts were observed during the transient depletion of platelets 1 day after anti-GP1bα injection; however, repeated injections of anti-platelet antibody caused severe bleeding, as expected.17 Importantly, we found that WT mice pretreated with anti-GP1bα prior to the initial CCl4 injection displayed less liver fibrosis than those pretreated with IgG (Figure 3C). Quantification of fibrotic areas showed ∼30% lower fibrosis in mAb anti-GP1bα–injected animals (n = 7) than in IgG-injected (n = 5) animals (P = .01) (Figure 3D). Next, we tested whether transient thrombocytopenia induced at the midpoint of CCl4 treatment (day 18; supplemental Figure 4C) affected liver fibrosis. We found that a single dose of mAb anti-GP1bα injected 6 hours prior to the seventh of 11 CCl4 treatments (day 18) also transiently reduced circulating platelet counts (data not shown), but did not decrease fibrosis (supplemental Figure 4D), suggesting that early platelet release of TGF-β1 is particularly important to initiate liver fibrosis.
Transient thrombocytopenia partially protects CCl4-induced liver fibrosis. (A) Experimental protocol showing IgG vs anti-GP-1bα (α-GP1bα) injection times and the CCl4 challenge time points (indicated by arrows) for the duration of the 36-day experiment. (B) Platelet counts showed marked thrombocytopenia in WT mice 1 day after α-GP1bα antibody administration (P = .01 for isotype-matched IgG vs α-GP1bα injections). (C) Picrosirius red staining images at 36 days (both light and polarized light microscopy) showed fibrotic areas in liver sections of WT mice pretreated with IgG or α-GP1bα and then subjected to CCl4 challenge. (D) Quantification of liver fibrosis showed that mice pretreated with α-GP1bα (n = 7) had significantly less fibrosis than those pretreated with IgG (n = 5; P = .01) at 36 days.
Transient thrombocytopenia partially protects CCl4-induced liver fibrosis. (A) Experimental protocol showing IgG vs anti-GP-1bα (α-GP1bα) injection times and the CCl4 challenge time points (indicated by arrows) for the duration of the 36-day experiment. (B) Platelet counts showed marked thrombocytopenia in WT mice 1 day after α-GP1bα antibody administration (P = .01 for isotype-matched IgG vs α-GP1bα injections). (C) Picrosirius red staining images at 36 days (both light and polarized light microscopy) showed fibrotic areas in liver sections of WT mice pretreated with IgG or α-GP1bα and then subjected to CCl4 challenge. (D) Quantification of liver fibrosis showed that mice pretreated with α-GP1bα (n = 7) had significantly less fibrosis than those pretreated with IgG (n = 5; P = .01) at 36 days.
Plasma levels of total TGF-β1 increase transiently 6 hours after CCl4 challenge in WT mice but not in PF4CreTgfb1f/f mice
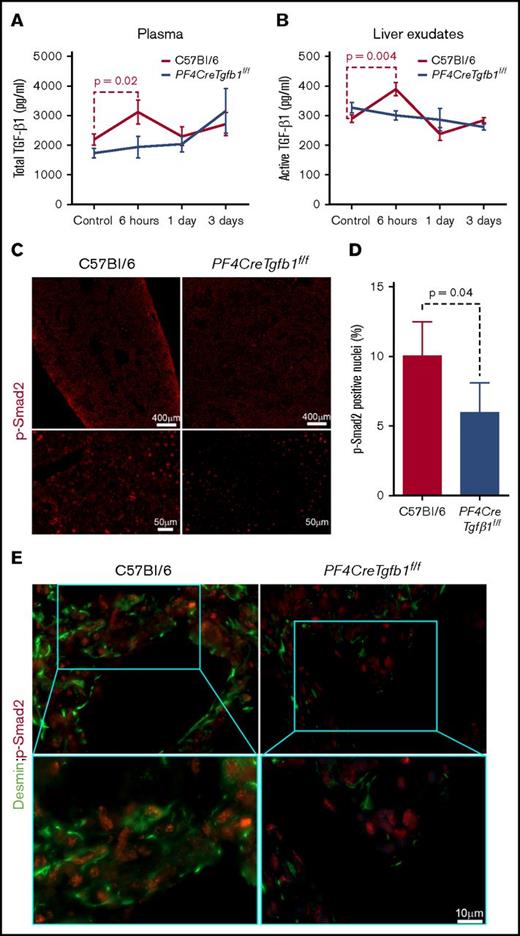
