Key Points
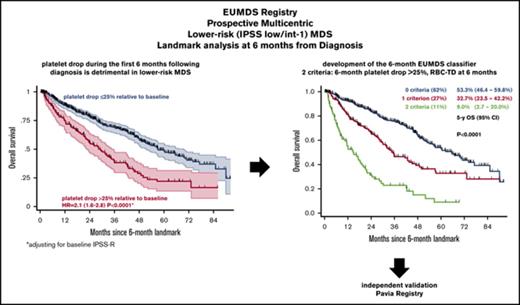
A drop in platelet count >25% relative to baseline at 6 months from diagnosis predicts inferior outcome in lower-risk MDS.
Platelet drop combined with RBC-TD at 6 months provides an inexpensive and validated classifier of outcome in lower-risk MDS.
Abstract
Prognosis of lower-risk (International Prognostic Scoring System [IPSS] low/intermediate-1) myelodysplastic syndrome (MDS) is heterogeneous and relies on steady-state assessment of cytopenias. We analyzed relative drops in neutrophil and platelet counts during the first 6 months of follow-up of lower-risk MDS patients. We performed a landmark analysis of overall survival (OS) of lower-risk MDS patients prospectively included in the European LeukaemiaNet MDS registry having a visit at 6 ± 1 month from inclusion to assess the prognostic relevance of relative drops in neutrophils and platelets, defined as (count at landmark − count at inclusion)/count at inclusion. Of 2102 patients, 807 were eligible for the stringent 6-month landmark analysis. Median age was 73 years. Revised IPSS was very low, low, and intermediate/higher in 26%, 43%, and 31% of patients, respectively. A relative drop in platelets >25% at landmark predicted shorter OS (5-year OS, 21.9% vs 48.6% with platelet drop ≤25%, P < 10−4), regardless of baseline IPSS-revised or absolute platelet counts. Relative neutrophil drop >25% had no significant impact on OS. We built a classifier based on red blood cell transfusion dependence (RBC-TD) and relative platelet drop >25% at landmark. Patients with none (62%), either (27%), or both criteria (11%) had 5-year OS of 53.3%, 32.7%, and 9.0%, respectively (P < 10−4). This classifier was validated in an independent cohort of 335 patients. Combining relative platelet drop >25% and RBC-TD at 6 months from diagnosis provides an inexpensive and noninvasive way to predict outcome in lower-risk MDS. This study was registered at www.clinicaltrials.gov as #NCT00600860.
Introduction
The prognosis of myelodysplastic syndromes (MDSs) defined as “lower-risk” per classical International Prognostic Scoring System (IPSS) criteria (IPSS low and intermediate-1) is heterogeneous.1 IPSS and revised IPSS (IPSS-R) rely on simple parameters, including complete blood count (CBC), bone marrow (BM) cytopathology, and cytogenetics.2 Flow cytometry or genomics could refine the prognosis of lower-risk MDS,3-5 but these techniques are limited by their cost and wide availability across health care systems. Time-dependent prognostic scores, applicable at any time during disease evolution, are helpful.6 However, they require repeated BM examinations, whose timing is not standardized, raising acceptability issues in this older patient population.
All current MDS prognostic scores rely on steady-state assessments of cytopenias (ie, hemoglobin [Hb] level or neutrophil or platelet counts) on the day of assessment. Conversely, the dynamics of tumor markers is instrumental in the prognostication of various malignancies.7,8 Here, we analyze, for the first time, the prognostic role of the kinetics of cytopenias during the first months following diagnosis in lower-risk MDS patients prospectively included in the European LeukaemiaNet MDS (EUMDS) registry.9 We performed a landmark analysis at 6 months from diagnosis to order simple prognostic criteria directly applicable in clinical practice.
Patients and methods
Patients
Since December 2007, patients from 16 European countries and Israel were included in the EUMDS registry, after signed informed consent according to the Declaration of Helsinki, within 100 days of the diagnosis of an MDS according to World Health Organization (WHO) 2001 criteria10 and with an IPSS risk of low or intermediate-1.1 Patients with an IPSS risk of intermediate-2 or high or with therapy-related MDS were excluded. Patients with cytogenetics failure, or without available cytogenetics were included if the diagnosis of MDS was morphologically proven, with <5% BM blasts and, at most, a single cytopenia according to IPSS. A post hoc central morphology review confirmed the accuracy of MDS diagnoses in the registry.11
The registry was approved by each institution’s ethics committee, according to the legislation of each country. It is registered at www.clinicaltrials.gov with the identifier NCT00600860.
Data collection, follow-up, and end points
Data were collected through a Web-based interface. Blast count was based on the local assessment of BM aspirates or, when unavailable, of BM biopsies.11 IPSS cytogenetic category was determined locally, whereas IPSS-R cytogenetic risks were retrospectively verified by an expert (D. Haase). IPSS and IPSS-R scores were computed automatically based on centralized data.
Patient-specific (including CBC) intervention and outcome data were collected at baseline and at each visit, which were to be repeated at 6-month intervals. Red blood cell transfusion dependence (RBC-TD) at any time point was defined as a requirement of ≥2 red blood cell units in the 8 weeks preceding the visit. There was no recommendation with respect to the timing of repeat BM evaluations. Interventions were based on each center’s routine care without guidelines from the registry.
All patients were prospectively followed until death, progression to higher-risk MDS (defined as IPSS risk intermediate-2/high), or transformation to acute myeloid leukemia per WHO criteria,10 until the last follow-up, or withdrawal of informed consent.
The present substudy was based on the 2102 patients included in the registry as of September 2016. The CONSORT diagram of the study population is reported in supplemental Figure 1. Inclusion criteria for the 6-month landmark analysis were a study visit at 6 months ± 30 days of study entry, available platelet counts at study entry and 6-month visit, and follow-up beyond the 6-month visit.
External validation cohort
The external validation cohort was extracted from the Pavia Registry, which accrued patients from 1992 to 20166,12 with the following criteria: diagnosis of an MDS according to WHO 2001 criteria10 with an IPSS risk of low or intermediate-1,1 and available CBC and information on RBC-TD at 6 ± 1 months from diagnosis. All definitions were identical to those used in EUMDS registry patients. Patients already included in the EUMDS registry were excluded from this validation cohort.
