Key Points
Allogeneic HCT can result in long-term survival for patients with sAML and prior MDS/MPN.
Myeloablative conditioning regimens should be selected for sAML and patients with prior MDS/MPN whenever possible.
Abstract
Patients with secondary AML (sAML) with antecedent myelodysplastic syndrome (MDS) or myeloproliferative neoplasms (MPNs) tend to have high-risk disease based on the older age of patients, high-risk cytogenetics, and higher number of prior treatments. Allogeneic hematopoietic cell transplant (HCT) is the only potentially curative therapy available. Eight hundred and two adults with sAML and prior MDS/MPN who received a first HCT between 2000 and 2016 were included in the European Society for Blood and Marrow Transplant (EBMT) Acute Leukemia Working Party (ALWP) study. Median age of the cohort was 59.6 years (range, 18.6-78.6 years). Myeloablative conditioning (MAC) was given to 40% of patients, and 60% received reduced-intensity conditioning (RIC). Overall, the 2-year cumulative incidence of relapse (RI) was 37%, leukemia-free survival (LFS) was 40%, overall survival (OS) was 46%, nonrelapse mortality (NRM) was 23%, and chronic graft-versus-host disease (cGVHD) was 39%. In univariate analysis, a statistical difference between conditioning regimens 6 months after HCT in favor of the MAC group was noted with regard to RI (hazard ratio [HR], 1.47; P = .03), LFS (HR, 1.43; P = .01), and OS (HR, 1.55; P < .05). There was no difference in the cumulative incidence of NRM (HR, 1.38; P = .15). This effect was similarly seen in multivariate analysis (MVA): cumulative incidence of relapse (HR, 1.79; P < .05), LFS (HR, 1.43; P = .02), and OS (HR, 1.53; P = .005) with no difference in NRM (HR, 1; P = .98). This EBMT ALWP analysis suggests that long-term survival can be achieved in patients with sAML with antecedent MDS/MPN and that MAC is a suitable conditioning regimen in patients with sAML.
Introduction
Acute myeloid leukemia (AML) remains one of the most lethal types of hematologic cancers in adults and remains challenging to treat. Patients with secondary AML (sAML) often have poorer outcomes compared with de novo AML. sAML has historically been defined as AML that arose from an antecedent myeloid neoplasm such as myelodysplastic syndrome (MDS) or a myeloproliferative neoplasm (MPN) or related to prior exposure to chemotherapy, radiation, or environmental toxins.1 Studies in the past have combined these 2 categories as sAML,2-5 and both categories have been shown to have inferior outcomes compared with de novo AML.6-9 However, they are similar in that sAML is also heterogeneous, and risk stratification is based on identified molecular and cytogenetic aberrations. Conversely, the effect of these aberrations is not as clearly defined as it is in patients with de novo AML. Our current understanding of sAML is that it is not so much the secondary nature of the disease that confers a significant effect on the prognosis and the natural history but, rather, the inherent genetic/molecular aberrations that have the most effect.
Data suggest that treated sAML arising from an antecedent hematologic disorder is particularly associated with being less responsive to current treatment strategies, and thus inferior outcomes in terms of overall survival (OS).10 Patients with adverse cytogenetics have particularly poor outcomes. Several studies have shown that incorporation of cytogenetic and molecular data in patients undergoing transplant into risk models can provide personalized prediction of outcomes after hematopoietic cell transplant (HCT). In particular, patients with RAS pathway and TP53 mutations, along with a complex karyotype, had poor outcomes even after transplant.11,12
In these high-risk groups, the optimal conditioning regimen remains unknown. High-intensity myeloablative regimens (MAC) tend to have significant toxicity and historically have been associated with increased nonrelapse mortality (NRM) compared with their reduced-intensity conditioning (RIC) counterparts, which rely more on the graft-versus-leukemia effect as opposed to the cytotoxic effect of MAC regimens. Over the years, increased use of RIC regimens that are associated with less toxicity have increased in usage to allow transplantation to be an option for older and less fit patients. In fact, the use of RIC preparative regimens now accounts for nearly 40% of all allogeneic HCT in the United States.13 Multiple retrospective studies have shown that in patients with MDS/AML who undergo transplantation after a RIC regimen, OS is similar to those who received MAC regimens. This is in large part because of the overall reduced NRM associated with RIC HCT.14-20 A recent phase 3 randomized trial comparing MAC with RIC for AML and MDS showed no statistical OS difference between the 2 conditioning regimens, but rather reduced NRM in the RIC groups. However, patients who underwent MAC did have improved relapse-free survival, suggesting that MAC should be the preferred conditioning regimen in patients with MDS/AML who are fit enough to tolerate it.21
In older and less fit patients in whom MAC is not an option, RIC regimens are effective for AML in first or second complete remission (CR1 or CR2). Although most available data are retrospective in nature and employ a variety of regimens, reported long-term survival rates at 5 years have been described to be approximately 40%.22-24 Similarly, a prospective phase 2 study by Cancer and Leukemia Group B showed that patients with a median age of 65 years had leukemia-free survival (LFS) and OS after 2 years of 42%, and that HCT was overall well tolerated, with superior outcomes when compared with nontransplant treatment.25
Although outcomes of patients with all subtypes of AML after MAC and RIC are well described, the outcomes of patients who are specifically transplanted with sAML are not. Thus, this study by the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplant (EBMT) network was undertaken.
Patients and methods
Study design and data collection
We used the EBMT registry to identify patients with a diagnosis of sAML who received HCT between 2000 and 2016 in this retrospective multicenter analysis. Data were provided by the ALWP of the EBMT registry. The EBMT registry is a voluntary working group of more than 500 transplant centers that are required to report all consecutive stem cell transplantations and follow-ups once a year. Audits are routinely performed to determine the accuracy of the data. Since 1990, patients have provided informed consent authorizing the use of their personal information for research purposes. The study was approved by the general assembly of the ALWP.
Eligibility criteria for this analysis included adult patients (ages >18 years) with a diagnosis of sAML and antecedent MDS or MPN who received either myeloablative or reduced-intensity conditioning as part of their matched sibling donor (MSD) or 9-10/10 HLA-matched unrelated donor (URD) transplant, availability of cytogenetic risk, and RIC/MAC information. MAC was defined as a conditioning regimen that contained either total body irradiation and/or alkylating agents at doses that would not allow autologous hematologic recovery, whereas RIC regimens were defined as regimens that could cause cytopenias, possibly prolonged, and that require stem cell support for hematologic recovery.26 Variables collected included recipient and donor characteristics (age, sex, cytomegalovirus serostatus), disease features, previous diagnosis of MDS/MPN, cytogenetics (favorable, intermediate, adverse), Karnofsky Performance Status at transplant, disease status at transplant (CR1 vs CR2/3 vs active disease), transplant-related factors including conditioning regimen (MAC or RIC), donor type (MSD, URD, degree of match [10/10, 9/10]), immunosuppression (in vivo T-cell depletion vs none), graft-versus-host-disease (GVHD) prophylaxis, and outcome variables (acute and chronic GVHD, relapse, NRM, LFS, OS, and causes of death). CR was defined as less than 5% bone marrow blasts at the time of transplantation.
