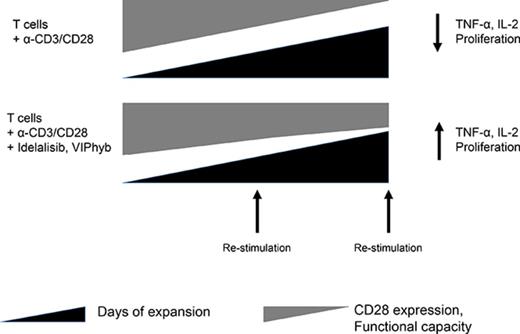
Key Points
Number of prior chemotherapy cycles in cancer patients correlates with T-cell senescent phenotype and loss of CD27 and CD28 expression.
Addition of PI3Kδ inhibitors and VIP antagonists increased ex vivo expansion, in vivo persistence, and anticancer cytotoxicity of T cells.
Abstract
Adoptive therapy with ex vivo–expanded genetically modified antigen-specific T cells can induce remissions in patients with relapsed/refractory cancer. The clinical success of this therapy depends upon efficient transduction and expansion of T cells ex vivo and their homing, persistence and cytotoxicity following reinfusion. Lower rates of ex vivo expansion and clinical response using anti-CD19 chimeric antigen receptor (CAR) T cells have been seen in heavily pretreated lymphoma patients compared with B-cell acute lymphoblastic leukemia patients and motivate the development of novel strategies to enhance ex vivo T cell expansion and their persistence in vivo. We demonstrate that inhibition of phosphatidylinositol 3-kinase δ (PI3Kδ) and antagonism of vasoactive intestinal peptide (VIP) signaling partially inhibits the terminal differentiation of T cells during anti-CD3/CD28 bead-mediated expansion (mean, 54.4% CD27+CD28+ T cells vs 27.4% in control cultures; P < .05). This strategy results in a mean of 83.7% more T cells cultured from lymphoma patients in the presence of PI3Kδ and VIP antagonists, increased survival of human T cells from a lymphoma patient in a murine xenograft model, enhanced cytotoxic activity of antigen-specific human CAR T cells and murine T cells against lymphoma, and increased transduction and expansion of anti-CD5 human CAR T cells. PI3Kδ and VIP antagonist-expanded T cells from lymphoma patients show reduced terminal differentiation, enhanced polyfunctional cytokine expression, and preservation of costimulatory molecule expression. Taken together, synergistic blockade of these pathways is an attractive strategy to enhance the expansion and functional capacity of ex vivo–expanded cancer-specific T cells.
Introduction
The early success of chimeric antigen receptor (CAR) T cell therapy has been greatest in the treatment of B-cell leukemias, most notably acute B-cell lymphoblastic leukemia (B-cell ALL) treated with anti-CD19 CAR T cells.1 Diffuse large B-cell lymphoma (DLBCL) is a CD19-positive non-Hodgkin B-cell lymphoma for which the use of anti-CD19 CAR T cell therapy is currently being evaluated.2,3 The efficacy of anti-CD19 CAR T cells in the treatment of adult B-cell lymphoma patients has been less than what has been observed in pediatric B-cell ALL patients, possibly due, in part, to differences in T-cell quality between pediatric patients with B-ALL and adult patients with DLBCL. Furthermore, tumor-specific differences between B-cell ALL and DLBCL may also contribute to different response rates observed in these entities following CD19 CAR T therapy. Patients with relapsed/refractory hematological cancer have been exposed to multiple rounds of cytotoxic therapies prior to the attempted manufacture of CAR T cells.3 Importantly, one of the major off-target effects of these therapies is damage to healthy T cells4 and loss of the naive and central memory T-cell subsets that have the most potent expansion potential and anticancer activity in vivo.5 Loss of naive and central memory T cells in previously treated cancer patients is particularly pronounced in adult patients with DLBCL and has been shown to a result of FasL-mediated fratricide from terminally differentiated effector cells.5 The end result of cell-intrinsic deficits in T-cell function in heavily pretreated patients can lead to inadequate ex vivo T-cell expansion, leading to CAR T-cell manufacturing failures and lack of adequate in vivo expansion of reinfused CAR T cells.6 Durable response rates of 30% to 40% have been reported for lymphoma patients treated with CAR T cells,3,7 with manufacturing failure rates of up to 6%.6 As the field of adoptive T-cell therapy expands to include older patients and those with solid tumors, it is imperative to devise methods that improve the overall quality and yield of T cells expanded from apheresis products of heavily pretreated cancer patients. Since the net expansion of T cells expanded in culture with anti-CD3/CD28 beads for 10 to 14 days is much less than what would be predicted based upon the cell cycle length of optimally activated T cells expanding in vivo to antigen, we hypothesized that adding agents that decrease activation-induced terminal differentiation and cell death8-10 and a peptide competitive antagonist of vasoactive intestinal polypeptide (VIP) that reverse immune suppression caused by native VIP11,12 would have favorable effects on net expansion of T cells with cytotoxic activity in vivo. The rationale for using these agents was previous data from our laboratory showing enhancement of CD8 T-cell dependent anticancer immunity in peptide antagonist to vasoactive intestinal peptide (VIPhyb)–treated mice13,14 and reports of autoimmunity after stopping PI3K δ inhibitor (idelalisib) in lymphoma and chronic lymphocytic leukemia (CLL) patients.15-17
To test this hypothesis, we studied blood samples from healthy volunteers, DLBCL patients prior to treatment, and samples from DLBCL patients who had received multiple courses of cytotoxic treatment. Of note, lymphoma patients who had received prior treatment had a significantly higher proportion of CD27−CD28− T cells, a marker for senescence, when compared with either healthy controls or newly diagnosed DLBCL patients. The overabundance of these cells was associated with failure of in vitro T-cell expansion, as loss of CD28 results in inadequate survival and expansion in cultures with anti-CD3/CD28 beads.18-20 We tested whether the addition of PI3Kδ inhibitors alone and in combination with VIPhyb during the expansion period would improve the quantity and quality of T cells using T cells from previously treated lymphoma patients and in the manufacture of anti-CD5 CAR T cells using a lentivirus construct.
We report herein that low concentrations of idelalisib, a PI3Kδ inhibitor, resulted in significantly increased yield of T cells with a less differentiated phenotype following ex vivo expansion. The addition of VIPhyb to idelalisib during T-cell expansion further increased the frequency of CD27+CD28+ T cells and their in vivo persistence when adoptively transferred to NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice, suggesting an additive or synergistic relationship between these 2 signaling pathways. The ex vivo cytotoxicity and in vivo antitumor activity of T cells expanded in idelalisib, VIPhyb, or a combination was significantly greater than that of T cells from control cultures. These results suggest that antagonism of the PI3Kδ and VIP signaling pathways may be a promising approach to limit T-cell exhaustion senescence during ex vivo expansion and improve the clinical efficacy of genetically modified cells.