Statistical analyses
Continuous and categorical variables are described as medians and interquartile ranges (IQRs) and number and percentages, respectively. Equality of variance between different continuous variables was assessed by the F test (variance ratio test). Group comparisons for categorical and continuous variables were done with χ2 tests and Student t tests, respectively. Monotonic correlations between continuous variables were investigated with Spearman correlation tests, with graphical description of linear regression and its 95% confidence interval (CI).
Relative platelet and neutrophil drop were defined as: (count at landmark − count at inclusion)/count at inclusion. Rates of relative platelet and neutrophil drops were defined as: relative platelet (or neutrophil) drop/interval between inclusion and landmark.
Multivariate analyses of factors associated with relative platelet and neutrophils drops above or below defined thresholds were performed by logistic regression, excluding the 41 patients having received blood count–modifying treatments (hypomethylating agents [HMAs, n = 16], lenalidomide [LEN, n = 22], or hydroxyurea [HY, n = 3]) between inclusion and landmark analysis.
For the landmark analyses, overall survival (OS) was estimated with the Kaplan-Meier method from the day of landmark visit censoring at last follow-up, considering death as event. Follow-up duration from the date of diagnosis was determined according to the inverse method.13 Cumulative incidence of progression (CIP) was calculated from landmark, considering progression to higher-risk MDS (IPSS intermediate-2 or high risk) or acute myeloid leukemia AML according to WHO criteria10 as events and considering death as a competing risk. Differences in CIP between groups were assessed with the Fine and Gray test.14
To dichotomize relative platelet and neutrophil drop, martingale residuals of univariate Cox models of OS using relative platelet or neutrophil drop as a continuous value were visually inspected. The relevance of the retained cutoff was validated by comparing Akaike information criteria (AIC) from univariate Cox models with continuous or dichotomized relative platelet (or neutrophil) drop.15 Sensitivity analyses were performed using different thresholds for relative platelet and neutrophil drops with 5% bins.
Univariate and multivariate analyses for OS were performed using log-rank tests and Cox models, respectively. The proportional hazard assumption was validated by graphical inspection of visual display of the Schönfeld residuals.16 Interactions were tested by comparing Cox models, with or without an interaction term, between the 2 variables analyzed through a likelihood ratio test, and forest plots were plotted with the ipdmetan package for STATA. Collinearity was estimated with variance inflation factors, retaining the conventional variance inflation factor threshold of 4 as indicative of unacceptable collinearity.17
Variable selection for multivariate Cox models was based on lasso penalized regression, using the 1 standard error rule with the glmnet package for R.18 All analyses were performed with STATA 12.0 (STATA Corporation, College Station, TX) or R version 3.3.2 (http://www.R-project.org) software.
Results
Landmark study population
From the first 2102 patients included in the registry, 807 fulfilled the criteria for the 6-month landmark analysis (“landmark cohort”). Characteristics of the landmark cohort at inclusion and at the 6-month visit are summarized in Table 1 and supplemental Table 1. Median age was 73 years; 497 (61.6%) patients were male. IPSS-R risk, available for 691 patients (85.6%), was very low, low, intermediate, high, and very high in 26.0%, 43.0%, 24.2%, 6.1%, and 0.7% of cases, respectively. The median interval between diagnosis and landmark visit was 183 days.
Main characteristics of the landmark cohort (n = 807) at inclusion and landmark
| Variable . | Inclusion visit (n = 807) . | 6-mo landmark visit . | ||
|---|---|---|---|---|
| n or median . | % or IQR . | n or median . | % or IQR . | |
| Sex | ||||
| Male | 497 | 61.6% | ||
| Female | 310 | 38.4% | ||
| Age, y | 73 | 67-79 | ||
| WHO classification at diagnosis | ||||
| RA | 141 | 17.5% | ||
| RARS | 139 | 17.2% | ||
| RCMD | 309 | 38.3% | ||
| RCMD-RS | 51 | 6.3% | ||
| 5q− syndrome | 49 | 6.1% | ||
| RAEB-1 | 94 | 11.7% | ||
| RAEB-2 | 1 | 0.1% | ||
| MDS-U | 23 | 2.9% | ||
| IPSS-R cytogenetic risk (n = 730) | ||||
| Very good | 84 | 11.5% | ||
| Good | 543 | 74.4% | ||
| Intermediate | 89 | 12.1% | ||
| Poor/very poor | 14 | 2.00% | ||
| IPSS-R risk (available in 691 patients*) | ||||
| Very low | 180 | 26.0% | ||
| Low | 297 | 43.0% | ||
| Intermediate | 167 | 24.2% | ||
| High | 42 | 6.1% | ||
| Very high | 5 | 0.7% | ||
| Hb level, g/dL | 10.1 | 9-11.3 | ||
| RBC-TD | ||||
| No | 601 | 74.5% | 592 | 73.4% |
| Yes | 206 | 25.5% | 215 | 26.6% |
| Platelets, ×109/L | 181 | 102-277 | 169 | 93-263 |
| Neutrophils, ×109/L | 2.4 | 1.4-3.9 | 2.3 | 1.4-3.7 |
| Treatment before visit | ||||
| ESA with or without G-CSF | 115 | 14.2% | 277 | 34.3% |
| G-CSF alone | 2 | 0.3% | 4 | 0.5% |
| HMA | 1 | 0.1% | 16 | 2.0% |
| HY | 0 | 0% | 3 | 0.4% |
| LEN | 3 | 0.4% | 22 | 2.7% |
| None | 686 | 85% | 485 | 60.1% |
| Time from diagnosis to inclusion, d | 37 | 19-61 | ||
| Time from inclusion to landmark, mo | 6.1 | 5.7-6.4 | ||
| Follow-up from landmark, mo | 37.8 | 17.0-58.8 | ||
| Variable . | Inclusion visit (n = 807) . | 6-mo landmark visit . | ||