Statistical analysis
The primary endpoint of the study was LFS. Secondary endpoints included OS, disease relapse incidence (RI), NRM, engraftment, and incidences and severity of acute and chronic GVHD (aGVHD, cGVHD). The starting point for time-to-event analysis was date of transplantation. OS was defined as the length of time the patients are still alive. LFS was defined as survival without relapse or progression; patients surviving without relapse were censored at time of last follow-up. RI was defined as time to onset of leukemic recurrence; NRM, defined as death without relapse or progression, was the competing risk. Surviving patients were censored at last contact.
Probabilities of OS and LFS were calculated using the Kaplan-Meier method. Cumulative incidence was used to estimate the endpoints of NRM, RI, aGVHD, and cGVHD to accommodate competing risks. To study aGVHD and cGVHD, we considered relapse and death to be competing events. Univariate analyses were performed using the Gray’s test for cumulative incidence functions and the log-rank test for OS and LFS. Multivariate analyses were conducted using Cox regression models; variables included were age, cytogenetic risk, year of HCT, donor to patient sex, HLA match, cytomegalovirus donor and patient, cell source, disease status at HCT, and in vivo T-cell depletion. Results were expressed as the hazard ratio (HR) with the 95% confidence interval (95% CI). Proportional hazards assumptions were checked systematically for all proposed models using the Grambsch-Therneau residual-based test. All tests were 2-sided. The type I error rate was fixed at 0.05 for the determination of factors associated with time-to-event outcomes. Statistical analyses were performed with R 3.4.1.27
Results
Patient, disease, and transplant characteristics
The details of patient, disease, and transplant characteristics are summarized in Table 1. A total of 802 patients with sAML who underwent HCT between 2000 and 2016 were included in this study. Of this total, 321 (40.0%) received MAC, whereas 481 (60.0%) received RIC preparative regimens. Median age at time of HCT was 59.6 years (range, 18.6-78.6 years) Median time from diagnosis to HCT was 4.47 months (interquartile ratio [IQR], 3.09-6.69 months).
Patient characteristics
| Variable . | Entire cohort (N = 802) . | MAC (n =321; 40%) . | RIC (n = 481; 60%) . | P . |
|---|---|---|---|---|
| Age at HCT, median, y | 59.58 (range, 18.58-78.64; IQR, 52.49-65.05) | 54.29 (range, 18.58-74.51; IQR, 45.48-61.14) | 61.98 (range, 19.96-78.64; IQR, 56.72-66.43) | <.0001 |
| Time to HCT, median, mo | 4.47 (range, 0.23-137.73; IQR, 3.09-6.69) | 4.37 (range, 0.26-108.83; IQR, 2.79-6.7) | 4.6 (range, 0.23-137.73; IQR, 3.19-6.67) | .2669 |
| Engraftment | 741 (95.2) | 297 (94.6) | 444 (95.7) | .5906 |
| Time to engraftment, median, d | 17 (range, 3-89; IQR, 14-20) | 17 (range, 3-89; IQR, 13.5-20) | 17 (range, 4-77; IQR, 14-20) | .8290 |
| Source of stem cells (peripheral blood) | 722 (90.1) | 274 (85.4) | 448 (93.3) | .0003 |
| Disease status | .1045 | |||
| Advanced | 364 (45.4) | 152 (47.4) | 212 (44.1) | |
| CR1 | 396 (49.4) | 147 (45.8) | 249 (51.8) | |
| CR1 | 42 (5.2) | 22 (6.9) | 20 (4.2) | |
| KPS* | .1088 | |||
| Poor (<80%) | 66 (9) | 20 (6.7) | 46 (10.5) | |
| Good (≥80%) | 671 (91) | 277 (93.3) | 394 (89.5) | |
| Donor HLA | .1358 | |||
| MSD | 340 (42.4) | 141 (43.9) | 199 (41.4) | |
| URD 10/10 | 370 (46.1) | 152 (47.4) | 218 (45.3) | |
| URD 9/10 | 92 (11.5) | 28 (8.7) | 64 (13.3) | |
| Donor to patient sex | .0251 | |||
| Female to male | 166 (20.9) | 53 (16.8) | 113 (23.6) | |
| Other | 628 (79.1) | 263 (83.2) | 365 (76.4) | |
| Cytogenetic risk | .0339 | |||
| Intermediate | 538 (67.1) | 201 (62.6) | 337 (70.1) | |
| Poor | 264 (32.9) | 120 (37.4) | 144 (29.9) | |
| T-cell depletion | <.0001 | |||
| No TCD | 263 (32.8) | 138 (43) | 125 (26) | |
| ATG/Campath | 539 (67.2) | 183 (57) | 356 (74) | |
| Use of TBI | .0008 | |||
| Yes | 624 (77.8) | 230 (71.7) | 394 (81.9) | |
| No | 178 (22.2) | 91 (28.3) | 87 (18.1) | |
| CMV status (patient) | .2589 | |||
| Negative | 248 (31.9) | 90 (29.4) | 158 (33.5) | |
| Positive | 529 (68.1) | 216 (70.6) | 313 (66.5) | |
| CMV status (donor) | .9626 | |||
| Negative | 383 (49.3) | 151 (49) | 232 (49.5) | |
| Positive | 394 (50.7) | 157 (51) | 237 (50.5) |
| Variable . | Entire cohort (N = 802) . | MAC (n =321; 40%) . | RIC (n = 481; 60%) . | P . |
|---|---|---|---|---|
| Age at HCT, median, y | 59.58 (range, 18.58-78.64; IQR, 52.49-65.05) | 54.29 (range, 18.58-74.51; IQR, 45.48-61.14) | 61.98 (range, 19.96-78.64; IQR, 56.72-66.43) | <.0001 |
| Time to HCT, median, mo | 4.47 (range, 0.23-137.73; IQR, 3.09-6.69) | 4.37 (range, 0.26-108.83; IQR, 2.79-6.7) | 4.6 (range, 0.23-137.73; IQR, 3.19-6.67) | .2669 |
| Engraftment | 741 (95.2) | 297 (94.6) | 444 (95.7) | .5906 |
| Time to engraftment, median, d | 17 (range, 3-89; IQR, 14-20) | 17 (range, 3-89; IQR, 13.5-20) | 17 (range, 4-77; IQR, 14-20) | .8290 |
| Source of stem cells (peripheral blood) | 722 (90.1) | 274 (85.4) | 448 (93.3) | .0003 |
| Disease status | .1045 | |||
| Advanced | 364 (45.4) | 152 (47.4) | 212 (44.1) | |
| CR1 | 396 (49.4) | 147 (45.8) | 249 (51.8) | |
| CR1 | 42 (5.2) | 22 (6.9) | 20 (4.2) | |
| KPS* | .1088 | |||
| Poor (<80%) | 66 (9) | 20 (6.7) | 46 (10.5) | |
| Good (≥80%) | 671 (91) | 277 (93.3) | 394 (89.5) | |
| Donor HLA | .1358 | |||
| MSD | 340 (42.4) | 141 (43.9) | 199 (41.4) | |
| URD 10/10 | 370 (46.1) | 152 (47.4) | 218 (45.3) | |
| URD 9/10 | 92 (11.5) | 28 (8.7) | 64 (13.3) | |
| Donor to patient sex | .0251 | |||
| Female to male | 166 (20.9) | 53 (16.8) | 113 (23.6) | |
| Other | 628 (79.1) | 263 (83.2) | 365 (76.4) | |
| Cytogenetic risk | .0339 | |||
| Intermediate | 538 (67.1) | 201 (62.6) | 337 (70.1) | |
| Poor | 264 (32.9) | 120 (37.4) | 144 (29.9) | |
| T-cell depletion | <.0001 | |||
| No TCD | 263 (32.8) | 138 (43) | 125 (26) | |
| ATG/Campath | 539 (67.2) | 183 (57) | 356 (74) | |
| Use of TBI | .0008 | |||
| Yes | 624 (77.8) | 230 (71.7) | 394 (81.9) | |
| No | 178 (22.2) | 91 (28.3) | 87 (18.1) | |
| CMV status (patient) | .2589 | |||
| Negative | 248 (31.9) | 90 (29.4) | 158 (33.5) | |
| Positive | 529 (68.1) | 216 (70.6) | 313 (66.5) | |
| CMV status (donor) | .9626 | |||
| Negative | 383 (49.3) | 151 (49) | 232 (49.5) | |
| Positive | 394 (50.7) | 157 (51) | 237 (50.5) |
Values are n (%) unless otherwise noted.