Methods
PBMC samples
Peripheral blood mononuclear cells (PBMCs) were obtained from consenting DLBCL patients and healthy controls via apheresis or phlebotomy and Ficoll-Hypaque separation of peripheral blood. All samples were processed immediately after collection and phenotyped by flow cytometry. An additional aliquot of mononuclear cells was frozen in Cryostor CS10 (STEMCELL Technologies, Vancouver, Canada) and stored in liquid nitrogen until use. The study was approved by Emory University’s Institutional Review Board (approval IDs IRB80716 and IRB60350).
Mice
Six- to 8-week-old male luciferase-expressing C57Bl/6 and male NSG mice were bred at the Emory University Animal Care Facility (Atlanta, GA). Male C57/Bl6 SJL (PepBoy) and T-cell receptor (TCR) transgenic OT-I and OT-II strains were purchased from The Jackson Laboratory. All procedures were approved by the Emory University Institutional Animal Care and Use Committee and conformed to the “Guide for the Care and Use of Laboratory Animals.”
Compounds
Idelalisib (CAL-101) was purchased from BocSci (Shirley, NY) and stored as a 10 mM stock solution in dimethyl sulfoxide (DMSO) at −20°C. Duvelisib was purchased from SelleckChem (Houston, TX) and was provided as a 10 mM stock solution in DMSO. Dilutions of idelalisib and duvelisib were performed in DMSO prior to addition to cell cultures. Mast cell chymase was purchased from Sigma-Aldrich (St. Louis, MO) and diluted in sterile phosphate-buffered saline (PBS). VIPhyb (KPRRPYTDNYRELRKQMAVKKYLNSILN) was purchased from New England Peptide (Gardner, MA) and reconstituted in sterile molecular grade water. VIPhyb dilutions were made in sterile PBS prior to addition to cell cultures.
Human T-cell cultures
Frozen PBMCs were thawed and rested overnight at 37°C in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol (complete media). Cells were then cultured in 96-well flat-bottom plates at 2 × 106 cells/mL in complete media containing 30 U/mL interleukin-2 (IL-2; Peprotech, Rocky Hill, NJ) and anti-CD3/CD28 beads (Life Technologies, Carlsbad, CA) at a 1:1 bead to cell ratio. Idelalisib or duvelisib was added at culture initiation, and VIPhyb was added daily. The final DMSO concentration was 0.1% in all wells. Cells were counted and subcultured on day 7 with the addition of fresh beads, IL-2, and compounds. The cells were then allowed to expand for an additional 3 or 7 days. Cultures were split during the expansion period as needed. Cells were counted and phenotyped on day 7 and on day 10 or 14. Cell numbers and fold expansion are reported as the final yield, back-calculated accounting for dilution and subculture.
Subsets of T cells from heavily treated DLBCL patients were sorted according to expression of CD27 and CD28 using a BD FACS Aria II (BD Biosciences, San Jose, CA). Two populations were sorted: T cells lacking expression of both CD27 and CD28 and the remaining cells (CD27+CD28−, CD27−CD28+, and CD27+CD28+). Gating strategies for cell sorting excluded other blood cells, including granulocytes, monocytes, natural killer cells, dendritic cells, and B cells. Three populations were expanded separately in culture under the conditions described above: the total T cell population including all subsets, the CD27−CD28− population, and the mixed population that did not contain CD27−CD28− cells. The cells were expanded for a period of 14 days whereupon they were analyzed for viability, total cell counts, and expression of surface markers.
CD5 CAR T-cell generation and cytotoxicity assay
The CD5 scFv complementary DNA sequence was derived from the protein sequence of a humanized version of the murine anti-human CD5 monoclonal antibody H65.21 The complementary DNA sequence was codon optimized for human cell expression and then cloned into our previously used vector encoding a second-generation CAR with CD28 as the costimulatory domain.22 The vector allowed for dual expression of enhanced green fluorescent protein and the CD5-CAR using a P2A sequence. High-titer, recombinant, self-inactivating HIV lentiviral vector was produced using a 4-plasmid system as we have previously described.22 Titering of the concentrated recombinant lentiviral vector was performed on HEK-293T cell genomic DNA using quantitative polymerase chain reaction with resulting titers of ∼1 × 107 TU/mL. Primary T cells were transduced on day 10 of expansion by incubating cells with the CAR-expressing lentiviral vector in the assigned culture media supplemented with 4 μg/mL polybrene (EMD Millipore, Billerica, MA). The transduced cells were cultured for at least 3 days before being used for downstream applications.
CD5-positive target Jurkat cells were labeled with the membrane dye PKH26 according to the manufacturer’s protocol (Sigma-Aldrich). The effector primary T cells were left unstained. Effector and target cells were counted and viability assessed using trypan blue. 50 000 labeled target cells were mixed with 250 000 effector cells in 12 × 75 mm fluorescence-activated cell sorting (FACS) tubes at an effector/target ratio of 5:1. The cell mixture was incubated for 4 hours at 37°C in 5% CO2. Baseline target cell death was measured by incubating 50 000 target cells without any effector cells over the same time period. After incubation, cells were washed and stained with the dead cell dye 7-aminoactinomycin D (7-AAD; BD Biosciences). Flow cytometry analysis was performed to assess 7-AAD–positive cells. All experiments were performed in triplicate. To calculate specific cytotoxicity, the number of spontaneously lysed target cells in the absence of effector cells was subtracted from the number of dead target cells, which were identified as PKH26 and 7-AAD double positive in the measured sample.
Mouse T-cell expansion and bioluminescent imaging
Splenic T cells from luciferase-positive, C57/Bl6, OT-I, and OT-II mice were purified using the EasySep T Cell Isolation Kit (STEMCELL Technologies). Cells were cultured in 96 well flat-bottom plates in complete RPMI 1640 containing 30 U/mL IL-2 (R&D Systems, Minneapolis, MN). T cells were stimulated with anti-CD3/CD28 beads (Life Technologies) at a 1:1 bead to cell ratio for 3 days then analyzed using imaging. d-luciferin (PerkinElmer, Waltham, MA) was added to each well at 150 μg/mL and luminescence in each well was measured using an IVIS spectrum imager (PerkinElmer). Quantification of relative cell numbers was performed by generating a region of interest over each well and measuring the luminescence. Measurements were given as photons per second per square centimeter and are reported as normalized to stimulated DMSO control. For experiments examining proliferation, T cells were labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Life Technologies) prior to stimulation.
Phenotypic analysis
Analysis of samples from patients, healthy volunteers, and mice prior to and during expansion was performed by flow cytometry. Anti-human CD3 phycoerythrin (PE)-CF594, CD4 allophycocyanin (APC)-Cy7, CD8 fluorescein isothiocyanate, CD27 PE, IL-2 PE-Cy7, interferon-γ (IFN-γ) PE, and tumor necrosis factor α V450 were purchased from BD Biosciences. Anti-human CD28 Alexa-Fluor 700 was purchased from BioLegend (San Diego, CA). Anti-mouse CD3 PE-Cy7, CD8 PerCP, and CD44 APC were purchased from BD Biosciences. Sytox blue was used for live-cell discrimination (Life Technologies).