|---|---|---|---|---|
| n or median . | % or IQR . | n or median . | % or IQR . | |
| Sex | ||||
| Male | 497 | 61.6% | ||
| Female | 310 | 38.4% | ||
| Age, y | 73 | 67-79 | ||
| WHO classification at diagnosis | ||||
| RA | 141 | 17.5% | ||
| RARS | 139 | 17.2% | ||
| RCMD | 309 | 38.3% | ||
| RCMD-RS | 51 | 6.3% | ||
| 5q− syndrome | 49 | 6.1% | ||
| RAEB-1 | 94 | 11.7% | ||
| RAEB-2 | 1 | 0.1% | ||
| MDS-U | 23 | 2.9% | ||
| IPSS-R cytogenetic risk (n = 730) | ||||
| Very good | 84 | 11.5% | ||
| Good | 543 | 74.4% | ||
| Intermediate | 89 | 12.1% | ||
| Poor/very poor | 14 | 2.00% | ||
| IPSS-R risk (available in 691 patients*) | ||||
| Very low | 180 | 26.0% | ||
| Low | 297 | 43.0% | ||
| Intermediate | 167 | 24.2% | ||
| High | 42 | 6.1% | ||
| Very high | 5 | 0.7% | ||
| Hb level, g/dL | 10.1 | 9-11.3 | ||
| RBC-TD | ||||
| No | 601 | 74.5% | 592 | 73.4% |
| Yes | 206 | 25.5% | 215 | 26.6% |
| Platelets, ×109/L | 181 | 102-277 | 169 | 93-263 |
| Neutrophils, ×109/L | 2.4 | 1.4-3.9 | 2.3 | 1.4-3.7 |
| Treatment before visit | ||||
| ESA with or without G-CSF | 115 | 14.2% | 277 | 34.3% |
| G-CSF alone | 2 | 0.3% | 4 | 0.5% |
| HMA | 1 | 0.1% | 16 | 2.0% |
| HY | 0 | 0% | 3 | 0.4% |
| LEN | 3 | 0.4% | 22 | 2.7% |
| None | 686 | 85% | 485 | 60.1% |
| Time from diagnosis to inclusion, d | 37 | 19-61 | ||
| Time from inclusion to landmark, mo | 6.1 | 5.7-6.4 | ||
| Follow-up from landmark, mo | 37.8 | 17.0-58.8 | ||
G-CSF, granulocyte colony-stimulating factor; MDS-U, MDS unclassified; RA, refractory anemia; RAEB, refractory anemia with excess of blasts; RARS, refractory anemia with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RD, refractory cytopenia with multilineage dysplasia and ringed sideroblasts.
Detailed cytogenetics missing in 77 patients; detailed BM blasts (total <5%) missing in 39 patients.
Evolution of cytopenias
A total of 206 patients (25.5%) patients had RBC-TD (≥2 red blood cell units during the previous 8 weeks) prior to inclusion, and 215 (26.6%) patients had RBC-TD at landmark, including 133 who already had RBC-TD at inclusion. Patients with RBC-TD at landmark were more likely to receive erythropoiesis-stimulating agent (ESA) compared with patients not transfused between the 2 visits (ESA treatment in 45.1% and 30.4% of cases respectively, P < 10−4). The median absolute drop in platelet count at landmark visit was 6 × 109/L (IQR of platelet change, −35 to +21), corresponding to a median relative drop of 4.6% (IQR, −22.2% to +12.5%). At landmark, neutrophil count was available in 774 patients. The median absolute and relative neutrophil drops were 0.11 × 109/L (IQR, −0.76 to +0.49) and 5.2% (IQR, −29.2% to +29.7%) (supplemental Figure 2).
To investigate platelet and neutrophil drops as surrogates of BM function irrespective of the risk for hemorrhage and infection, which may differ for the same absolute platelet and neutrophil drop depending on baseline values, we focused on relative drops compared with baseline. There was a modest, yet significant, correlation between 6-month changes in platelets and neutrophils (R2 = +0.048, P < 10−4), but no correlation between time to landmark and relative platelet or neutrophil drop (Spearman correlation tests, P = .51 and P = .16, respectively; supplemental Figure 2). The use of rates of platelet or neutrophil drop, by normalizing according to the interval between inclusion and landmark did, not affect our findings (data not shown).
To exclude physiologic intrapatient variability in blood counts,19-21 we defined a >25% drop in platelets or in neutrophils over the first 6 months of follow-up as relevant cutoffs for prognostic analyses. Relative platelet and neutrophil drops >25% were present in 21.7% and 29.1% of evaluable patients, respectively. A univariate Cox model for OS using relative platelet or neutrophil drop did not reveal any specific cutoff by visual inspection of martingale residuals. AIC, a measure of information loss,15 was lower when relative platelet drop was dichotomized at 25% compared with the continuous variable (3540 and 3575, respectively). AIC for continuous and dichotomized relative neutrophil drop was similar (3372 and 3370, respectively).
Age, sex, BM (or peripheral blood) blasts, and cytogenetic risk did not significantly influence early platelet drop (Table 2). Platelet drop varied across diagnostic categories (P = .015) and was more frequent in patients with intermediate IPSS-R risk or higher (P = .024). Platelet drop was more frequent in the presence of multilineage dysplasia (MLD; 67.9% vs 53.4% in the absence of MLD, P = .005) but less frequent in MDS with ring sideroblasts (21.3% vs 31.9% without ring sideroblasts, P = .01). An increasing number of cytopenias was associated with more frequent platelet drop (P < 10−4). Patients with lower baseline Hb levels (P < 10−4) and those with RBC-TD at inclusion (P < 10−4) also had a platelet drop > 25% more frequently, whereas baseline platelet count did not affect relative platelet drop. Expectedly, treatment during the first 6 months had a strong impact on platelet and neutrophil drops (both P < 10−4), especially in patients treated with HY, LEN, or HMAs. In a multivariate logistic regression accounting for WHO, number of cytopenias, RBC-TD, and baseline Hb level, RBC-TD at inclusion stood out as the main determinant of early platelet drop (odds ratio [OR], 1.7; 95% confidence interval [CI] 1.1-2.6, P = .02).