ATG, antithymocyte globulin; CMV, cytomegalovirus; CR, complete remission; KPS, Karnofsky Performance Status; TBI, total body irradiation.
Data missing on 65 patients in registry.
At the time of transplantation for the entire cohort, a significant proportion of patients had active disease (n = 364, 45.4%), while 396 (49.4%) were in CR1 and 42 (5.2%) were in CR2. As expected, patients who received RIC regimens were significantly older (median age, 61.9 years) than patients who received MAC regimens (median age, 54.3 years; P < .0001). In the MAC group, 201 (62.6%) had intermediate-risk disease compared with 337 (70.1%) in the RIC group, and 120 (37.4%) in the MAC group had poor risk cytogenetics compared with 144 (29.9%) in the RIC group (P = .0339). Donor sources were not different between the 2 groups, with MAC having 141 (43.9%) with MSD, 10/10 URD in 152 (47.4%) and 9/10 URD in 28 (8.7%), whereas the RIC group had 199 (41.4%) with MSD, 218 (45.3%) with 10/10 URD, and 64 (13.3%) with 9/10 URD (P = .1358). Most of the patients received peripheral blood grafts (n = 722, 90.1%), with only 79 (9.9%) receiving bone marrow grafts. Forty-seven patients (14.6%) in the MAC group had bone marrow as the source of stem cells compared with 32 (6.7%) in the RIC group (P = .0003). Female donor to male recipients were used less frequently in the MAC group as compared with RIC, at 16.8% vs 23.6%, respectively (P = .0251).
All patients received calcineurin inhibitor-based GVHD prophylaxis, and 539 (67.2%) received in vivo T-cell depletion (TCD; the majority with rabbit anti-thymocyte globulin or alemtuzumab). There was a difference between the MAC and RIC groups in terms of who received TCD (MAC, 57% vs RIC, 74%; P < .0001). The majority of patients achieved myeloid engraftment (95.2%), and the median time to myeloid engraftment was 17 days in both the MAC and RIC groups (IQR, 13.5-20 and 14-20 days, respectively). There were a variety of conditioning regimens used, and the use of total body irradiation was seen more commonly in the MAC group vs the RIC group (28.3% and 18.1%, respectively; P = .0008).
Outcomes (overall and according to conditioning)
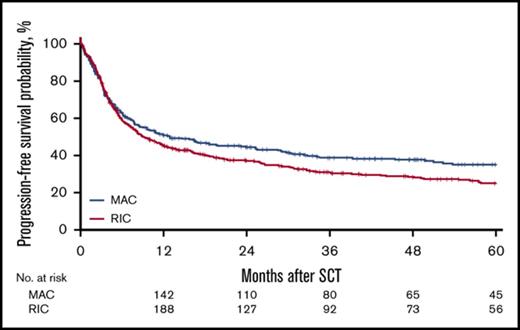
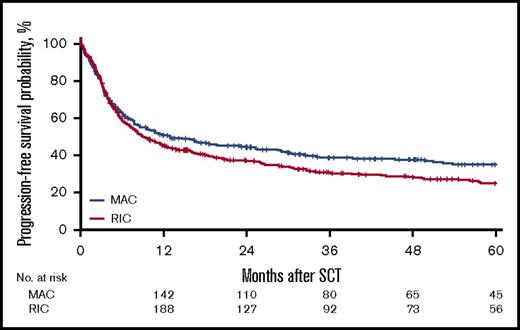
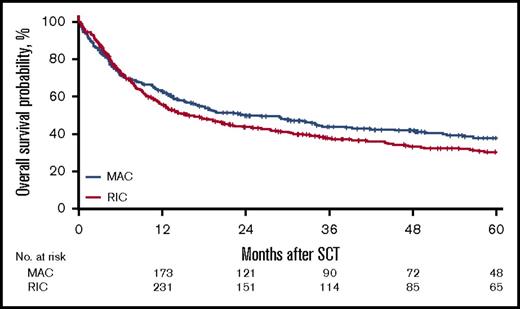
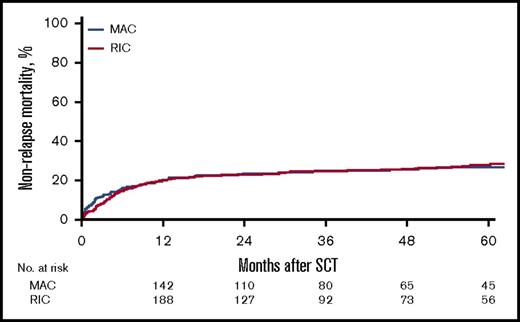
For the entire cohort, the 2-year cumulative incidence of relapse was 37% (95% CI, 33%-40%); NRM, 23% (95% CI, 20%-26%), LFS, 40% (95% CI, 37%-44%), OS, 46% (95% CI, 43%-50%). See Table 2 for complete details. Grades II-IV aGVHD by day 100 occurred in 25% of all the patients, with 11% experiencing at least grade III aGVHD and 3.9% grade IV.