T-cell xenografts
T cells from 1 DLBCL patient apheresis sample (38.7% CD27−CD28−) were expanded over the course of 14 days as described above. On the final day of expansion, the cells were thoroughly washed, counted, and resuspended in sterile PBS. 3 × 106 cells were injected intravenously into NSG mice via the lateral tail vein. 14 days following adoptive transfer, blood was collected and the frequencies, absolute numbers, and phenotypes of persisting human T cells were determined by flow cytometry using CD45 APC and CD3 PE-CF594 (BD Biosciences).
Tumor challenge and adoptive transfer
E.G7 ovalbumin (OVA) tumor cells were purchased from the American Tissue Type Culture Collection and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 4 mg/mL G418, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol. To establish tumors, 5 × 105 E.G7 OVA cells were injected in sterile PBS subcutaneously into the right flank of C57Bl/6 SJL (PepBoy) mice. Seven days later, 3-day-expanded C57Bl/6, OT-I, and OT-II T cells (2 × 106 C57Bl/6, 2 × 106 OT-I, and 1 × 106 OT-II) were injected IV with measurable tumors. Tumor growth was monitored using calipers. Tumor volume was calculated as (length × width2)/2. Mice were sacrificed once the tumors reached Institutional Animal Care and Use Committee guidelines.
Statistical analysis
Statistical analyses were performed using Prism version 5d for Mac (GraphPad, La Jolla, CA). Significant differences between 2 groups were determined using a 2-tailed Student t test. Statistical significance among 3 or more groups was determined using a 1-way or 2-way analysis of variance with a Tukey or Dunnet post-test. Significant survival differences were calculated in a pairwise fashion comparing each group to a control. Each experiment was repeated at least twice, and P < .05 was considered significant.
Results
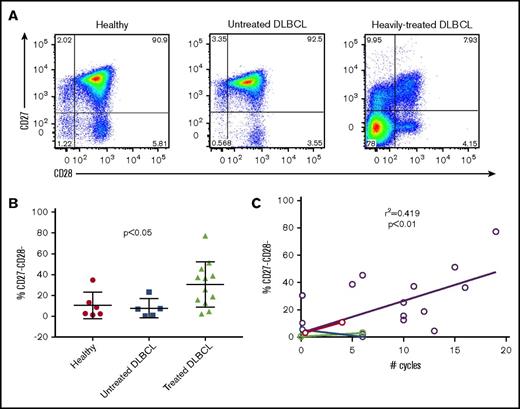
T cells from heavily treated DLBCL patients show loss of CD27 and CD28 expression and decreased ex vivo expansion in response to stimulation with anti-CD3/CD28 beads
Based on our institutional clinical experience that up to 1 out of 3 apheresis products collected from heavily pretreated DLBCL patients failed to adequately expand T cells during CAR T cell manufacturing, we hypothesized that the intensity and frequency of prior cytotoxicity chemotherapy would be associated with the phenotype and expansion properties of cultured T cells. We studied T cells from 6 normal volunteers, a cohort of 5 DLBCL patients identified at diagnosis, prior to cytotoxic chemotherapy, and 12 patients who had received prior chemotherapy, including patients relapsed after autologous stem cell transplantation (Tables 1 and 2). Phenotypic analysis of patients’ T cells by flow cytometry showed several phenotypic abnormalities from chemotherapy-treated DLBCL patients (Figure 1A). Most striking was the high frequency of CD27−CD28− T cells (median, 30.9%; range, 2.13-77.3), which was not observed to the same extent in either healthy controls (median, 5.6%; range, 1.22-34.7) or untreated DLBCL patients (median, 6.9%; range, 0.5-23.1) (Figure 1B). The frequency of CD27−CD28− T cells correlated significantly to the total number of chemotherapy cycles, with a significantly higher frequency in treated patients than healthy controls and newly diagnosed DLBCL patient samples (Figure 1C). T cells from DLBCL patients’ blood with a high frequency of CD27−CD28− T cells failed to adequately expand in cultures with anti-CD3/CD28 beads (Figure 2A). To determine whether the presence of CD27−CD28− (double-negative) T cells limited expansion of CD27+CD28+ T cells, we FACS-purified T cells from 4 cases of heavily pretreated lymphoma into 2 populations (CD27−CD28− or T cells expressing CD27 and/or CD28) and expanded them alongside unsorted cells for 14 days using anti-CD3/CD28 beads and low-dose IL-2 (Figure 2B). Depletion of CD27−CD28− cells led to a significant increase in viability at the end of the expansion period as well as a significant increase in expansion (Figure 2C-E). These results are consistent with the fratricide of naive T cells by T effector memory cells that has been recently described.5
Patient demographics and treatment information for treated DLBCL patients
| Patient . | Sex . | Age, y . | Total cycles of chemotherapy . | WBC, × 109/L . | ALC, × 109/L . | Absolute CD3, per μL . | Absolute CD4, per μL . | Absolute CD8, per μL . | Absolute CD19, per μL . | Absolute CD56, per μL . | Absolute CD16, per μL . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 33 | 12 | 6.1 | 1.281 | 343 | 99 | 233 | 0 | 206 | 214 |
| 2 | M | 61 | 19 | 11.6 | 1.856 | 1013 | 440 | 512 | 156 | 219 | 256 |
| 3 | M | 64 | 7 | 6.5 | .455 | 259 | 110 | 149 | 0 | 89 | 92 |
| 4 | F | 52 | 5 | 5.4 | 1.728 | 1030 | 366 | 355 | 326 | 124 | 214 |
| 5 | F | 64 | 11 | 3.4 | 1.650 | 1576 | 423 | 1091 | 0 | 40 | 55 |
| 6 | F | 33 | 21 | 2.7 | .648 | 706 | 70 | 636 | 0 | 94 | 118 |
| 7 | M | 41 | 12 | 3.1 | .690 | 202 | 112 | 87 | 0 | 147 | 133 |
| 8 | F | 38 | 12 | 5 | 1.100 | 792 | 261 | 496 | 0 | 176 | 211 |
| 9 | M | 36 | 10 | 5.4 | 2.240 | 405 | 257 | 139 | N/A | N/A | N/A |
| 10 | F | 47 | 6 | 5.8 | 0.600 | 430 | 246 | 139 | N/A | N/A | N/A |
| 11 | F | 63 | 6 | 5.8 | 1.140 | 829 | 342 | 464 | N/A | N/A | N/A |
| 12 | M | 56 | 15 | 2.5 | 0.540 | 198 | 93 | 94 | N/A | N/A | N/A |