Clinical and biological predictors of early platelet drop
| Variable . | Relative platelet drop . | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤25% (n = 632) . | >25% (n = 175) . | P . | Multivariate . | |||||
| n or median . | % or IQR . | n or median . | % or IQR . | OR . | 95% CI . | P . | ||
| Sex | .4 | |||||||
| Male | 394 | 62.3% | 103 | 58.9% | ||||
| Female | 238 | 37.7% | 72 | 41.1% | ||||
| Age, y | 73 | 67-79 | 73 | 66-79 | .5 | |||
| WHO classification at diagnosis | .015 | |||||||
| RA | 113 | 17.9% | 28 | 16.0% | 1 | |||
| RARS | 122 | 19.3% | 17 | 9.7% | 0.7 | 0.3-1.3 | .2 | |
| RCMD | 230 | 36.4% | 79 | 45.1% | 1.3 | 0.8-2.1 | .4 | |
| RCMD-RS | 39 | 6.1% | 12 | 6.9% | 1.2 | 0.5-2.5 | .7 | |
| 5q− syndrome | 34 | 5.4% | 15 | 8.6% | 1.0 | 0.4-.26 | .9 | |
| RAEB-1 | 74 | 11.7% | 20 | 11.4% | 0.7 | 0.4-1.5 | .4 | |
| RAEB-2 | 0 | 0.0% | 1 | 0.6% | ||||
| MDS-U | 20 | 3.2% | 3 | 1.7% | 0.6 | 0.2-2.4 | .45 | |
| IPSS at inclusion | <10−4 | |||||||
| Low | 332 | 55.5% | 61 | 36.7% | ||||
| Intermediate-1 | 266 | 44.5% | 105 | 63.3% | ||||
| BM blasts, % | .1 | |||||||
| ≤2 | 362 | 60.7% | 85 | 51.8% | ||||
| >2 to <5 | 162 | 27.2% | 51 | 31.1% | ||||
| 5-10 | 69 | 11.6% | 28 | 17.1% | ||||
| >10 | 3 | 0.5% | 0 | 0.0% | ||||
| Peripheral blasts, % | .4 | |||||||
| <1 | 477 | 75.5% | 127 | 72.6% | ||||
| ≥1 | 155 | 24.5% | 48 | 27.4% | ||||
| No. of cytopenias | <10−4 | |||||||
| 0 | 132 | 20.9% | 19 | 10.9% | 1 | |||
| 1 | 332 | 52.5% | 84 | 48.0% | 1.3 | 0.7-2.4 | .3 | |
| 2 | 126 | 19.9% | 46 | 26.2% | 1.7 | 0.9-3.2 | .1 | |
| 3 | 42 | 6.7% | 26 | 14.9% | 2.4 | 1.1-5.2 | .03 | |
| IPSS-R cytogenetic risk | .5 | |||||||
| Very good | 64 | 11.2% | 20 | 12.5% | ||||
| Good | 431 | 75.6% | 112 | 70.0% | ||||
| Intermediate | 65 | 11.4% | 24 | 15.0% | ||||
| Poor/very poor | 10 | 1.8% | 4 | 2.5% | ||||
| IPSS-R at inclusion | .024 | |||||||
| Very low | 151 | 27.8% | 29 | 19.7% | ||||
| Low | 238 | 43.7% | 59 | 40.1% | ||||
| Intermediate | 118 | 21.7% | 49 | 33.4% | ||||
| High | 32 | 5.9% | 10 | 6.8% | ||||
| Very high | 5 | 0.9% | 0 | 0% | ||||
| Hb level, g/dL | 10.2 | 9.3-11.4 | 9.5 | 8.5-10.7 | <10−4 | 0.9 | 0.8-1.1 | .2 |
| RBC-TD at inclusion | <10−4 | .02 | ||||||
| No | 499 | 79.0% | 102 | 58.3% | 1 | |||
| Yes | 133 | 21.0% | 73 | 41.7% | 1.7 | 1.1-2.6 | ||
| Platelets, ×109/L | 184 | 108-277 | 155 | 89-261 | .6 | |||
| Neutrophils, ×109/L | 2.4 | 1.5-3.9 | 2.1 | 1.2-3.8 | .8 | |||
| Treatment before landmark | <10−4 | |||||||
| ESA with or without G-CSF | 205 | 32.4% | 72 | 41.1% | ||||
| G-CSF alone | 3 | 0.5% | 1 | 0.6% | ||||
| HMA | 8 | 1.3% | 8 | 4.6% | ||||
| HY | 1 | 0.2% | 2 | 1.2% | ||||
| LEN | 7 | 1.1% | 15 | 8.6% | ||||
| None | 408 | 64.5% | 77 | 44.0% | ||||
| Variable . | Relative platelet drop . | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤25% (n = 632) . | >25% (n = 175) . | P . | Multivariate . | |||||
| n or median . | % or IQR . | n or median . | % or IQR . | OR . | 95% CI . | P . | ||
| Sex | .4 | |||||||
| Male | 394 | 62.3% | 103 | 58.9% | ||||
| Female | 238 | 37.7% | 72 | 41.1% | ||||
| Age, y | 73 | 67-79 | 73 | 66-79 | .5 | |||
| WHO classification at diagnosis | .015 | |||||||
| RA | 113 | 17.9% | 28 | 16.0% | 1 | |||
| RARS | 122 | 19.3% | 17 | 9.7% | 0.7 | 0.3-1.3 | .2 | |
| RCMD | 230 | 36.4% | 79 | 45.1% | 1.3 | 0.8-2.1 | .4 | |
| RCMD-RS | 39 | 6.1% | 12 | 6.9% | 1.2 | 0.5-2.5 | .7 | |
| 5q− syndrome | 34 | 5.4% | 15 | 8.6% | 1.0 | 0.4-.26 | .9 | |
| RAEB-1 | 74 | 11.7% | 20 | 11.4% | 0.7 | 0.4-1.5 | .4 | |
| RAEB-2 | 0 | 0.0% | 1 | 0.6% | ||||
| MDS-U | 20 | 3.2% | 3 | 1.7% | 0.6 | 0.2-2.4 | .45 | |
| IPSS at inclusion | <10−4 | |||||||
| Low | 332 | 55.5% | 61 | 36.7% | ||||
| Intermediate-1 | 266 | 44.5% | 105 | 63.3% | ||||
| BM blasts, % | .1 | |||||||
| ≤2 | 362 | 60.7% | 85 | 51.8% | ||||
| >2 to <5 | 162 | 27.2% | 51 | 31.1% | ||||
| 5-10 | 69 | 11.6% | 28 | 17.1% | ||||