Transplant outcomes
| Outcome . | Entire cohort . | MAC . | RIC . | P . |
|---|---|---|---|---|
| Incidence of aGVHD, n (%) | ||||
| Grade I | 140 (18) | 67 (21.8) | 73 (15.6) | .0129 |
| Grade II | 110 (14.2) | 52 (16.9) | 58 (12.4) | |
| Grade III | 54 (7) | 26 (8.5) | 28 (6) | |
| Grade IV | 30 (3.9) | 9 (2.9) | 21 (4.5) | |
| None | 433 (55.8) | 149 (48.5) | 284 (60.6) | |
| Present grade unknown | 9 (1.2) | 4 (1.3) | 5 (1.1) | |
| Chronic GVHD, n (%) | ||||
| No | 467 (63.9) | 187 (64.9) | 280 (63.2) | |
| Yes | 264 (36.1) | 101 (35.1) | 163 (36.8) | .6923 |
| 2-y OS (95% CI), % | 46 (43-50) | 50 (45-56) | 44 (39-49) | .039 |
| 2-y LFS (95% CI), % | 40 (37-44) | 45 (39-51) | 37 (33-42) | .029 |
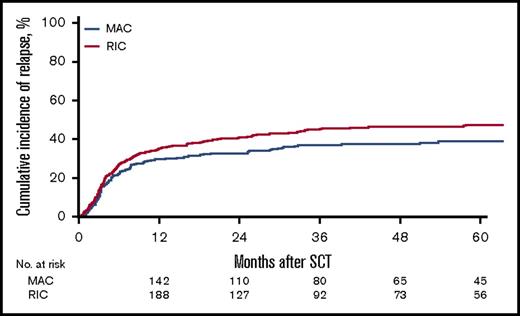
| 2-y cumulative incidence of relapse (95% CI), % | 37 (33-40) | 32 (27-38) | 40 (36-45) | .0341 |
| 2-y cumulative incidence of NRM (95% CI), % | 23 (20-26) | 23 (19-28) | 23 (19-27) | .8561 |
| aGVHD at 100 d (95% CI), % | 11 (9-13) | 12 (8-15) | 10 (8-13) | .6443 |
| 2-y cumulative incidence of cGVHD (95% CI), % | 39 (35-42) | 36 (31-42) | 40 (35-45) | .3906 |
| 2-y cumulative incidence of extensive cGVHD (95% CI), % | 17 (15-20) | 17 (13-22) | 17 (14-21) | .8851 |
| Outcome . | Entire cohort . | MAC . | RIC . | P . |
|---|---|---|---|---|
| Incidence of aGVHD, n (%) | ||||
| Grade I | 140 (18) | 67 (21.8) | 73 (15.6) | .0129 |
| Grade II | 110 (14.2) | 52 (16.9) | 58 (12.4) | |
| Grade III | 54 (7) | 26 (8.5) | 28 (6) | |
| Grade IV | 30 (3.9) | 9 (2.9) | 21 (4.5) | |
| None | 433 (55.8) | 149 (48.5) | 284 (60.6) | |
| Present grade unknown | 9 (1.2) | 4 (1.3) | 5 (1.1) | |
| Chronic GVHD, n (%) | ||||
| No | 467 (63.9) | 187 (64.9) | 280 (63.2) | |
| Yes | 264 (36.1) | 101 (35.1) | 163 (36.8) | .6923 |
| 2-y OS (95% CI), % | 46 (43-50) | 50 (45-56) | 44 (39-49) | .039 |
| 2-y LFS (95% CI), % | 40 (37-44) | 45 (39-51) | 37 (33-42) | .029 |
| 2-y cumulative incidence of relapse (95% CI), % | 37 (33-40) | 32 (27-38) | 40 (36-45) | .0341 |
| 2-y cumulative incidence of NRM (95% CI), % | 23 (20-26) | 23 (19-28) | 23 (19-27) | .8561 |
| aGVHD at 100 d (95% CI), % | 11 (9-13) | 12 (8-15) | 10 (8-13) | .6443 |
| 2-y cumulative incidence of cGVHD (95% CI), % | 39 (35-42) | 36 (31-42) | 40 (35-45) | .3906 |
| 2-y cumulative incidence of extensive cGVHD (95% CI), % | 17 (15-20) | 17 (13-22) | 17 (14-21) | .8851 |
Univariate analysis
For the MAC group, the 2-year cumulative incidence of relapse was 32% (95% CI, 27%-38%); LFS, 45% (95% CI, 39%-51%); OS, 50% (95% CI, 45%-56%); and NRM, 23% (95% CI, 19%-28%). In contrast, for the RIC group, the 2-year CI of relapse was 40% (95% CI, 36%-45%); LFS, 37% (95% CI, 33%-42%); OS, 44% (95% CI, 39%-49%); NRM, 23% (95% CI, 19%-27%). However, the effect of RIC vs MAC was found to be different before and after 6 months in LFS, OS, RI, and NRM. Before 6 months after transplantation, there was no difference in LFS (HR, 1.08; 95% CI, 0.85-1.35; P = NS), OS (HR, 0.91; 95% CI, 0.69-1.20; P = NS), RI (HR, 1.18; 95% CI, 0.88-1.59; P = NS), and NRM (HR, 0.93; 95% CI, 0.64-1.34; P = NS) between the RIC and MAC groups. However, after 6 months, there was a significant difference in the 2 groups in favor of the MAC group with regard to LFS (HR, 1.43; 95% CI, 1.09-1.89; P = .01), OS (HR, 1.55; 95% CI, 1.19 - 2.01; P < .001), and RI (HR, 1.47; 95% CI, 1.03-2.11; P = .04; Figures 1-4).
Table 3 gives details of additional factors that were tested in univariate analysis for their effect on outcomes. In addition to MAC conditioning, variables that affected the cumulative incidence of relapse included poor cytogenetic risk (HR, 2.09; 95% CI, 1.67-2.61; P < .0001), use of a MSD vs a 10/10 URD (HR, 0.77; 95% CI, 0.61-0.98; P = .03), and active disease pretransplant (HR, 1.57; 95% CI, 1.25-1.97; P = .0001).