| 13* | M | 55 | 6 | 5.6 | 0.980 | 851 | 719 | 87 | N/A | N/A | N/A |
| 14* | F | 52 | 4 | 5.9 | 1.050 | 915 | 573 | 197 | N/A | N/A | N/A |
| 15* | M | 71 | 6 | 3.9 | 0.772 | 585 | 472 | 99 | N/A | N/A | N/A |
| 46.5 | 10 | 5.4 | 1.050 | 706 | 261 | 197 | 0 | 124 | 133 | ||
| Patient . | Sex . | Age, y . | Total cycles of chemotherapy . | WBC, × 109/L . | ALC, × 109/L . | Absolute CD3, per μL . | Absolute CD4, per μL . | Absolute CD8, per μL . | Absolute CD19, per μL . | Absolute CD56, per μL . | Absolute CD16, per μL . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 33 | 12 | 6.1 | 1.281 | 343 | 99 | 233 | 0 | 206 | 214 |
| 2 | M | 61 | 19 | 11.6 | 1.856 | 1013 | 440 | 512 | 156 | 219 | 256 |
| 3 | M | 64 | 7 | 6.5 | .455 | 259 | 110 | 149 | 0 | 89 | 92 |
| 4 | F | 52 | 5 | 5.4 | 1.728 | 1030 | 366 | 355 | 326 | 124 | 214 |
| 5 | F | 64 | 11 | 3.4 | 1.650 | 1576 | 423 | 1091 | 0 | 40 | 55 |
| 6 | F | 33 | 21 | 2.7 | .648 | 706 | 70 | 636 | 0 | 94 | 118 |
| 7 | M | 41 | 12 | 3.1 | .690 | 202 | 112 | 87 | 0 | 147 | 133 |
| 8 | F | 38 | 12 | 5 | 1.100 | 792 | 261 | 496 | 0 | 176 | 211 |
| 9 | M | 36 | 10 | 5.4 | 2.240 | 405 | 257 | 139 | N/A | N/A | N/A |
| 10 | F | 47 | 6 | 5.8 | 0.600 | 430 | 246 | 139 | N/A | N/A | N/A |
| 11 | F | 63 | 6 | 5.8 | 1.140 | 829 | 342 | 464 | N/A | N/A | N/A |
| 12 | M | 56 | 15 | 2.5 | 0.540 | 198 | 93 | 94 | N/A | N/A | N/A |
| 13* | M | 55 | 6 | 5.6 | 0.980 | 851 | 719 | 87 | N/A | N/A | N/A |
| 14* | F | 52 | 4 | 5.9 | 1.050 | 915 | 573 | 197 | N/A | N/A | N/A |
| 15* | M | 71 | 6 | 3.9 | 0.772 | 585 | 472 | 99 | N/A | N/A | N/A |
| 46.5 | 10 | 5.4 | 1.050 | 706 | 261 | 197 | 0 | 124 | 133 | ||
The bottom row shows median values. All procedures were approved by the Institutional Review Board of Emory University (approval IRB0716).
Paired samples pre- and posttreatment.
ALC, absolute lymphocyte count; F, female; M, male; N/A, not available; WBC, white blood cell count.
Patient demographics and treatment information for untreated DLBCL patients
| Patient . | Sex . | Age, y . | Total cycles of chemotherapy . | WBC, × 109/L . | ALC, × 109/L . | Absolute CD3, per μL . | Absolute CD4, per μL . | Absolute CD8, per μL . | Absolute CD19, per μL . | Absolute CD56, per μL . | Absolute CD16, per μL . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | M | 55 | 0 | 8.1 | 2.660 | 1085 | 933 | 107 | N/A | N/A | N/A |
| 2* | F | 51 | 0 | 8.8 | 2.675 | 2517 | 1734 | 604 | N/A | N/A | N/A |
| 3 | F | 75 | 0 | 8.5 | 1.173 | 652 | 538 | 91 | N/A | N/A | N/A |
| 4* | M | 70 | 0 | 15.1 | 6.886 | 4968 | 4645 | 247 | N/A | N/A | N/A |
| 5 | F | 67 | 0 | 8.5 | 3.332 | 2465 | 1038 | 789 | N/A | N/A | N/A |
| 67 | 0 | 8.5 | 2.675 | 2465 | 1038 | 247 | N/A | N/A | N/A | ||
| Patient . | Sex . | Age, y . | Total cycles of chemotherapy . | WBC, × 109/L . | ALC, × 109/L . | Absolute CD3, per μL . | Absolute CD4, per μL . | Absolute CD8, per μL . | Absolute CD19, per μL . | Absolute CD56, per μL . | Absolute CD16, per μL . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | M | 55 | 0 | 8.1 | 2.660 | 1085 | 933 | 107 | N/A | N/A | N/A |
| 2* | F | 51 | 0 | 8.8 | 2.675 | 2517 | 1734 | 604 | N/A | N/A | N/A |
| 3 | F | 75 | 0 | 8.5 | 1.173 | 652 | 538 | 91 | N/A | N/A | N/A |
| 4* | M | 70 | 0 | 15.1 | 6.886 | 4968 | 4645 | 247 | N/A | N/A | N/A |
| 5 | F | 67 | 0 | 8.5 | 3.332 | 2465 | 1038 | 789 | N/A | N/A | N/A |
| 67 | 0 | 8.5 | 2.675 | 2465 | 1038 | 247 | N/A | N/A | N/A | ||
The bottom row shows median values. All procedures were approved by the Institutional Review Board of Emory University (approval IRB0716).
Paired samples pre- and posttreatment.
T cells from DLBCL patients who have received multiple rounds of chemotherapy show loss of CD27 and CD28 expression. Peripheral blood samples from healthy controls, untreated DLBCL patients, and heavily-treated DLBCL patients were examined for the expression of CD27 and CD28 by flow cytometry. (A) Representative flow plots of CD27 and CD28 expression gated on CD3+ cells. (B) Quantification of CD27−CD28− cells from healthy controls (n = 6), untreated DLBCL patients (n = 5), and treated DLBCL patients (n = 12). Horizontal bar shows mean values; vertical “whiskers” show 95% confidence interval. (C) Linear regression showing the correlation between number of total chemotherapy cycles and frequency of CD27−CD28− cells in DLBCL patient samples. Colored symbols and connecting lines indicate paired blood samples taken from DLBCL prior to and following cytotoxic chemotherapy.
T cells from DLBCL patients who have received multiple rounds of chemotherapy show loss of CD27 and CD28 expression. Peripheral blood samples from healthy controls, untreated DLBCL patients, and heavily-treated DLBCL patients were examined for the expression of CD27 and CD28 by flow cytometry. (A) Representative flow plots of CD27 and CD28 expression gated on CD3+ cells. (B) Quantification of CD27−CD28− cells from healthy controls (n = 6), untreated DLBCL patients (n = 5), and treated DLBCL patients (n = 12). Horizontal bar shows mean values; vertical “whiskers” show 95% confidence interval. (C) Linear regression showing the correlation between number of total chemotherapy cycles and frequency of CD27−CD28− cells in DLBCL patient samples. Colored symbols and connecting lines indicate paired blood samples taken from DLBCL prior to and following cytotoxic chemotherapy.