| >10 | 3 | 0.5% | 0 | 0.0% | ||||
| Peripheral blasts, % | .4 | |||||||
| <1 | 477 | 75.5% | 127 | 72.6% | ||||
| ≥1 | 155 | 24.5% | 48 | 27.4% | ||||
| No. of cytopenias | <10−4 | |||||||
| 0 | 132 | 20.9% | 19 | 10.9% | 1 | |||
| 1 | 332 | 52.5% | 84 | 48.0% | 1.3 | 0.7-2.4 | .3 | |
| 2 | 126 | 19.9% | 46 | 26.2% | 1.7 | 0.9-3.2 | .1 | |
| 3 | 42 | 6.7% | 26 | 14.9% | 2.4 | 1.1-5.2 | .03 | |
| IPSS-R cytogenetic risk | .5 | |||||||
| Very good | 64 | 11.2% | 20 | 12.5% | ||||
| Good | 431 | 75.6% | 112 | 70.0% | ||||
| Intermediate | 65 | 11.4% | 24 | 15.0% | ||||
| Poor/very poor | 10 | 1.8% | 4 | 2.5% | ||||
| IPSS-R at inclusion | .024 | |||||||
| Very low | 151 | 27.8% | 29 | 19.7% | ||||
| Low | 238 | 43.7% | 59 | 40.1% | ||||
| Intermediate | 118 | 21.7% | 49 | 33.4% | ||||
| High | 32 | 5.9% | 10 | 6.8% | ||||
| Very high | 5 | 0.9% | 0 | 0% | ||||
| Hb level, g/dL | 10.2 | 9.3-11.4 | 9.5 | 8.5-10.7 | <10−4 | 0.9 | 0.8-1.1 | .2 |
| RBC-TD at inclusion | <10−4 | .02 | ||||||
| No | 499 | 79.0% | 102 | 58.3% | 1 | |||
| Yes | 133 | 21.0% | 73 | 41.7% | 1.7 | 1.1-2.6 | ||
| Platelets, ×109/L | 184 | 108-277 | 155 | 89-261 | .6 | |||
| Neutrophils, ×109/L | 2.4 | 1.5-3.9 | 2.1 | 1.2-3.8 | .8 | |||
| Treatment before landmark | <10−4 | |||||||
| ESA with or without G-CSF | 205 | 32.4% | 72 | 41.1% | ||||
| G-CSF alone | 3 | 0.5% | 1 | 0.6% | ||||
| HMA | 8 | 1.3% | 8 | 4.6% | ||||
| HY | 1 | 0.2% | 2 | 1.2% | ||||
| LEN | 7 | 1.1% | 15 | 8.6% | ||||
| None | 408 | 64.5% | 77 | 44.0% | ||||
A 6-month drop in neutrophils >25% was more frequent in patients with higher baseline neutrophil count (P < 10−4) and was associated with worse IPSS-R cytogenetic risk (P = .015). In a multivariate regression, only a higher baseline neutrophil count (OR, 1.2; 95% CI, 1.1-1.4; P < 10−4) maintained its predictive value (supplemental Table 2), suggesting that the dynamic range of neutrophils is not sufficient to capture meaningful changes in patients with baseline neutropenia.
Prognostic relevance of early drop in platelets and neutrophils
In the landmark cohort (n = 807), with a median follow-up of 37.8 months, 5-year OS was 42.8% (95% CI, 37.6-47.9) and 5-year CIP was 21.4% (95% CI, 17.8-25.0). Patients with a platelet drop >25% had a 5-year OS of 21.9% (95% CI, 13.6-31.4) compared with 48.6% (95% CI, 42.5-54.4) in patients with platelet drop ≤25% (P < 10−4; Figure 1). Five-year OS was 40.9% (95% CI, 30.7-50.8) in patients with a >25% neutrophil drop and 45.1% (95% CI, 38.9-51.1) in those with a ≤25% drop (P = .12). CIP was also higher in patients with platelet drop >25% (hazard ratio [HR], 2.5; 95% CI, 1.7-3.7; P < 10−4) and, to a lesser extent, with neutrophil drop >25% (HR, 1.5; 95% CI, 1.0-2.2; P = .03; Figure 1). Sensitivity analyses using different cutoff values for relative platelet and neutrophil drop found a robust prognostic role for relative platelet drop in OS across a large spectrum of thresholds (5%-40% relative drop), whereas a significantly shorter OS was only found in patients with a neutrophil drop >30% (data not shown). Because of the stronger prognostic impact of platelet kinetics, we next focused on the relevance of early platelet drop in defined subgroups.
Prognostic impact of relative drop in platelets and neutrophils. Kaplan-Meier plots of OS from landmark (with 95% CIs) according to 6-month relative platelet (A) and neutrophil (B) drop. CIP since landmark according to 6-month platelet (C) and neutrophil (D) drop. Blue, relative platelet or neutrophil drop ≤25%; red, relative platelet or neutrophil drop >25%.
Prognostic impact of relative drop in platelets and neutrophils. Kaplan-Meier plots of OS from landmark (with 95% CIs) according to 6-month relative platelet (A) and neutrophil (B) drop. CIP since landmark according to 6-month platelet (C) and neutrophil (D) drop. Blue, relative platelet or neutrophil drop ≤25%; red, relative platelet or neutrophil drop >25%.