Univariate analysis of transplant outcomes
| Variables . | LFS . | OS . | Incidence of relapse . | Nonrelapse mortality . |
|---|---|---|---|---|
| RIC vs MAC | ||||
| Before 6 months | 1.08 (0.85-1.35), P = .5401 | 0.91 (0.69-1.20), P = .4898 | 1.18 (0.88-1.59), P = .273 | 0.93 (0.64-1.34), P = .6824 |
| After 6 months | 1.43 (1.09-1.89), P = .0108 | 1.55 (1.19-2.01), P = .0009 | 1.47 (1.03-2.11), P = .0353 | 1.38 (0.89-2.14), P = .1476 |
| Age | ||||
| 10-y increase | 1.16 (1.06-1.27), P = .0009 | 1.22 (1.12-1.34), P < .0001 | 1.04 (0.93-1.15), P = .5273 | 1.42 (1.22-1.66), P < .0001 |
| Time from diagnosis to HCT | ||||
| 1-mo increase | 1 (1-1.01), P = .2125 | 1.01 (1-1.01), P = .1586 | 1.01 (1-1.02), P = .1015 | 1 (0.98-1.01), P = .9685 |
| Cytogenetic risk | ||||
| Intermediate | ref | ref | ref | ref |
| Poor | 1.75 (1.47-2.09), P < .0001 | 1.79 (1.49-2.15), P < .0001 | 2.09 (1.67-2.61), P < .0001 | 1.32 (0.98-1.77), P = .064 |
| HLA | ||||
| Identical sibling | ref | ref | ref | ref |
| URD 10/10 | 0.96 (0.8-1.16), P = .6649 | 1.12 (0.92-1.37), P = .2496 | 0.77 (0.61-0.98), P = .033 | 1.39 (1.02-1.89), P = .0386 |
| URD 9/10 | 1.36 (1.03-1.78), P = .0282 | 1.59 (1.2-2.11), P = .0014 | 1.05 (0.73-1.51), P = .7802 | 2.04 (1.34-3.1), P = .0009 |
| Donor to patient sex | ||||
| Other | ref | ref | ref | ref |
| Female to male | 1.01 (0.82-1.25), P = .9288 | 1 (0.8-1.25), P = .9749 | 0.87 (0.66-1.16), P = .3554 | 1.24 (0.9-1.72), P = .1882 |
| CMV patient | ||||
| Negative | ref | ref | ref | ref |
| Positive | 1.13 (0.93-1.37), P = .2069 | 1.14 (0.93-1.39), P = .2056 | 1.05 (0.82-1.34), P = .6966 | 1.27 (0.93-1.74), P = .1338 |
| CMV donor | ||||
| Negative | ref | ref | ref | ref |
| Positive | 1.04 (0.87-1.24), P = .6913 | 1.07 (0.89-1.29), P = .4515 | 1.15 (0.92-1.44), P = .2176 | 0.88 (0.66-1.17), P = .3838 |
| Cell source | ||||
| BM | ref | ref | ref | ref |
| PB | 0.99 (0.74-1.31), P = .9319 | 0.92 (0.69-1.22), P = .5618 | 0.99 (0.69-1.43), P = .9553 | 0.98 (0.62-1.54), P = .9371 |
| Disease status at HCT | ||||
| CR1 | ref | ref | ref | ref |
| CR2+ | 1.17 (0.78-1.74), P = .4436 | 1.25 (0.83-1.88), P = .2883 | 1.09 (0.64-1.85), P = .7602 | 1.3 (0.71-2.37), P = .3961 |
| Active | 1.5 (1.25-1.79), P < .0001 | 1.49 (1.24-1.8), P < .0001 | 1.57 (1.25-1.97), P = .0001 | 1.39 (1.05-1.86), P = .0225 |
| TCD | ||||
| No | ref | ref | ref | ref |
| ATG/alemtuzumab | 1.07 (0.89-1.29), P = .4588 | 1.14 (0.94-1.39), P = .1742 | 0.96 (0.76-1.22), P = .757 | 1.29 (0.95-1.75), P = .1052 |
| Year of HCT | ||||
| One-year increase | 1.01 (0.99-1.04), P = .3365 | 1.01 (0.99-1.04), P = .302 | 1.01 (0.98-1.05), P = .4074 | 1.01 (0.97-1.05), P = .6019 |
| Variables . | LFS . | OS . | Incidence of relapse . | Nonrelapse mortality . |
|---|---|---|---|---|
| RIC vs MAC | ||||
| Before 6 months | 1.08 (0.85-1.35), P = .5401 | 0.91 (0.69-1.20), P = .4898 | 1.18 (0.88-1.59), P = .273 | 0.93 (0.64-1.34), P = .6824 |
| After 6 months | 1.43 (1.09-1.89), P = .0108 | 1.55 (1.19-2.01), P = .0009 | 1.47 (1.03-2.11), P = .0353 | 1.38 (0.89-2.14), P = .1476 |
| Age | ||||
| 10-y increase | 1.16 (1.06-1.27), P = .0009 | 1.22 (1.12-1.34), P < .0001 | 1.04 (0.93-1.15), P = .5273 | 1.42 (1.22-1.66), P < .0001 |
| Time from diagnosis to HCT | ||||
| 1-mo increase | 1 (1-1.01), P = .2125 | 1.01 (1-1.01), P = .1586 | 1.01 (1-1.02), P = .1015 | 1 (0.98-1.01), P = .9685 |
| Cytogenetic risk | ||||
| Intermediate | ref | ref | ref | ref |
| Poor | 1.75 (1.47-2.09), P < .0001 | 1.79 (1.49-2.15), P < .0001 | 2.09 (1.67-2.61), P < .0001 | 1.32 (0.98-1.77), P = .064 |
| HLA | ||||
| Identical sibling | ref | ref | ref | ref |
| URD 10/10 | 0.96 (0.8-1.16), P = .6649 | 1.12 (0.92-1.37), P = .2496 | 0.77 (0.61-0.98), P = .033 | 1.39 (1.02-1.89), P = .0386 |
| URD 9/10 | 1.36 (1.03-1.78), P = .0282 | 1.59 (1.2-2.11), P = .0014 | 1.05 (0.73-1.51), P = .7802 | 2.04 (1.34-3.1), P = .0009 |
| Donor to patient sex | ||||
| Other | ref | ref | ref | ref |
| Female to male | 1.01 (0.82-1.25), P = .9288 | 1 (0.8-1.25), P = .9749 | 0.87 (0.66-1.16), P = .3554 | 1.24 (0.9-1.72), P = .1882 |
| CMV patient | ||||
| Negative | ref | ref | ref | ref |
| Positive | 1.13 (0.93-1.37), P = .2069 | 1.14 (0.93-1.39), P = .2056 | 1.05 (0.82-1.34), P = .6966 | 1.27 (0.93-1.74), P = .1338 |
| CMV donor | ||||
| Negative | ref | ref | ref | ref |
| Positive | 1.04 (0.87-1.24), P = .6913 | 1.07 (0.89-1.29), P = .4515 | 1.15 (0.92-1.44), P = .2176 | 0.88 (0.66-1.17), P = .3838 |
| Cell source | ||||
| BM | ref | ref | ref | ref |
| PB | 0.99 (0.74-1.31), P = .9319 | 0.92 (0.69-1.22), P = .5618 | 0.99 (0.69-1.43), P = .9553 | 0.98 (0.62-1.54), P = .9371 |
| Disease status at HCT | ||||
| CR1 | ref | ref | ref | ref |
| CR2+ | 1.17 (0.78-1.74), P = .4436 | 1.25 (0.83-1.88), P = .2883 | 1.09 (0.64-1.85), P = .7602 | 1.3 (0.71-2.37), P = .3961 |
| Active | 1.5 (1.25-1.79), P < .0001 | 1.49 (1.24-1.8), P < .0001 | 1.57 (1.25-1.97), P = .0001 | 1.39 (1.05-1.86), P = .0225 |
| TCD | ||||
| No | ref | ref | ref | ref |
| ATG/alemtuzumab | 1.07 (0.89-1.29), P = .4588 | 1.14 (0.94-1.39), P = .1742 | 0.96 (0.76-1.22), P = .757 | 1.29 (0.95-1.75), P = .1052 |
| Year of HCT | ||||
| One-year increase | 1.01 (0.99-1.04), P = .3365 | 1.01 (0.99-1.04), P = .302 | 1.01 (0.98-1.05), P = .4074 | 1.01 (0.97-1.05), P = .6019 |
BM, bone marrow; dx, diagnosis; PB, peripheral blood; ref, reference category.