Depletion of CD27−CD28−cells improves the expansion of T cells from heavily pretreated DLBCL patients. PBMCs from pretreated DLBCL patients were rested overnight followed by FACS sorting to separate CD27−CD28− cells from the remaining populations. Cells were then stimulated with anti-CD3/CD28 beads with IL-2 for 14 days. (A) Comparison of normal T-cell expansion from healthy donors, untreated DLBCL patients, and treated DLBCL patients. (B) Flow plots showing the sorting strategy and postsort purity. (C) T-cell viability at the end of the expansion period as assessed by Sytox blue staining. (D) Quantitation of cell viabilities for the indicated populations on day 14 of expansion (n = 4 DLBCL patient donors). (E) Cell counts of cultures consisting of the indicated T-cell populations from 4 patient sorts. **P < .01, ****P < .001.
Depletion of CD27−CD28−cells improves the expansion of T cells from heavily pretreated DLBCL patients. PBMCs from pretreated DLBCL patients were rested overnight followed by FACS sorting to separate CD27−CD28− cells from the remaining populations. Cells were then stimulated with anti-CD3/CD28 beads with IL-2 for 14 days. (A) Comparison of normal T-cell expansion from healthy donors, untreated DLBCL patients, and treated DLBCL patients. (B) Flow plots showing the sorting strategy and postsort purity. (C) T-cell viability at the end of the expansion period as assessed by Sytox blue staining. (D) Quantitation of cell viabilities for the indicated populations on day 14 of expansion (n = 4 DLBCL patient donors). (E) Cell counts of cultures consisting of the indicated T-cell populations from 4 patient sorts. **P < .01, ****P < .001.
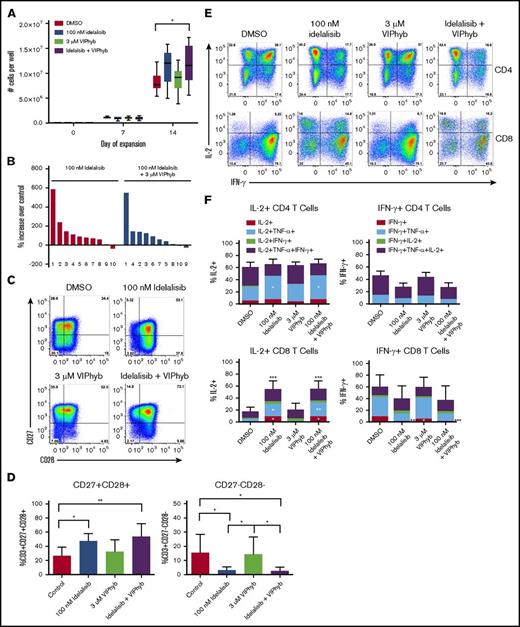
Idelalisib and VIPhyb significantly improved numbers of viable T cells and delayed terminal differentiation during ex vivo T-cell expansion
We next tested the effects of idelalisib and VIPhyb on the ability to expand T cells from DLBCL patients and healthy volunteers. T cells were expanded for 10 or 14 days with CD3/CD28 beads and low-dose IL-2 in the presence or absence of idelalisib and/or VIPhyb. T-cell expansion from healthy volunteers was significantly increased with the addition of idelalisib and VIPhyb (Figure 3A). Additionally, the inclusion of idelalisib significantly increased T-cell yield in cultures from lymphoma patients (Figure 3B). In order to account for the increased T-cell yields, we examined proliferation of CFSE-labeled murine T cells expanded for 3 days in the presence of idelalisib. There was no significant difference in dye dilution despite significant increases in yield in this system (supplemental Figure 1A-C).
Pharmacological blockade of PI3K δ and VIP signaling results in increased cell yield, preservation of CD27+CD28+cells and enhanced IL-2 production during ex vivo T-cell expansion. T cells from healthy donors and DLBCL patients were expanded in vitro in the presence or absence of idelalisib and/or VIPhyb for 14 days. Cells were counted on days 7 and 14 of expansion and phenotyped by flow cytometry for surface markers or cytokine production on day 14. (A) Expansion profiles of healthy controls under the indicated culture conditions. (B) Waterfall plot showing the expansion of DLBCL patient samples relative to DMSO controls. The numbers below the bars indicate specific patient samples. (C) Representative flow plots showing the expression of CD27 and CD28 on the total T-cell population. (D) Quantification of double-positive and double-negative T cells from 10 lymphoma patients. (E) Representative flow plots of IL-2 and IFN-γ production by human CD4 and CD8 T cells after 14 days of expansion under the indicated culture conditions. (F) Quantitation of total frequencies of cells producing the indicated cytokines as well as single, double, and triple cytokine-producing T cells (n = 6 healthy donors). *P < .05, **P < .01, ***P < .001. TNF, tumor necrosis factor.
Pharmacological blockade of PI3K δ and VIP signaling results in increased cell yield, preservation of CD27+CD28+cells and enhanced IL-2 production during ex vivo T-cell expansion. T cells from healthy donors and DLBCL patients were expanded in vitro in the presence or absence of idelalisib and/or VIPhyb for 14 days. Cells were counted on days 7 and 14 of expansion and phenotyped by flow cytometry for surface markers or cytokine production on day 14. (A) Expansion profiles of healthy controls under the indicated culture conditions. (B) Waterfall plot showing the expansion of DLBCL patient samples relative to DMSO controls. The numbers below the bars indicate specific patient samples. (C) Representative flow plots showing the expression of CD27 and CD28 on the total T-cell population. (D) Quantification of double-positive and double-negative T cells from 10 lymphoma patients. (E) Representative flow plots of IL-2 and IFN-γ production by human CD4 and CD8 T cells after 14 days of expansion under the indicated culture conditions. (F) Quantitation of total frequencies of cells producing the indicated cytokines as well as single, double, and triple cytokine-producing T cells (n = 6 healthy donors). *P < .05, **P < .01, ***P < .001. TNF, tumor necrosis factor.
Cell yields from cultures containing a combination of idelalisib and VIPhyb did not differ significantly from cultures containing idelalisib alone (Figure 3B), but the addition of idelalisib or a combination of idelalisib and VIPhyb resulted in significantly increased frequencies of CD27+CD28+ T cells and reduced the proportion of CD27−CD28− T cells (Figure 3C-D). Similar results were obtained with the addition of the dual PI3Kδ/γ inhibitor duvelisib (supplemental Figure 5). Expression of PD-1 on T cells expanded in the presence of idelalisib or a combination of idelalisib and VIPhyb was lower after 10 days of culture, whereas the addition of VIPhyb alone resulted in lower expression of PD-1 during short-term expansion (supplemental Figure 2). We next examined the functional capacity of T cells expanded in the presence of idelalisib and VIPhyb by performing intracellular cytokine staining at the end of the expansion period. Expansion of T cells in the presence of idelalisib alone or in combination with VIPhyb resulted in significantly more IL-2+ CD8 T cells with a trend toward reduced frequencies of IFN-γ-positive cells (Figure 3E-F). Additionally, IL-2–producing T cells expanded in the presence of idelalisib or idelalisib and VIPhyb had significantly increased frequencies of dual-cytokine–producing CD8 T cells (Figure 3F).