We followed our previously published method of plasma preparation17 and then measured the levels of total TGF-β1 in plasma, which showed higher total TGF-β1 in WT mice than PF4CreTgfb1f/f mice under basal condition. Oil challenge did not affect plasma TGF-β1 levels in either WT or PF4CreTgfb1f/f mice. CCl4 challenge, however, led to a transient increase (of ∼20%) in total plasma TGF-β1 at 6 hours in WT mice (2.2 ng/mL before and 3.1 ng/mL after), but no increase in total plasma TGF-β1 in PF4CreTgfb1f/f mice (Figure 4A). We also observed higher levels of PF4 and thrombospondin 1 (TSP-1) 6 hours after CCl4 challenge (data not shown). These data are consistent with transient platelet activation 6 hours after a single CCl4 challenge.
Total and active TGF-β1 and p-Smad2 levels increase transiently after acute CCl4 challenge in WT mice but not in PF4CreTgfb1f/fmice. (A) Total TGF-β1 levels in the plasma of WT and PF4CreTgfb1f/f mice at the indicated time points were measured with a modified ELISA protocol using DuoSet kits from R&D Systems. There was a transient increase in the total plasma TGF-β1 in WT mice (n = 7) but not in PF4CreTgfb1f/f mice at 6 hours (n = 9). (B) Both active and total TGF-β1 levels in liver exudates were measured by ELISA. Active TGF-β1 levels were higher in WT liver exudates than in PF4CreTgfb1f/f liver exudates at 6 hours (n = 5; P = .004). (C) p-Smad2 immunostaining showed that most of the phosphorylated signals were in the nuclei (red dots in the tiled pictures of the whole-liver section in the top panels and magnified injured areas in the bottom panels), showing lower levels of p-Smad2 in the injured liver areas of PF4CreTgfb1f/f vs WT mice at 3 days after CCl4 challenge. (D) Quantification of p-Smad2 intensity in the injured areas of the liver sections showed that PF4CreTgfb1f/f livers had fewer p-Smad2 cells than did those of WT mice (n = 4; P = .04). (E) Sequential dual staining with p-Smad2 and desmin antibodies showed that most of the cells expressing p-Smad2 were desmin-positive HSCs in the injured areas of the liver at 3 days after CCl4 challenge.
Total and active TGF-β1 and p-Smad2 levels increase transiently after acute CCl4 challenge in WT mice but not in PF4CreTgfb1f/fmice. (A) Total TGF-β1 levels in the plasma of WT and PF4CreTgfb1f/f mice at the indicated time points were measured with a modified ELISA protocol using DuoSet kits from R&D Systems. There was a transient increase in the total plasma TGF-β1 in WT mice (n = 7) but not in PF4CreTgfb1f/f mice at 6 hours (n = 9). (B) Both active and total TGF-β1 levels in liver exudates were measured by ELISA. Active TGF-β1 levels were higher in WT liver exudates than in PF4CreTgfb1f/f liver exudates at 6 hours (n = 5; P = .004). (C) p-Smad2 immunostaining showed that most of the phosphorylated signals were in the nuclei (red dots in the tiled pictures of the whole-liver section in the top panels and magnified injured areas in the bottom panels), showing lower levels of p-Smad2 in the injured liver areas of PF4CreTgfb1f/f vs WT mice at 3 days after CCl4 challenge. (D) Quantification of p-Smad2 intensity in the injured areas of the liver sections showed that PF4CreTgfb1f/f livers had fewer p-Smad2 cells than did those of WT mice (n = 4; P = .04). (E) Sequential dual staining with p-Smad2 and desmin antibodies showed that most of the cells expressing p-Smad2 were desmin-positive HSCs in the injured areas of the liver at 3 days after CCl4 challenge.