For LFS, variables that adversely affected LFS included age at transplant with each 10-year increase (HR, 1.16; 95% CI, 1.06-1.27; P = .0009), use of a 9/10 URD (HR, 1.36; 95% CI, 1.03-1.78; P = .03), poor-risk cytogenetics (HR, 1.75; 95% CI, 1.47-2.09; P < .0001), and active disease pretransplant (HR, 1.5; 95% CI, 1.25-1.79; P < .05).
Similarly, for OS, transplant variables that adversely affected OS were age at transplant with each 10- year increase (HR, 1.22; 95% CI, 1.12-1.34; P < .05), use of a 9/10 URD (HR, 1.59; 95% CI, 1.2-2.11; P = .001), poor cytogenetic risk (HR, 1.79; 95% CI, 1.49-2.15; P < .0001), and active disease at the time of transplant (HR, 1.5; 95% CI 1.25-1.79; P < .0001).
With regard to NRM, no difference was seen in between the conditioning regimen groups (HR, 1.38; 95% CI, 0.89-2.14; P = .15), but variables that affected NRM included each 10-year increase in age (HR, 1.42; 95% CI, 1.22-1.66; P < .0001), use of a 10/10 URD (HR, 1.39; 95% CI, 1.02-1.89; P = .03) or 9/10 URD (HR, 2.04; 95% CI, 1.34-3.1; P < .0009), and active disease pretransplant (HR, 1.39; 95% CI, 1.05-1.86; P = .02).
The 2-year cumulative incidence of cGVHD of the entire cohort was 39% (95% CI, 35%-42%). There was no difference between the MAC and RIC groups with regard to the 2-year CI of cGVHD (MAC: 36% [95% CI, 31%-42%] vs RIC: 40% [95% CI, 35%-45%]; P = .39). Similarly, there was no difference in the 2-year cumulative incidence of extensive cGHVD between the 2 groups (MAC: 17% [95% CI, 13%-22%] vs RIC: 17% [95% CI, 14%-21%]; P = .88). The only transplant variable that affected GVHD outcomes was the use of T-cell depletion (either antithymocyte globulin or alemtuzumab) with a HR of 0.67 (95% CI, 0.45-0.98; P = .04) for grades II-IV acute GVHD and 0.55 (95% CI, 0.4-0.76; P = .0003) for chronic GVHD.
Multivariate analysis
The outcomes of multivariate analysis are summarized in Tables 4 and 5. In multivariate analysis, the effect of MAC similarly was significant after the 6-month posttransplant mark with regard to improved outcomes of LFS (HR, 1.43; 95% CI, 1.05-1.94; P = .02), OS (HR, 1.53; 95% CI, 1.14-2.05; P = .005), and RI (HR, 1.79; 95% CI, 1.19-2.68; P = .005). Again, there was no difference in NRM (HR, 1; 95% CI, 0.62-1.6; P = .98) between the RIC and MAC groups. In terms of GVHD, there was no significant difference between the 2 groups with regard to the incidence of aGVHD grades II-IV (HR, 0.83; 95% CI, 0.6-1.14; P = .25). After adjustment for in vivo TCD and other variables, a higher cumulative incidence of cGVHD (HR, 1.49; 95% CI 1.11-2; P = .0075) was noted in the RIC group.
Multivariate analysis of transplant outcomes
| . | LFS . | OS . | Incidence of relapse . | Non-relapse mortality . |
|---|---|---|---|---|
| Age (10-y increase) | 1.09 (0.99-1.21), P = .0812 | 1.17 (1.05-1.3), P = .0042 | 0.95 (0.84-1.07), P = .3871 | 1.41 (1.18-1.68), P = .0002 |
| Cytogenetic risk: poor vs intermediate | 1.68 (1.4-2.03), P < .0001 | 1.71 (1.41-2.07), P < .0001 | 2.06 (1.63-2.6), P < .0001 | 1.23 (0.9-1.68), P = .1964 |
| Year of HCT (1-y increase) | 1.02 (0.99-1.05), P = .1439 | 1.01 (0.98-1.04), P = .4641 | 1.04 (1.01-1.08), P = .0252 | 0.98 (0.94-1.03), P = .5343 |
| Female donor to male patient vs other | 0.98 (0.79-1.23), P = .8942 | 1.04 (0.82-1.31), P = .7596 | 0.79 (0.58-1.06), P = .117 | 1.36 (0.96-1.93), P = .079 |
| URD 10/10 vs MSD | 0.84 (0.66-1.07), P = .1606 | 1.04 (0.81-1.34), P = .7394 | 0.63 (0.47-0.86), P = .0029 | 1.36 (0.91-2.03), P = .1302 |
| URD 9/10 vs IMSD | 1.1 (0.8-1.5), P = .5561 | 1.32 (0.95-1.84), P = .0938 | 0.81 (0.54-1.21), P = .3015 | 1.86 (1.13-3.06), P = .0154 |
| CMV patient: positive vs negative | 1.14 (0.93-1.4), P = .2154 | 1.14 (0.92-1.41), P = .2405 | 1.05 (0.81-1.36), P = .7273 | 1.3 (0.93-1.81), P = .1219 |
| CMV donor: positive vs negative | 1 (0.82-1.21), P = .974 | 1.05 (0.86-1.28), P = .6647 | 1.13 (0.89-1.45), P = .3129 | 0.84 (0.62-1.13), P = .2492 |
| Cell source: PB vs BM | 0.88 (0.64-1.2), P = .4076 | 0.78 (0.57-1.07), P = .1202 | 0.89 (0.59-1.32), P = .5536 | 0.87 (0.53-1.43), P = .5891 |
| Disease status at HCT: CR2 vs CR1 | 1.21 (0.79-1.85), P = .3758 | 1.31 (0.85-2.03), P = .2166 | 1.18 (0.66-2.1), P = .5834 | 1.18 (0.64-2.2), P = .5986 |
| Disease status at HCT: active vs CR1 | 1.48 (1.22-1.78), P < .0001 | 1.43 (1.17-1.74), P = .0005 | 1.69 (1.33-2.15), P < .0001 | 1.19 (0.88-1.62), P = .2638 |
| TCD: ATG/alemtuzumab vs no | 0.95 (0.74-1.21), P = .6648 | 0.91 (0.71-1.18), P = .4741 | 0.95 (0.7-1.29), P = .7492 | 0.95 (0.64-1.41), P = .7959 |
| RIC vs MAC (6 mo before HCT) | 1.1 (0.84-1.43), P = .4868 | 0.89 (0.66-1.2), P = .4423 | 1.34 (0.96-1.88), P = .0874 | 0.8 (0.52-1.21), P = .2856 |
| RIC vs MAC (6 mo after HCT) | 1.43 (1.05-1.94), P = .0243 | 1.53 (1.14-2.05), P = .005 | 1.79 (1.19-2.68), P = .0052 | 1 (0.62-1.6), P = .9862 |
| . | LFS . | OS . | Incidence of relapse . | Non-relapse mortality . |
|---|---|---|---|---|
| Age (10-y increase) | 1.09 (0.99-1.21), P = .0812 | 1.17 (1.05-1.3), P = .0042 | 0.95 (0.84-1.07), P = .3871 | 1.41 (1.18-1.68), P = .0002 |