Prognostic relevance of early platelet drop in subgroups
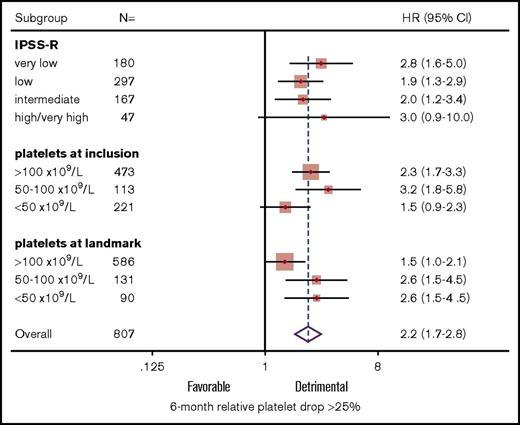
Six-month relative platelet drop retained significant poor prognostic value (HR, 2.1; 95% CI, 1.6-2.8; P < 10−4) at landmark when adjusting for IPSS-R risk at inclusion (HR, 1.1; 95% CI, 1.0-1.3; P = .054). In subgroup analysis, there was no significant interaction between IPSS-R risk and relative platelet drop (P = .4), and 6-month platelet drop was associated with shorter OS in each IPSS-R stratum (all P < .007; Figure 2). There were nonsignificant interaction trends between relative platelet drop and baseline (P = .1) or landmark platelet counts (P = .07). In particular, early platelet drop had limited impact in the 221 (27.4%) patients with a baseline platelet count <50 × 109/L (P = .10) and a lower, but nevertheless significant, impact in patients retaining a platelet count >100 × 109/L at landmark (P = .051). Importantly, excluding the 45 patients (5.6%) having received HY, LEN, or HMAs during the 6-month interval did not affect the poor prognosis of patients with a platelet drop >25% and confirmed the prognostic relevance of platelet drop in patients retaining platelets >100 × 109/L at landmark (HR, 1.6; 95% CI, 1.1-2.4; P = .023).
Prognostic impact of relative platelet drop in defined subgroups. Forest plot of HRs of relative platelet drop >25% according to baseline IPSS-R risk and absolute platelet count at inclusion and landmark.
Prognostic impact of relative platelet drop in defined subgroups. Forest plot of HRs of relative platelet drop >25% according to baseline IPSS-R risk and absolute platelet count at inclusion and landmark.
Sensitivity analysis of the timing of landmark
To generalize the finding that early platelet and, to a lesser extent, neutrophil drop is associated with poor prognosis in lower-risk MDS, we performed a sensitivity analysis by relaxing the inclusion criteria for landmark analysis. All patients with a second visit within 10 months of registry inclusion with available platelet counts were included, regardless of follow-up (supplemental Figure 1). This “sensitivity cohort” included 1610 patients, with a median time to second visit of 5.2 months (IQR, 4.1-6.1). Baseline characteristics of this sensitivity cohort are summarized in supplemental Table 1. Expectedly, time to landmark was much more variable in this sensitivity cohort than in the 6-month landmark cohort (variance ratio test: P < 10−4; supplemental Figure 3).
In this sensitivity cohort, 340 (21.1%) patients had a >25% platelet drop and 424 (26.3%) had a >25% neutrophil drop at landmark visit. With a median follow-up of 40.9 months, the poor prognosis of patients with platelet drop, as previously defined, was confirmed in univariate analysis (HR, 2.3; 95% CI, 2.0-2.8; P < 10−4; supplemental Figure 4), and it remained strongly significant after adjusting for baseline IPSS-R risk and platelet count at baseline or at landmark (all P < 10−4). In a sensitivity analysis considering the rate of platelet decline, a decline in platelets of 4.2% per month (corresponding to a 25% decline in 6 months) retained its prognostic impact, even in patients assessed >6 months after the inclusion visit (P = .05). Time to second visit had no prognostic impact per se (P = .12), also suggesting that the prognostic impact of platelet decline is not due to a poorer outcome of patients with delayed study visits.
Multivariate analysis of OS at the 6-month landmark
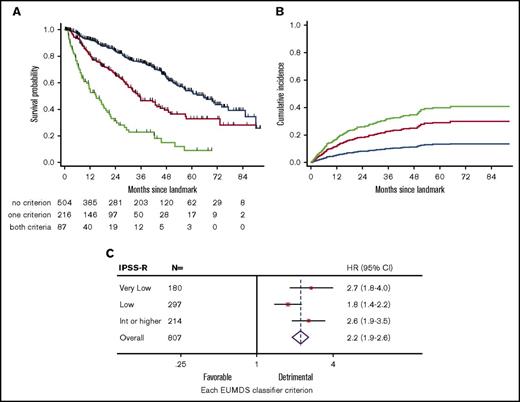
In the stringent landmark cohort of 807 patients, only 95 (11.8%) patients had a control of BM blast percentage by aspirate or biopsy, including 50 with informative cytogenetics, allowing stringent reassessment of IPSS-R in only 6.2% of patients. Only 24 of the 95 patients with BM reassessment had progressed to IPSS-R high or very high risk. Therefore, we sought to define a simple prognostic index based on age, sex, baseline IPSS-R, and noninvasive hematological parameters available at the 6-month visit, including RBC-TD, absolute neutrophil and platelet counts at landmark, and 6-month relative neutrophil and platelet drops. In univariate analysis, older age, RBC-TD, early platelet drop >25%, and lower platelet value at landmark had significant adverse prognostic impact (supplemental Table 3). Multivariate analysis after lasso penalized regression18 identified older age (HR, 1.04; 95% CI, 1.02-1.05; P < 10−4), RBC-TD at landmark (HR, 2.8; 95% CI, 2.2-3.5; P < 10−4), and platelet drop > 25% (HR, 1.7; 95% CI, 1.3-2.2; P < 10−4) as independent predictors of poor outcome. Of note, replacing the global IPSS-R risk group with its individual components, including baseline platelet count group (given the association between baseline platelet count and 6-month platelet drop) also led to the identification of RBC-TD at landmark and platelet drop >25% as significant variables after lasso regression. Although platelet drop was more frequent in patients with RBC-TD at landmark (41.5% vs 15.0% in those without RBC-TD; P < 10−4), there was no significant interaction between these 2 parameters (P = .19) and limited collinearity in the multivariate model (mean variance inflation coefficient, 1.46). Therefore, we could design an age-independent 6-month EUMDS classifier combining RBC-TD and platelet drop at landmark to discriminate 3 groups with none (n = 504, 62%), 1 (n = 216, 27%), or 2 (n = 87, 11%) of these criteria, with significantly different 5-year OS of 53.3% (95% CI, 46.4-59.8), 32.7% (95% CI, 23.5-42.2), and 9.0% (95% CI, 2.7-20.0), respectively, after the 6-month landmark (P < 10−4; Figure 3A). The EUMDS 6-month classifier was also predictive of CIP (P < 10−4; Figure 3B). Importantly, the EUMDS 6-month classifier successfully discriminated outcome in each IPSS-R risk category (Figure 3C) and retained prognostic value independently of the MD Anderson Prognostic Score for lower-risk MDS (data not shown).22 Excluding the minority of patients treated with HMAs, LEN, or HY prior to landmark, with or without stratification on ESA treatment, did not affect these conclusions, nor did exclusion of all 322 patients treated prior to landmark (data not shown). The EUMDS classifier could also be applied to the sensitivity cohort of 1610 patients (P < 10−4), where it could also discriminate outcome within each baseline IPSS-R stratum (all P < 10−4; supplemental Figure 5).