Active TGF-β1 levels increase transiently in liver tissue 6 hours after CCl4 challenge in WT mice but not in PF4CreTgfb1f/f mice
To assess whether active TGF-β1 levels increased in liver tissue, the total and active TGF-β1 levels in liver exudates were measured before, 6 hours, 1 day, and 3 days after CCl4 challenge. Active TGF-β1 levels increased in WT mice but not in PF4CreTgfb1f/f mice 6 hours after CCl4 challenge (Figure 4B). The percentage of active TGF-β1 compared with total TGF-β1 was also increased in WT mice but not in PF4CreTgfb1f/f mice (data not shown). These data indicate the occurrence of transient activation of TGF-β1 concomitant with the peak release of total TGF-β1 and platelet activation.
PF4CreTgfb1f/f mice have lower p-Smad2 signaling than WT mice
The consequence of active TGF-β1 generation in liver tissue was also assessed by measuring p-Smad2 as a primary TGF-β1 signaling event during acute injury after CCl4 challenge. Representative images showed a lower number of p-Smad2+ nuclei in the injured areas in PF4CreTgfb1f/f mice than in WT mice (Figure 4C). Quantification of p-Smad2+ nuclei in the injured areas showed ∼20% fewer p-Smad2+ nuclei in PF4CreTgfb1f/f mice compared with WT mice 3 days after CCl4 challenge (Figure 4D). Dual staining of p-Smad2+ cells for desmin was also positive, indicating that p-Smad2+ cells are HSCs that are stimulated by platelet-derived TGF-β1 (Figure 4E).
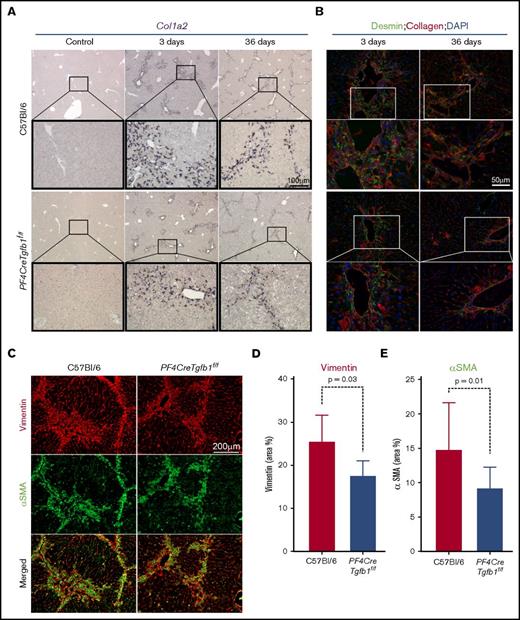
PF4CreTgfb1f/f mice have lower numbers of collagen-expressing activated HSCs and myofibroblasts than WT mice
Because HSCs are the primary cells that transdifferentiate into myofibroblasts and produce collagen during the initial phase of TGF-β1 signaling,15 in situ hybridization was performed to assess collagen (Col1a2) mRNA expression. The results showed a higher number of Col1a2-expressing cells in the injured areas of the liver in WT mice than in PF4CreTgfb1f/f mice at both 3 days and 36 days after CCl4 challenge (Figure 5A). To confirm that those Col1a2-expressing cells were HSCs, the cells were coimmunostained with desmin and collagen antibodies. A higher number of desmin-positive HSCs (some also positive for collagen) were seen at 3 days in WT mice compared with PF4CreTgfb1f/f mice; however, most cells expressed collagen at 36 days and few desmin-positive HSCs were present (Figure 5B), indicating that HSCs were transformed into myofibroblasts. Myofibroblast accumulation is a hallmark of tissue fibrosis, which causes loss of the original markers of the parent cells, gained expression of α–smooth muscle actin (αSMA) and vimentin, and the production of collagen.32 To confirm whether myofibroblasts were present in the fibrotic areas, liver sections after 36 days of repeated CCl4 challenge were stained with αSMA and vimentin. Higher numbers of αSMA and vimentin double-positive myofibroblasts were observed in the fibrotic areas of WT mice compared with PF4CreTgfb1f/f mice (Figure 5C). The numbers of both αSMA- and vimentin-positive cells were significantly lower in PF4CreTgfb1f/f mice than in WT mice (Figure 5D-E).