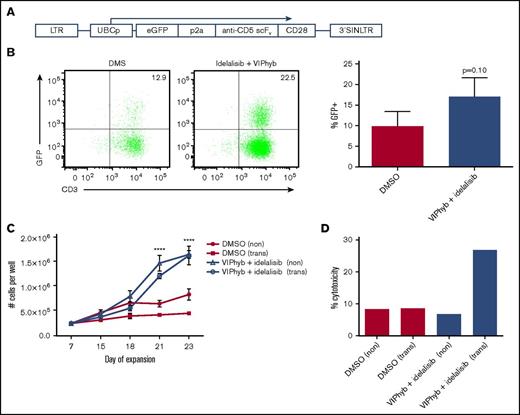
Addition of VIPhyb and idelalisib to T-cell cultures significantly enhanced expansion and transduction of human anti-CD5 CAR T cells and their cytotoxicity against CD5+ lymphoma
To determine whether the effects of idelalisib and VIPhyb would translate to T-cell expansion and CAR T cell viral transduction, we expanded T cells from 3 healthy donors in the presence of DMSO or a combination of idelalisib and VIPhyb and transduced them with a construct encoding an anti-CD5 CAR (Figure 4A). Transduction of T cells expanded in the presence of idelalisib and VIPhyb resulted in an increased (but not statistically significant) transduction efficiency, as evidenced by increased frequencies of GFP-positive T cells (Figure 4B). In addition, long-term expansion of virally transduced T cells was significantly enhanced in cultures containing idelalisib and VIPhyb (Figure 4C). Interestingly, the reduced proliferation of transduced T cells observed in control cultures was not observed at the end of the expansion period in cultures containing idelalisib and VIPhyb (Figure 4C). To determine the functional capacity of the generated CAR T cells, the cells were cocultured with CD5+ Jurkat cells as targets, and cytotoxicity was assessed as described in “Methods.” Figure 4D shows a representative example of cytotoxicity demonstrating enhanced cytotoxic activity of CAR T cells expanded in the presence of idelalisib and VIPhyb. Taken together, inclusion of idelalisib and VIPhyb to expansion cultures enhanced the transduction efficiency, yield, and function of CD5 CAR T cells.
Idelalisib and VIPhyb enhance the expansion and functionality of CAR T cells in vitro. CD5 CAR T cells were produced from healthy donors (n = 3) in the presence or absence of idelalisib and VIPhyb and evaluated for growth, transduction efficiency, and in vitro cytotoxicity. (A) CD5 CAR vector design. (B) Transduction efficiency as assessed by GFP expression. (C) Expansion profiles of CD5 CAR T cells under the indicated culture conditions. (D) In vitro cytotoxicity of CD5 CAR T cells generated from healthy controls and expanded under the indicated conditions. eGFP, enhanced green protein; LTR, long terminal repeat; SIN, self-inactivating.
Idelalisib and VIPhyb enhance the expansion and functionality of CAR T cells in vitro. CD5 CAR T cells were produced from healthy donors (n = 3) in the presence or absence of idelalisib and VIPhyb and evaluated for growth, transduction efficiency, and in vitro cytotoxicity. (A) CD5 CAR vector design. (B) Transduction efficiency as assessed by GFP expression. (C) Expansion profiles of CD5 CAR T cells under the indicated culture conditions. (D) In vitro cytotoxicity of CD5 CAR T cells generated from healthy controls and expanded under the indicated conditions. eGFP, enhanced green protein; LTR, long terminal repeat; SIN, self-inactivating.
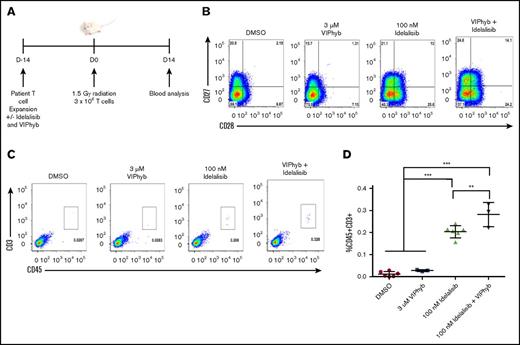
DLBCL patient T cells expanded in the presence of idelalisib and VIPhyb had enhanced persistence in vivo in immune-deficient mice
To determine whether the preservation of less terminally differentiated T cells in cultures expanded in the presence of idelalisib and VIPhyb had an effect on T-cell persistence in vivo, we expanded T cells from a DLBCL patient and injected them into irradiated NSG mice. Blood was collected 14 days postinjection and analyzed for the presence of human CD45+CD3+ cells by flow cytometry (Figure 5A). Prior to injection, the expression of CD27 and CD28 was highest on T cells expanded in the presence of idelalisib and VIPhyb (Figure 5B). Addition of VIPhyb alone did not have a significant effect on T-cell recovery, whereas the addition of idelalisib significantly increased the frequency of human T cells in mouse peripheral blood (Figure 5C-D). Interestingly, human T cells were higher in mice that received T cells expanded in both idelalisib and VIPhyb than in mice injected with T cells expanded in the presence of idelalisib alone (Figure 5C-D).
Expansion of DLBCL patient T cells in the presence of idelalisib and VIPhyb significantly enhances their in vivo persistence following adoptive transfer to NSG mice. T cells from a heavily treated DLBCL patient were expanded in the presence or absence of idelalisib and/or VIPhyb for 14 days followed by transfer to irradiated NSG mice. Blood was analyzed 14 days posttransfer for the presence of human CD45+CD3+ cells. (A) Experimental outline. (B) Flow plots showing the phenotype of T cells on day 14 of expansion just prior to adoptive transfer. (C) Representative flow plots from the blood of NSG mice showing the frequency of human T cells. (D) Quantification of T-cell frequencies in NSG mice 14 days after adoptive transfer from 1 of 3 independent experiments. **P < .01; ***P < .001.
Expansion of DLBCL patient T cells in the presence of idelalisib and VIPhyb significantly enhances their in vivo persistence following adoptive transfer to NSG mice. T cells from a heavily treated DLBCL patient were expanded in the presence or absence of idelalisib and/or VIPhyb for 14 days followed by transfer to irradiated NSG mice. Blood was analyzed 14 days posttransfer for the presence of human CD45+CD3+ cells. (A) Experimental outline. (B) Flow plots showing the phenotype of T cells on day 14 of expansion just prior to adoptive transfer. (C) Representative flow plots from the blood of NSG mice showing the frequency of human T cells. (D) Quantification of T-cell frequencies in NSG mice 14 days after adoptive transfer from 1 of 3 independent experiments. **P < .01; ***P < .001.