Design of the 6-month EUMDS classifier. Kaplan-Meier plots of OS (A) and CIP (B) from landmark according to the 6-month EUMDS classifier based on platelet drop >25% and RBC-TD at landmark. (C). Forest plots of HRs of each additional criterion in the 6-month EUMDS classifier according to baseline IPSS-R risk in the landmark cohort. Blue, no criteria; red, either criteria, green, both criteria.
Design of the 6-month EUMDS classifier. Kaplan-Meier plots of OS (A) and CIP (B) from landmark according to the 6-month EUMDS classifier based on platelet drop >25% and RBC-TD at landmark. (C). Forest plots of HRs of each additional criterion in the 6-month EUMDS classifier according to baseline IPSS-R risk in the landmark cohort. Blue, no criteria; red, either criteria, green, both criteria.
External validation of the 6-month EUMDS score
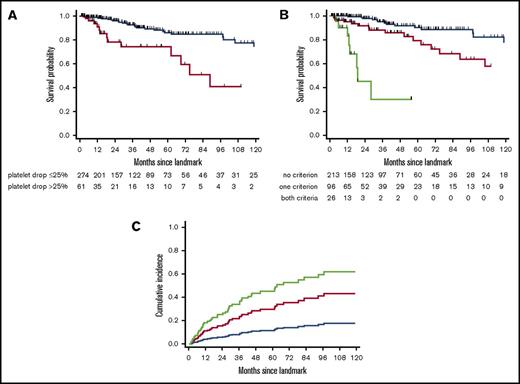
We next validated the 6-month EUMDS score in an independent cohort from the Pavia Registry of lower-risk MDS. The validation cohort included 335 lower-risk (IPSS low, n = 182; intermediate-1, n = 153) MDS patients accrued since 1992, with a follow-up visit at 6 ± 1 month from diagnosis. Their detailed characteristics at diagnosis and 6-month landmark are shown in supplemental Table 4. The median interval from diagnosis to landmark was 6.4 months. Eighty-seven (26%) patients had RBC-TD at landmark. A platelet drop > 25% at landmark was found in 61 (18%) patients. With a median follow-up of 31.4 months after landmark, the 5-year OS of those patients was 74.1% (95% CI, 55.2-86.0) compared with 86.0% (95% CI, 78.6-90.1) for those with slower platelet decline (P < 10−4; Figure 4A); 213 (63%), 96 (29%), and 26 (8%) patients had neither, 1, or both 6-month EUMDS criteria. Their 5-year OS was 90.3% (95% CI, 82.5-94.8), 79.4% (95% CI, 64.2-88.7), and 30.3% (95% CI, 5.8-60.6), respectively (P < 10−4; Figure 4B). The EUMDS classifier also successfully predicted CIP, with HR = 2.9 (95% CI, 1.6-6.3) and HR = 5.0 (95% CI, 1.9-13.0) in patients with 1 and 2 criteria, respectively (P = .0002; Figure 4C). Restricting analyses to the 273 patients diagnosed since 2000 led to similar conclusions (data not shown).
Validation of the 6-month EUMDS classifier. Kaplan-Meier plots of OS in the external validation cohort (n = 335) according to platelet drop >25% at the 6-month landmark (blue, relative platelet drop ≤25%; red, relative platelet drop >25%) (A) and the 6-month EUMDS classifier (B). (C) CIP according to the 6-month EUMDS classifier. (B-C) Blue, no criteria; red, either criteria; green: both criteria.
Validation of the 6-month EUMDS classifier. Kaplan-Meier plots of OS in the external validation cohort (n = 335) according to platelet drop >25% at the 6-month landmark (blue, relative platelet drop ≤25%; red, relative platelet drop >25%) (A) and the 6-month EUMDS classifier (B). (C) CIP according to the 6-month EUMDS classifier. (B-C) Blue, no criteria; red, either criteria; green: both criteria.
Discussion
The prognosis of lower-risk MDS is heterogeneous.1 Early identification of “lower-risk” MDS patients at risk for rapid progression should rely on universal, affordable, and noninvasive tools to gain acceptance in an elderly population often managed in community care centers. The EUMDS registry provided a unique opportunity to address, for the first time, the potential prognostic role of the dynamics of cytopenias in lower-risk MDS.
We have identified a 25% platelet drop during the first 6 months of lower-risk MDS diagnosis as an independent poor prognostic feature in MDS. Combining early platelet drop with the presence of RBC-TD after 6 months of follow-up allowed us to design a robust cross-validated prognostic classifier that could be successfully applied to all lower-risk MDS patients, regardless of IPSS-R risk category. Sensitivity analyses confirmed the applicability of this simple classifier even when follow-up visits were planned any time during the first 10 months after diagnosis, thus capturing most situations encountered in daily practice. This score was validated in an independent cohort.