PF4CreTgfb1f/fmice have fewer collagen-expressing HSCs and myofibroblasts than WT mice. (A) In situ hybridization of the Col1a2 probe showed higher collagen-expressing cell accumulation in the injured areas of the liver in WT vs PF4CreTgfb1f/f mice at 3 days and 36 days after CCl4 challenge. Higher-magnification images (boxed) showed that the morphology of those cells resembled HSCs. (B) To confirm that they were HSCs, liver sections were costained with anti-desmin and collagen antibodies; double-positive cells started to appear at 3 days and in the fibrotic areas at 36 days after CCl4 challenge. (C) Dual staining with myofibroblast markers αSMA (green) and vimentin (red) showed double-positive (yellow) cells at 36 days after CCl4 challenge in the WT and PF4CreTgfb1f/f mice. Quantification of vimentin- (D) and αSMA- (E) positive cells in PF4CreTgfb1f/f (n = 7) vs WT (n = 12) mice. DAPI, 4′,6-diamidino-2-phenylindole.
PF4CreTgfb1f/fmice have fewer collagen-expressing HSCs and myofibroblasts than WT mice. (A) In situ hybridization of the Col1a2 probe showed higher collagen-expressing cell accumulation in the injured areas of the liver in WT vs PF4CreTgfb1f/f mice at 3 days and 36 days after CCl4 challenge. Higher-magnification images (boxed) showed that the morphology of those cells resembled HSCs. (B) To confirm that they were HSCs, liver sections were costained with anti-desmin and collagen antibodies; double-positive cells started to appear at 3 days and in the fibrotic areas at 36 days after CCl4 challenge. (C) Dual staining with myofibroblast markers αSMA (green) and vimentin (red) showed double-positive (yellow) cells at 36 days after CCl4 challenge in the WT and PF4CreTgfb1f/f mice. Quantification of vimentin- (D) and αSMA- (E) positive cells in PF4CreTgfb1f/f (n = 7) vs WT (n = 12) mice. DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
Our finding that PF4CreTgfb1f/f mice exhibited significantly less liver fibrosis than WT or littermate control mice after chronic CCl4 challenge provides evidence that platelet-derived TGF-β1 partially contributes to liver fibrosis in this model. Our new homozygous PF4CreTgfb1f/f mice had much lower levels of TGF-β1 in platelets (∼94% lower) and in basal plasma (∼50% lower) than the levels observed in our previous study using heterozygous PF4Cre+/−Tgfb1f/f mice, in which TGF-β1 levels were decreased by ∼80% in platelets and ∼40% in plasma.17 This difference indicates that introducing 2 alleles of PF4Cre+/+ recombinase more efficiently depleted TGF-β1 in platelets, resulting in a dramatic reduction in systemic TGF-β1 levels by almost half and confirming our previous finding that platelets contribute significantly to basal plasma levels of TGF-β1.17
Our fibrosis quantification methodology is unique and robust because the visible parenchymal fibrosis in the entire lobe of the liver was quantified after exclusion of the visible basal collagen associated with large vessels in the liver (supplemental Figure 1A). Although the hydroxyproline assay is commonly used to quantify tissue collagen content, it measures both natural basal collagen associated with the basement membrane and blood vessels and pathologic collagen accumulation in fibrosis. Although our visual and quantified data from picrosirius red staining showed a significant difference in liver fibrosis in PF4CreTgfb1f/f mice compared with WT and littermate control mice after chronic CCl4 (Figure 1C-D), our hydroxyproline data from the same groups of mice showed only a decreasing trend (Figure 1E; P = .1 and .06). These discrepancies may have been due to some liver sections containing more blood vessels, thus accounting for basal collagen, which may be a confounding factor in quantifying collagen accumulation in pathologic fibrotic phenotypes. Therefore, our visual image processing, including carefully removing blood vessels, and computational quantification data for fibrosis are much more reliable. Our in situ hybridization results showing expression of Cyp2e1 (which changes upon CCl4 challenge) and Alb genes indicated that WT and PF4CreTgfb1f/f mice sustained equivalent acute liver injury induced by CCl4 challenge, thus suggesting that the lower amount of fibrosis observed in PF4CreTgfb1f/f mice may not be due to less severe initial injury caused by CCl4 but instead may be the result of platelet-derived TGF-β1–mediated profibrotic signaling.