| Cytogenetic risk: poor vs intermediate | 1.68 (1.4-2.03), P < .0001 | 1.71 (1.41-2.07), P < .0001 | 2.06 (1.63-2.6), P < .0001 | 1.23 (0.9-1.68), P = .1964 |
| Year of HCT (1-y increase) | 1.02 (0.99-1.05), P = .1439 | 1.01 (0.98-1.04), P = .4641 | 1.04 (1.01-1.08), P = .0252 | 0.98 (0.94-1.03), P = .5343 |
| Female donor to male patient vs other | 0.98 (0.79-1.23), P = .8942 | 1.04 (0.82-1.31), P = .7596 | 0.79 (0.58-1.06), P = .117 | 1.36 (0.96-1.93), P = .079 |
| URD 10/10 vs MSD | 0.84 (0.66-1.07), P = .1606 | 1.04 (0.81-1.34), P = .7394 | 0.63 (0.47-0.86), P = .0029 | 1.36 (0.91-2.03), P = .1302 |
| URD 9/10 vs IMSD | 1.1 (0.8-1.5), P = .5561 | 1.32 (0.95-1.84), P = .0938 | 0.81 (0.54-1.21), P = .3015 | 1.86 (1.13-3.06), P = .0154 |
| CMV patient: positive vs negative | 1.14 (0.93-1.4), P = .2154 | 1.14 (0.92-1.41), P = .2405 | 1.05 (0.81-1.36), P = .7273 | 1.3 (0.93-1.81), P = .1219 |
| CMV donor: positive vs negative | 1 (0.82-1.21), P = .974 | 1.05 (0.86-1.28), P = .6647 | 1.13 (0.89-1.45), P = .3129 | 0.84 (0.62-1.13), P = .2492 |
| Cell source: PB vs BM | 0.88 (0.64-1.2), P = .4076 | 0.78 (0.57-1.07), P = .1202 | 0.89 (0.59-1.32), P = .5536 | 0.87 (0.53-1.43), P = .5891 |
| Disease status at HCT: CR2 vs CR1 | 1.21 (0.79-1.85), P = .3758 | 1.31 (0.85-2.03), P = .2166 | 1.18 (0.66-2.1), P = .5834 | 1.18 (0.64-2.2), P = .5986 |
| Disease status at HCT: active vs CR1 | 1.48 (1.22-1.78), P < .0001 | 1.43 (1.17-1.74), P = .0005 | 1.69 (1.33-2.15), P < .0001 | 1.19 (0.88-1.62), P = .2638 |
| TCD: ATG/alemtuzumab vs no | 0.95 (0.74-1.21), P = .6648 | 0.91 (0.71-1.18), P = .4741 | 0.95 (0.7-1.29), P = .7492 | 0.95 (0.64-1.41), P = .7959 |
| RIC vs MAC (6 mo before HCT) | 1.1 (0.84-1.43), P = .4868 | 0.89 (0.66-1.2), P = .4423 | 1.34 (0.96-1.88), P = .0874 | 0.8 (0.52-1.21), P = .2856 |
| RIC vs MAC (6 mo after HCT) | 1.43 (1.05-1.94), P = .0243 | 1.53 (1.14-2.05), P = .005 | 1.79 (1.19-2.68), P = .0052 | 1 (0.62-1.6), P = .9862 |
Multivariate analysis of graft versus host disease
| . | cGVHD . | aGVHD grades II-IV . |
|---|---|---|
| Age (10-y increase) | 0.97 (0.85-1.1), P = .6249 | 0.93 (0.81-1.08), P = .3388 |
| Cytogenetic risk: poor vs intermediate | 0.79 (0.59-1.05), P = .1072 | 0.97 (0.71-1.33), P = .8714 |
| Year of HCT (1-y increase) | 0.99 (0.95-1.03), P = .4605 | 1.04 (0.99-1.09), P = .0902 |
| Female donor to male patient vs other | 1.29 (0.96-1.73), P = .0939 | 1.15 (0.81-1.64), P = .4243 |
| URD 10/10 vs MSD | 1.14 (0.82-1.59), P = .4232 | 1.45 (0.97-2.16), P = .0697 |
| URD 9/10 vs MSD | 0.88 (0.53-1.46), P = .6092 | 1.81 (1.08-3.05), P = .024 |
| CMV patient: positive vs negative | 0.96 (0.72-1.29), P = .8033 | 1.09 (0.78-1.52), P = .6232 |
| CMV donor: positive vs negative | 1.08 (0.83-1.4), P = .5887 | 1.12 (0.82-1.52), P = .4789 |
| Cell source: PB vs BM | 1.06 (0.68-1.65), P = .8041 | 1.21 (0.71-2.05), P = .4805 |
| Disease status at HCT: CR2 vs CR1 | 0.96 (0.53-1.73), P = .8827 | 1.06 (0.57-1.96), P = .851 |
| Disease status at HCT: active vs CR1 | 1.2 (0.92-1.57), P = .1753 | 1.09 (0.8-1.48), P = .5787 |
| TCD: ATG/alemtuzumab vs none | 0.55 (0.4-0.76), P = .0003 | 0.67 (0.45-0.98), P = .0369 |
| RIC vs MAC (overall effect) | 1.49 (1.11-2), P = .0075 | 0.83 (0.6-1.14), P = .2533 |
| . | cGVHD . | aGVHD grades II-IV . |
|---|---|---|
| Age (10-y increase) | 0.97 (0.85-1.1), P = .6249 | 0.93 (0.81-1.08), P = .3388 |
| Cytogenetic risk: poor vs intermediate | 0.79 (0.59-1.05), P = .1072 | 0.97 (0.71-1.33), P = .8714 |
| Year of HCT (1-y increase) | 0.99 (0.95-1.03), P = .4605 | 1.04 (0.99-1.09), P = .0902 |
| Female donor to male patient vs other | 1.29 (0.96-1.73), P = .0939 | 1.15 (0.81-1.64), P = .4243 |
| URD 10/10 vs MSD | 1.14 (0.82-1.59), P = .4232 | 1.45 (0.97-2.16), P = .0697 |
| URD 9/10 vs MSD | 0.88 (0.53-1.46), P = .6092 | 1.81 (1.08-3.05), P = .024 |
| CMV patient: positive vs negative | 0.96 (0.72-1.29), P = .8033 | 1.09 (0.78-1.52), P = .6232 |
| CMV donor: positive vs negative | 1.08 (0.83-1.4), P = .5887 | 1.12 (0.82-1.52), P = .4789 |
| Cell source: PB vs BM | 1.06 (0.68-1.65), P = .8041 | 1.21 (0.71-2.05), P = .4805 |
| Disease status at HCT: CR2 vs CR1 | 0.96 (0.53-1.73), P = .8827 | 1.06 (0.57-1.96), P = .851 |
| Disease status at HCT: active vs CR1 | 1.2 (0.92-1.57), P = .1753 | 1.09 (0.8-1.48), P = .5787 |
| TCD: ATG/alemtuzumab vs none | 0.55 (0.4-0.76), P = .0003 | 0.67 (0.45-0.98), P = .0369 |
| RIC vs MAC (overall effect) | 1.49 (1.11-2), P = .0075 | 0.83 (0.6-1.14), P = .2533 |
Variables other than conditioning regimen intensity that also independently affected outcomes included both poor cytogenetic risk and active disease at the time of transplant: LFS (HR, 1.8 [95% CI, 1.4-2.03; P < .0001] and HR, 1.48 [95% CI, 1.22-1.78; P < .0001], respectively) and OS (HR, 1.71 [95% CI, 1.41-2.07; P < .0001] and HR, 1.43 [95% CI, 1.17-1.74; P = .0005], respectively). In addition, older age adversely affected OS with each 10-year increase in age (HR, 1.17; 95% CI, 1.05-1.3; P = .004).