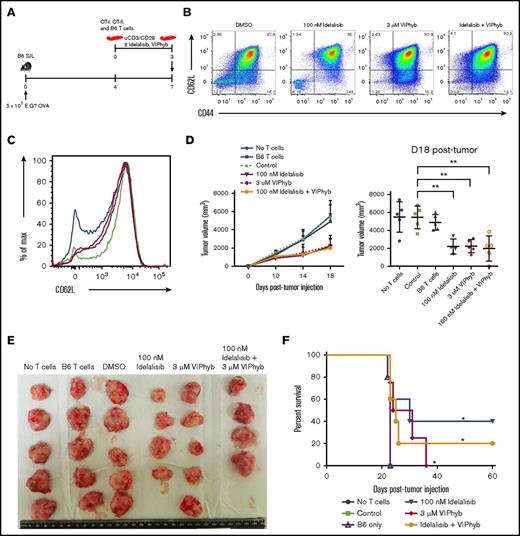
Addition of either VIPhyb or idelalisib to T-cell expansion cultures significantly enhanced antigen-specific antitumor activity in a murine lymphoma model
Having established an effect on polyfunctional cytokine expression and in vivo persistence of T cells expanded in the presence of idelalisib and/or VIPhyb, we next determined the effect of these culture conditions on antitumor activity in vivo. CD45 congenic B6 SJL mice were subcutaneously injected with OVA-expressing E.G7 lymphoma cells in the right flank. Tumors were allowed to grow for 7 days, during which time a mixture of ex vivo–expanded OT-I (CD8), OT-II (CD4), and nonspecific B6 T cells (in a 2:1:2 ratio; 5 × 106 total T cells) were injected IV (Figure 6A). A mixture of antigen-specific transgenic CD8, CD4, and wild-type (nonspecific) T cells was used in these experiments to mimic what is seen clinically using genetically modified T cells that express a tumor-specific antigen receptor following lentivirus transduction in which a portion of the T cells are antigen specific and a portion are polyclonal. Mice receiving no T cells or only cultured wild-type B6 T cells were used as negative controls. T cells were expanded for 3 days in the presence of DMSO, idelalisib, VIPhyb, or a combination of idelalisib and VIPhyb prior to injection. The phenotype of the cells just prior to injection is shown in Figure 6B-C. Measured tumor growth was slower in mice receiving T cells expanded in the presence of idelalisib, VIPhyb, or a combination of both (Figure 6D), and tumors removed at necropsy were smaller (Figure 6E). Mice that received T cells expanded in idelalisib, VIPhyb, or a combination of both survived significantly longer than mice that received no T cells, B6 T cells alone, or control mixtures of OT-I, OT-II, and B6 cells expanded in DMSO (Figure 6F). In summary, addition of VIPhyb and idelalisib either alone or in combination to T-cell expansion cultures significantly enhanced the antitumor response following adoptive transfer to lymphoma-bearing mice.
Expansion of T cells in the presence of idelalisib and/or VIPhyb significantly enhances their anti-tumor activity in a murine lymphoma model. OT-I, OT-II, and B6 T cells were expanded for 3 days using anti-CD3/CD28 beads in the presence or absence of idelalisib and/or VIPhyb. Cells were then mixed (2 OT-I:1 OT-II:2 B6 ratio) and injected into B6 SJL mice bearing subcutaneous E.G7 OVA tumors. Growth of tumors was monitored by caliper measurement with volume calculated as (length × width2)/2. (A) Experimental outline. (B) Flow plots showing the phenotype of T cells on day 3 of expansion just prior to injection into tumor-bearing mice. (C) Histogram showing the expression of CD62L on T cells on day 3 of expansion. Blue, DMSO; green, 100 nM idelalisib; purple, 3 μM VIPhyb; red, Idelalisib + VIPhyb. (D) Tumor growth curve and quantification of tumor volume on the final day of allowable growth. (E) Images of tumors removed from sacrificed mice on day 18 of growth. Note that the tumor from 1 mouse from the idelalisib + VIPhyb group regressed and was undetectable. (F) Survival curve of tumor-bearing mice receiving T cells expanded under the indicated conditions. **P < .01; n = 4 or 5 mice per group from 2 independent experiments.
Expansion of T cells in the presence of idelalisib and/or VIPhyb significantly enhances their anti-tumor activity in a murine lymphoma model. OT-I, OT-II, and B6 T cells were expanded for 3 days using anti-CD3/CD28 beads in the presence or absence of idelalisib and/or VIPhyb. Cells were then mixed (2 OT-I:1 OT-II:2 B6 ratio) and injected into B6 SJL mice bearing subcutaneous E.G7 OVA tumors. Growth of tumors was monitored by caliper measurement with volume calculated as (length × width2)/2. (A) Experimental outline. (B) Flow plots showing the phenotype of T cells on day 3 of expansion just prior to injection into tumor-bearing mice. (C) Histogram showing the expression of CD62L on T cells on day 3 of expansion. Blue, DMSO; green, 100 nM idelalisib; purple, 3 μM VIPhyb; red, Idelalisib + VIPhyb. (D) Tumor growth curve and quantification of tumor volume on the final day of allowable growth. (E) Images of tumors removed from sacrificed mice on day 18 of growth. Note that the tumor from 1 mouse from the idelalisib + VIPhyb group regressed and was undetectable. (F) Survival curve of tumor-bearing mice receiving T cells expanded under the indicated conditions. **P < .01; n = 4 or 5 mice per group from 2 independent experiments.