Sterile inflammation (SI) is a key process in drug- or toxin-induced liver injury in the absence of pathogens,33 and SI-driven liver diseases are common in industrially developed countries; unfortunately, there is no specific treatment of fibrosis. We used the CCl4-induced liver fibrosis model because it simulates SI-induced pathology and is a reproducible model,34 whereas the commonly used bile-duct ligation model requires surgery and can often be associated with mortality and platelet activation associated with surgery.35 Although our model involves an artificial and aggressive liver injury, other natural chronic liver injury models should be tested in the future to assess the role of platelet-derived TGF-β1 in models of alcohol-induced, acetaminophen overdose–induced, and viral or parasite infection–induced liver fibrosis.34
Recently, Ito et al have shown that platelets can pass through toxin-induced injured liver sinusoidal endothelial layers.36 These data are consistent with our finding that platelets in injured areas were activated, which is unsurprising given that platelets are activated after contact with extracellular matrix.37 However, the timing of initial transient platelet activation is interesting because it correlated with transient increases in total TGF-β1 in both plasma and liver tissue 6 hours after the first CCl4 challenge, indicating that the release of TGF-β1 from platelets occurs in acute liver injury as early as 6 hours after CCl4 challenge.
Our experiments with transient platelet depletion before injury using mAb anti-GP1bα are protective, indicating that initial platelet activation is important, although the possibility of later contributions of platelets in the process cannot be excluded. More importantly, the detection of active TGF-β1 in liver exudates in the basal condition supports the importance of TGF-β1 signaling in liver homeostasis,16 but the transient spike in TGF-β1 activity in WT mice and increased p-Smad2+ cells during the initial liver injury suggests that platelet-derived TGF-β1 release and subsequent activation most likely initiates profibrogenic signaling that may lead to liver fibrosis. However, further time-course and systematic analyses using lineage-tracing experiments are required to determine the mechanism by which platelet TGF-β1 initiates signaling that leads to activation of cellular transformation, which ultimately results in liver fibrosis.
A redox environment during liver injury can be assumed, as supported by our data that upregulation of Gpx4, an oxidoreductase enzyme capable of catalyzing oxidation, activates TGF-β1.38 A high-redox environment generates reactive oxygen species, which also activate latent TGF-β1.8,39 Other possible mechanisms of TGF-β1 activation in the liver may include changes in shear force; previously, we have shown that shear can activate latent TGF-β1.18 It has been demonstrated that during liver injury, sinusoidal endothelial cells sense changes in shear stress,40 but measuring shear stress change and correlating this change with active TGF-β1 is challenging in vivo.41 Moreover, our group and others have shown that TSP-1 activates TGF-β1,42,43 and a recent review has summarized the role of TSP-1 in liver fibrosis.44 Therefore, TSP-1–mediated TGF-β1 activation under shear stress change may also be a potential mechanism of TGF-β1 activation in the liver. Moreover, protease-degraded fragments of latency-associated protein/TGF-β1 have been detected using specific epitope-recognizing antibodies in patients and animals with liver fibrosis, thus suggesting that protease-mediated TGF-β1 activation occurs during liver fibrosis, which can be detected in plasma.45 Further experiments are required to identify the mechanism(s) of TGF-β1 activation in the liver.