Dissecting the contribution of disease progression vs cytopenia-specific complications remains challenging in MDS.6,23 Although platelet drop increases bleeding risk,24-26 we believe that our results support the contention that early platelet drop is a surrogate of the global pace of MDS progression. We measured platelet drop relative to baseline value, because an absolute drop does not carry the same risk for bleeding, depending on baseline platelet count. In multivariate analysis, relative platelet drop, rather than steady-state platelet value at landmark, affected prognosis. Relative platelet drop retained prognostic value even in patients with platelets counts >50 × 109/L at landmark, a threshold above which bleeding is infrequent. Finally, early platelet drop also predicted a higher incidence of MDS progression. Our findings resonate with previous reports that short-term platelet changes induced by specific drugs instruct prognosis, stressing that platelet kinetics is a good surrogate of BM failure in MDS.27-29
Although early neutrophil drop had a prognostic impact on CIP, it is likely that the prognostic impact of neutrophil drop is confounded by inflammation or infection episodes. Platelet transfusions may confound assessment of platelet dynamics, possibly explaining the limited prognostic value of platelets drop in patients already presenting with <50 × 109/L platelets at inclusion. Treatments such as HY, HMAs, and LEN can also induce thrombocytopenia; however, few patients (5.1%) received these treatments during the 6 months following MDS diagnosis, and excluding them did not change our results.
The stringent 6-month visit spacing recommended by the registry was not realized in all patients; however, there was no correlation between relative platelet drop and time to second evaluation. A sensitivity analysis including all patients with a second visit within 10 months from inclusion (77% of patients) did not affect our conclusions nor did considering the rate of platelet drop per month in patients evaluated >6 months later.
Early platelet drop was only modestly linked to IPSS-R, suggesting that dynamic parameters capture additional information. In fact, early platelet drop was tightly correlated to dyserythropoiesis, as assessed by baseline Hb value and RBC-TD. Our study also confirmed that RBC-TD is a key prognostic feature in lower-risk MDS.6,30 Although correlated, early platelet drop >25% and RBC-TD at 6 months from diagnosis could nevertheless be combined in a simple EUMDS classifier, without significant interaction or collinearity. This classifier could discriminate 3 groups of patients with different OS, including a subgroup with RBC-TD and early platelet drop at 6 months accounting for ∼10% of “lower-risk” MDS patients, whose OS from landmark did not exceed 2 years. Importantly, our classifier retained its significance, regardless of the baseline IPSS-R risk category of patients. We ensured the robustness of our model by basing variable selection on penalized regression and by performing external validation in an independent cohort from the Pavia Registry.6,12 Further validation of this classifier in recently accrued cohorts receiving novel treatments for lower-risk MDS will be required.
More sophisticated methodologies are required to describe patterns in the trajectories of cytopenias in MDS31 and to determine whether platelet drop can be used at any time of MDS evolution.32 Our proposed EUMDS classifier nevertheless offers a simple universal noninvasive and robust strategy to identify, early in the disease course, the minority of “lower-risk” MDS patients who may require close monitoring and potentially specific therapeutic interventions, such as early start of HMAs or allogeneic stem cell transplantation. Further studies, such as the ongoing prospective MDS-RIGHT project, are required to determine whether somatic mutations induce different kinetics of thrombocytopenia or whether they can be combined with our EUMDS classifier to refine the prognostic assessment of lower-risk MDS.
Presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9 December 2017.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all local investigators, operational team members, and Jackie Droste for their contribution to the registry.
The EUMDS Registry is supported by an educational grant from Novartis Pharmacy B.V. Oncology Europe and Amgen Limited. This work is part of the MDS-RIGHT activities, which have received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634789—“Providing the right care to the right patient with myelodysplastic syndrome at the right time.” The Pavia Registry is supported by a grant from Associazione Italiana per la Ricerca sul Cancro (IG 20125) (L.M.)
Authorship
Contribution: R.I., P.F., D.B., and T.d.W. designed the study; R.I. and S.C. performed analyses; A. Smith and C.v.M. managed the EUMDS database; L.M., E.T., and C.E. accrued patients into the Pavia Registry and managed the validation database; A. Symeonidis, E.H.-L., G.S., J.C., R.S., L.M., U.G., M.M., S.L., K.M., A.T., M.S.H., A.M.A., A. Savic, N.G.S., E.L., D.C., and A.G.-B. accrued patients; R.I. drafted the manuscript; and all authors revised the manuscript and agreed to its final version.
Conflict-of-interest disclosure: R.I. has received research funding from Novartis and Janssen, honoraria from Celgene, BMS, and Sanofi, and consulting fees from Novartis, Otsuka, and Karyopharm. P.F. has received research funding from Amgen, Astex, Celgene, Janssen, and Novartis and honoraria from Janssen. A.M.A. has received honoraria from BMS and Alexion and consulting fees from Novartis, Celgene, and Servier. A. Symeonidis has received honoraria and consulting fees from Amgen, Celgene/GenesisPharma, Janssen-Cilag, Gilead, Pfizer, MSD, Novartis, and Genzyme/Sanofi. E.H.-L. has received research funding from Celgene. G.S. has received research funding and honoraria from Celgene, Novartis, Amgen, and consulting fees from Amgen, Boehringer-Ingelheim, Celgene, MSD, and Novartis. M.S.H. has received research funding Celgene. A. Savic has received honoraria and consulting fees from Pfizer, MSD, and Hoffmann-La Roche. U.G. has received research funding and honorarium from Novartis. C.v.M., project manager of the EUMDS Registry, is funded from the EUMDS project budget. T.d.W. has received research funding from Novartis, Celgene, and Amgen, and honorarium from Novartis as project coordinator EUMDS. The remaining authors declare no competing financial interests.
A list of the members of the European MDS Registry appears in the online appendix.
Correspondence: Raphael Itzykson, INSERM/CNRS UMR 944/7212, Hematology Department, Hôpital Saint-Louis, Assistance Publique–Hôpitaux de Paris, Université Paris Diderot, 1, Ave Claude Vellefaux, 75010 Paris, France; e-mail: raphael.itzykson@aphp.fr.