Discussion
Patients with relapsed refractory cancer have been exposed to multiple rounds of cytotoxic combination chemotherapy that potently deplete T cells, especially when administered at high doses.23-25 Patients with highly chemorefractory disease, including those patients relapsed after high-dose alkylator-based conditioning regimens given prior to autologous stem cell transplant, are the initial patient population for whom novel T-cell therapies are currently being explored.6,26 We found that patients with relapsed/refractory DLBCL have depressed T-cell counts and phenotypic abnormalities in T cells that may result in insufficient yield or poor quality products during CAR T cell manufacturing.27,28 Patients with non-Hodgkin lymphoma have an overabundance of memory T cells when compared with patients with other malignancies, presumably due to prior exposure to alkylator or alkylator-like chemotherapies.5 These memory populations interfere with expansion of naive T cells through FasL-mediated fratricide, thereby limiting the efficacy of therapies that use autologous T cells.5 Loss of CD27 and CD28 has also been observed in patients as they age and is considered to be a marker for senescence.29,30 We found that the frequency of CD27/CD28 double-negative T cells in untreated DLBCL patients was low and similar to frequencies seen in a younger cohort of healthy controls. The frequency of CD27/CD28 double-negative T cells was increased significantly in previously treated DLBCL and was proportional to the number of prior chemotherapy cycles, consistent with chemotherapy-induced damage to T cells (Figure 1A-C). We found that T cells lacking both CD27 and CD28 costimulatory domains appear be immunosuppressive, as their removal improved ex vivo expansion of T cells from heavily pretreated lymphoma patients (Figure 2E). The loss of CD28 from T cells is particularly relevant to novel therapies employing autologous T cells genetically modified to express a tumor-specific antigen receptor, as many of the currently used manufacturing methods use anti-CD3/CD28 bead-mediated stimulation,31 and signaling through CD3 in the absence of a costimulatory signal such as CD28 leads to anergy and an inability to proliferate.20,32
Although the lymphoid-lineage–specific PI3Kδ inhibitor idelalisib is currently used as a therapeutic to treat CLL, we reasoned that it could be used to augment T-cell numbers and function during their ex vivo expansion. The clinical activity of PI3Kδ inhibitors in B-cell malignancies is believed to be via blocking downstream survival and differentiation signals in malignant B cells.33-35 In addition to B cells, the δ isoform of PI3K is also expressed in T cells and natural killer cells.36 PI3Kδ signaling has been shown to be crucial for CD8 T-cell–mediated antilisteria responses and also in the function of regulatory T cells in murine solid tumor models.37,38 However, the differentiation signals downstream of PI3Kδ, including AKT, make it an attractive target to modulate during anti-CD3/CD28 bead stimulation, as strong TCR stimulation leads to exhaustion and terminal differentiation of T cells.39 Naive and central memory T cells are the most effective in T-cell–based therapies, and partially blocking terminal differentiation during CD3/C28 bead-mediated expansion is thus an attractive strategy to improve the quality of T cells used for adoptive cancer therapy.5 Addition of idelalisib to T-cell expansion cultures resulted in significantly increased frequencies of CD27 and CD28 coexpressing cells, likely due to a partial blockade of activation signals through the TCR that are mediated by PI3K.40 A similar block or delay in differentiation was observed in p110δ knockout murine T cells stimulated with anti-CD3 antibodies in vitro.37 One caveat to this culture strategy is the potential to expand autoreactive cells, as autoimmune pathology has been observed in CLL patients taking idelalisib.15-17 Whether addition of idelalisib to T-cell expansion cultures preferentially expands autoreactive cells will need to be determined prior to employment of this culture strategy in a clinical setting.
Strong in vitro stimulation of the TCR leads to activation-induced cell death (AICD), the extent of which can be reduced by the anti-apoptotic protein Bcl-2.41 PI3K δ inhibition resulted in increased Bcl-2 levels (data not shown) suggesting a reduction in AICD. Higher frequencies of less terminally differentiated cells suggest that addition of idelalisib helped to partially block terminal differentiation. Increased signaling through CD28 cannot account for the increased levels of Bcl-2, as CD28 signaling was shown not to have an effect on protein levels in T cells18 ; thus, the mechanisms underlying increased Bcl-2 expression need further exploration. Increased cell viability helps explain increased yield, as there was no significant difference in CFSE dilution in stimulated murine T cells. However, the increased production of IL-2 in restimulated CD8 T cells expanded in idelalisib and VIPhyb could also account for increased yield and explain the lack of significant differences prior to restimulation at day 7. We observed similar yield increases during the expansion of CD5 CAR T cells over longer periods of expansion, which may be partly accounted for by a reduction in fratricide, as T cells dynamically express CD5, the target for the expressed CAR. As such, the culture strategy employed in this study may yield different results depending on the CAR construct used. Taken together, blockade of PI3Kδ likely reduced TCR signal strength, reducing terminal differentiation and AICD as well as increased IL-2 production.
We found that daily addition of a VIP antagonist during ex vivo expansion further augmented the proportion of T cells coexpressing CD27 and CD28 and enhanced their ability to persist in vivo following adoptive transfer to NSG mice. VIP is a neuropeptide that signals through a cyclic adenosine 5′-monophosphate/cyclic adenosine 5′-monophosphate–dependent protein kinase pathway and has a large variety of anti-inflammatory effects.42,43 Among the observed effects of VIP on T cells is a reduction in proinflammatory cytokine secretion, reduced proliferation, reduced cytotoxicity, and regulatory T-cell generation, making the pathway an attractive target for T-cell–based therapies.44-48 T cells expanded in VIPhyb alone had significantly increased antitumor activity, consistent with prior reports that VIPhyb enhances both autologous antitumor T-cell responses and graft-versus-leukemia responses in murine leukemia models.13,14 Utilizing an alternative approach to block VIP signaling through cleavage of endogenously produced VIP peptide with mast cell chymase gave similar results (supplemental Figure 6).49 One mechanism by which VIP exerts its anti-inflammatory effect is through phosphorylation and activation of CREB, reducing downstream nuclear factor κB signaling.14,50 VIP antagonists reduce CREB signaling, relieving pCREB-mediated inhibition of the nuclear factor κB pathway and enhancing survival of T cells activated in vitro.51 Therefore, we postulate that idelalisib added during ex vivo T cell expansion partially blocks terminal differentiation of T cells, whereas the addition of VIPhyb increases the in vivo persistence and cytotoxicity of expanded T cells. In support of this notion is the enhanced antitumor responses observed following adoptive transfer of expanded TCR transgenic T cells to OVA-expressing tumor-bearing mice. Although this model is of little relevance to CAR T therapy, it is highly relevant to alternative approaches to tumor-specific T-cell therapies, including T cells genetically modified with clonal expression of T-cell receptors. The in vivo antitumor activity of CAR T cells expanded in the presence of idelalisib and VIPhyb against a variety of B-cell lymphomas is currently being evaluated by our group using a PDX model.
In summary, we show herein that the combination of idelalisib and VIPhyb significantly increases the quantity and quality of ex vivo–expanded T cells. Antagonism of these 2 pathways may be an attractive strategy to expand polyfunctional T cells used to treat patients with cancer.
Acknowledgments
This study was supported by the Coulter Foundation (project 60934), the Katz Foundation (Principal Investigator, E.K.W.), and the National Institutes of Health, National Institute of General Medical Sciences (MARC program T34GM105550).
Authorship
Contribution: C.T.P. designed and performed research, analyzed data, and wrote the manuscript; M.H. performed research and analyzed data; A.B.M. designed and performed research and analyzed data; J.J. performed research and analyzed data; K.L. analyzed data; N.J. analyzed data; A.D.S. analyzed data and helped obtain samples; S.S.R. designed and performed research and analyzed data; H.T.S. designed research; T.S. helped fund the study and reviewed the data; C.R.F. helped obtain samples and edited the manuscript; and E.K.W. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edmund K. Waller, Winship Cancer Institute, Emory University School of Medicine, 1365B Clifton Rd NE, Room B519, Atlanta, GA 30322; e-mail: ewaller@emory.edu.