TGF-β1 signaling is a strong stimulator of HSC transdifferentiation into collagen-producing myofibroblasts,15 the primary cell type involved in pathologic liver fibrosis.46-48 Our findings showed that mice deficient in platelet TGF-β1 (PF4CreTgfb1f/f) have less p-Smad2 signaling in the injured areas, lower expression of Col1a2 genes in HSCs, fewer collagen-producing myofibroblasts, and less fibrosis in the liver, as compared with WT mice, thus suggesting that platelet TGF-β1 may have direct and/or indirect effects on HSCs. However, the possible effects of platelet TGF-β1 on other cell types in the liver, including portal fibroblasts, macrophages, hepatocytes, endothelial cells, and stem cells, cannot be eliminated in the pathogenesis of liver fibrosis.49 Indeed, both in vitro and in vivo evidence have shown that sinusoidal endothelial cells and hepatocytes can internalize platelets,41,50 thereby indicating that direct contact of platelets with other liver cells is possible during injury. Moreover, a recent study showed that liver macrophages activated platelets via a receptor that requires glycosylation,51 suggesting a possible role of liver macrophages in regulating liver fibrosis. Because platelet TGF-β1 does not account for all of the induction of liver fibrosis, the possibility that other cellular sources of TGF-β1 might influence liver fibrosis remains to be determined. Future studies with conditional deletion of TGF-β1 in other liver cells, along with inflammatory cell depletion and pharmacological inhibitor experiments, are required to address these issues.
The role of platelets in liver fibrosis is controversial, with some studies showing a beneficial role of platelets on liver function,21-23 as suggested by a clinical trial indicating that increased platelet numbers induced by platelet transfusion improve liver function in patients with chronic liver disease,52 whereas other studies have shown detrimental effects.24-27 These conflicting findings could suggest a dual role of platelets that may be explained by their differential activation status and potential ability to release various factors. As shown by Italiano et al, platelets contain both antiangiogenic and proangiogenic factors that are differentially released after platelet activation by stimulation of various receptors,53 in agreement with the concept that platelets play a dual role in regulating angiogenesis. A similar scenario may occur in terms of platelets regulating fibrosis vs liver regeneration, by releasing factors that cause fibrosis, such as TGF-β1, or factors that mobilize and promote proliferation of stem cells and progenitor cells during regeneration, such as serotonin or stromal-cell derived factor-1. A recent study reported that reprogramming myofibroblasts and stimulating stem cells differentially regulates liver fibrosis and regeneration in a manner dependent on transcriptional expression and the duration of TGF-β1 stimulation,54,55 thus supporting the dual-role concept of platelets, which might be differentially mediated by platelet-derived TGF-β1. Further studies with genetically modified mouse models, including lineage-tracing experiments, are required to resolve these complicated issues.
This study provides new insights into the mechanism by which platelet-derived TGF-β1 may induce liver fibrosis. Our findings suggest that future studies blocking activation of platelets and/or TGF-β1 may lead to new therapeutic approaches to prevent hepatic fibrosis.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the members of the Cardiovascular Biology Research Program at Oklahoma Medical Research Foundation for their kind assistance in allowing the use of their instruments. The authors also thank Melinda West for mouse colony maintenance and M. K. Occhipinti and A. Anderson (life science editors) for editorial assistance.
This work was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (grant HL123605) (J.A.), National Institute of General Medical Sciences (grant GM114731), and National Cancer Institute (grant CA213987).
Authorship
Contribution: S.G. performed the experiments, interpreted the data, created figures, and assisted in drafting the manuscript; R.V. and T.R. assisted with and performed some experiments and provided valuable suggestions; B.M., K.K., and S.W. performed some experiments; L.X. provided intellectual feedback and valuable comments; and J.A. conceived the idea, provided intellectual feedback, supervised the project, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jasimuddin Ahamed, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: ahamedj@omrf.org.