With regard to relapse incidence, poor cytogenetic risk (HR, 2.06; 95% CI, 1.63-2.6; P < .0001), each subsequent year of transplant (HR, 1.04; 95% CI, 1.01-1.08; P = .02), use of a MSD (HR, 0.63; 95% CI, 0.47-0.86; P = .003), and active disease status at transplant (HR, 1.69; 95% CI, 1.33-2.15; P < .0001) were all statistically significant. Variables that negatively affected NRM were older age (10-year increase; HR, 1.4; 95% CI, 1.18-1.68; P = .0002) and use of a 9/10 URD (HR, 1.86; 95% CI, 1.13-3.06; P = .02). The incidence of grades II-IV aGVHD were affected by the use of a 9/10 URD vs MRD (HR, 1.81; 95% CI, 1.08-3.05; P = .02) and the use of TCD (HR, 0.67; 95% CI, 0.45-0.98; P = .04). As previously noted, the only statistically significant variable that affected the incidence of cGVHD in multivariate analysis was the use of TCD (HR, 0.55; 95% CI, 0.4-0.79; P = .0003).
Discussion
Our data demonstrate that approximately 45% of these patients can achieve long-term survival after HCT even in patients with high-risk disease, based on cytogenetics or active disease pre-HCT. This supports the current understanding that allogeneic HCT is likely the best long-term treatment strategy for this highest-risk patient population28,29 compared with only 10% long-term survival in patients who receive chemotherapy alone.6 This study indicates that patients who received myeloablative regimens had a lower risk for relapse and superior LFS and OS compared with those who received RIC regimens without a statistically significant difference in NRM. The decreased incidence of relapse is concordant with a recent phase 3 study by Scott et al in which patients with AML/MDS who received MAC had a statistically significantly higher relapse-free survival rate and a nonsignificant trend toward improved OS.21 This present study also showed no difference in NRM between the 2 conditioning groups, which is different from many prior retrospective studies in patients with AML/MDS who received allogeneic HCT.14-20,30 In the current era of improved supportive care and understanding of management of posttransplantation complications, NRM has overall declined compared with years past, although prospective data still suggest that NRM is generally less in patients who received RICs.25
These data suggest that select patients who are fit enough to tolerate MAC should be offered this conditioning regimen to optimize their outcomes posttransplantation, particularly given that our data did not show a difference with regard to NRM. This is especially important given previous data, which showed that patients who experienced relapsed disease within 18 months of HCT for AML or MDS have a dismal 2-year OS of only 9% to 14%.31 Although our study was not able to evaluate the particular variables that may have contributed to similar NRM between the 2 groups, it is possible that improved supportive care measures over the years and physician selection of patients contributed to similar NRM in the 2 groups.
Some limitations of our study include those that are typically inherent in a retrospective registry study. Although our study included only patients with sAML with high-risk cytogenetics and active disease going into transplant, which typically is considered a very high-risk group, our cumulative incidence of relapse was not higher than what is typically noted in the literature for this population, regardless of the preparative regimen received.21,25 This may suggest that even in these very high-risk patients, long-term survival can be achieved. However, our current knowledge of AML now includes a robust understanding of the importance of molecular data in risk stratification of patients. Unfortunately, our study was not able to include molecular characterization in an attempt to further delineate whether it would impact outcomes. Another important factor in determining transplantation outcomes is comorbidities. We had Karnofsky Performance Status for nearly all patients but were missing the Sorror score in more than half of the patients and therefore were unable to include this information in the analysis. We also did not conduct analyses according to disease risk index or the hematopoietic cell transplant-comorbidity index, but rather only according to cytogenetic risk score, as this is the current method used by the EBMT, although we acknowledge this is a limitation given their value in interpreting transplant outcomes.
As expected, other transplant variables such as older age, poor risk cytogenetics, and active disease were associated with significantly poorer outcomes in the entire cohort, and these variables should also be taken into account during physician-patient counseling regarding their individual risks and possible outcomes after transplant. In summary, our study showed that patients with AML with an antecedent diagnosis of MDS or MPN had good 2-year OS of more than 45% after either RIC or MAC allogeneic HCT. Our data demonstrate the utility of MAC in patients with sAML to reduce the risk for posttransplantation relapse without significantly increasing NRM.32 Further prospective studies are needed to study preemptive strategies to reduce the risk for relapse in high-risk groups.
Authorship
Contribution: S.S. wrote the paper in equal contribution with K.S.G.; A.B. and M.L. analyzed the data; J.F., A.G., M.S., G.E., D.B., D.N., D.B., P.D., G.M., P.C., A.M., M.H.G., N.G., J.E., F.C., F.B., C.S., S.G., and M.M. designed the research; and B.N.S. and A.N. designed the research and assisted in writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katie S. Gatwood, Vanderbilt University Medical Center, 1301 Medical Center Dr, Room 2664, Nashville, TN 37232; e-mail: katie.s.gatwood@vanderbilt.edu.
References
Author notes
S.S. and K.S.G. contributed equally to this manuscript.