Key Points
DNA ligase 4 deficiency is a defect causing lymphopenia (T-B-NK+) and a radiosensitive severe combined immunodeficiency phenotype.
Flow cytometric analysis of phosphorylation/dephosphorylation states of histone H2AX allows for in-depth lineage-specific assessment.
Abstract
DNA ligase 4 deficiency (LIG4-SCID) causes lymphopenia (T-B-NK+) and a radiosensitive SCID (RS-SCID) phenotype. We demonstrate, for the first time, flow cytometric-based kinetic analysis of phosphorylated H2AX (γH2AX) in lymphocyte subsets, especially NK cells, for the assessment of LIG4-SCID. Measurement of phosphorylated (p) ATM, SMC1, and H2AX (γH2AX) was performed by flow cytometry to assess DNA repair defects in a 3-year-old girl. Functional assessment (phosphorylation) was measured in T and NK cells (B cells were absent) before irradiation (background control) or after low-dose (2Gy) irradiation (1 and 24 hours). We observed maximal γH2AX at 1 hour postirradiation, with dephosphorylation at 24 hours postirradiation in healthy control patients. The patient showed normal frequencies (percentage) of T cells and NK cells for γH2AX, but increased levels of γH2AX compared with control patients at 1 hour postirradiation. At 24 hours postirradiation, there was a lack of dephosphorylation in a substantial proportion of lymphocytes (with differences observed between T and NK cells) compared with healthy control patients. Although there was dephosphorylation of γH2AX at 24 hours in patient lymphocytes compared with 1 hour, the amount remained elevated at 24 hours compared with in control patients. The data from pATM and pSMC1 were uninformative. Flow-based kinetic analysis of γH2AX is a useful marker for the diagnosis of LIG4-SCID.
Introduction
DNA double-strand breaks are part of cellular physiology, but if not repaired correctly, they can affect the ability of a cell to proliferate, differentiate, and function. There are several monogenic disorders caused by mutations in genes that control DNA repair.1 These include forms of radiosensitive severe combined immunodeficiency (RS-SCID) such as DNA ligase 4 deficiency (LIG4-SCID) and other nonhomologous DNA repair defects.2 The assignment of a timely diagnosis is vital in the management of patients with RS-SCID, as hematopoietic cell transplantation may be considered.3 The current laboratory assessment of radiosensitivity in lymphocytes includes proliferation assays and/or irradiation-induced immunofluorescence foci, counting using fibroblasts or lymphoblastoid cell lines, which can take 1 to 2 weeks4 in addition to the 1 to 2 months it may require to generate a fibroblast or Epstein-Barr virus cell line.
H2AX is a component of the histone octamer in nucleosomes and is phosphorylated by ATM (ataxia-telangiectasia mutated) and ATR (ATM-Rad3-related) and serves as the initiation step in the mobilization of DNA repair proteins.5 Flow cytometric quantitation of phosphorylated H2AX (γH2AX) and SMC1, on induction of DNA damage, has been used for the diagnosis of ataxia-telangiectasia (AT).6,7 Assessment of γH2AX by flow has been used previously to assess the functional effect of mutations in the DCLRE1C gene in Artemis-deficient patients.8 Functional assessments of patients with LIG4-SCID have included quantification of DNA double-strand break rejoining, assessed using pulsed field gel electrophoresis in a several patients with LIG4-SCID,9 as well as flow cytometric assessment of γH2AX in CD3+ T cells or peripheral blood mononuclear cells in a family of 3 individuals with LIG4 deficiency.10 However, in this published report, the dose of irradiation was significantly higher (10 Gy) compared with the dose used in this report (2 Gy). Here, we demonstrate for the first time the application of flow cytometric-based kinetic analysis of γH2AX in individual lymphocyte subsets, especially NK cells, which is particularly relevant in T-B-NK+ SCID, for the diagnostic assessment of LIG4-SCID.
Methods
Simultaneous assessment of a multimarker (phosphorylated [p]ATM, pSMC1, γH2AX) flow assay was used to assess DNA repair defects in a 3-year-old Korean girl (see supplemental Materials; supplemental Table 1 for additional clinical and laboratory details) who was evaluated for recurrent fevers, chronic respiratory tract infections, chronic diarrhea, and a rash in association with compound heterozygous LIG4 variants (NM_001098268, c.1341G>T, p.Trp447Cys and NM_001098268, c.1103A>T, p.Asp368Val). Phosphorylation of these markers was measured in T and NK cells (B cells were undetectable in this patient), without (unirradiated control) or with exposure to low-dose (2 Gy) irradiation. We have observed that the amount of γH2AX linearly correlates with radiation dose (data not shown), which has also been observed by others.11 We assessed phosphorylation of these proteins preirradiation (0 hours) and at 1 hour and 24 hours postirradiation. The data from pATM and pSMC1 were uninformative for the evaluation of LIG4-SCID (data not shown). Patient blood samples and biopsy material were obtained after provision of informed consent, using an Institutional Review Board approved consent form.
Results
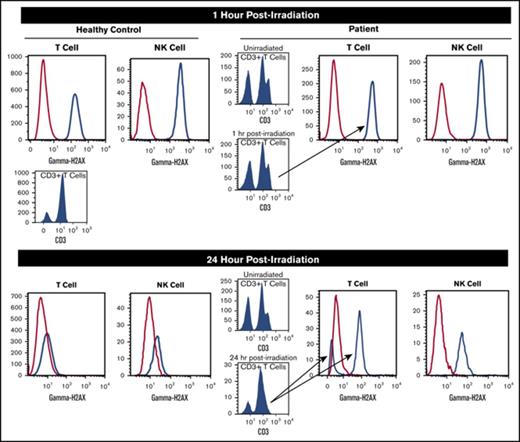
Kinetic analysis of γH2AX in T and NK cells was found to be critical in the diagnosis and evaluation of LIG4-SCID. As depicted in Figure 1A, we observed maximal γH2AX at 1 hour postirradiation, with progressive dephosphorylation at 24 hours postirradiation in a healthy control cohort (range of γH2AX in healthy pediatric controls [n = 8] at 1 and 24 hours postirradiation in T [mean fluorescence intensity (MFI) ratio of irradiated to nonirradiated, 50.09 and 2.62, respectively] and NK [59.94 and 2.87, respectively] cells). Two experimental healthy controls (HC1 and HC2) were also assessed and showed similar findings in T cells (HC1, 30.14 and 2.94 at 1 and 24 hours, respectively; HC2, 27.58 and 2.39 at 1 and 24 hours, respectively) and NK cells (HC1, 49.80 and 3.26 at 1 and 24 hours, respectively; HC2, 58.38 and 2.57 at 1 and 24 hours, respectively). The LIG4-SCID patient showed comparable MFI ratios to the experimental healthy control patients (HC1 and HC2) at 1 hour for T cells (28.47) and NK cells (52.39). However, at 24 hours postirradiation, there is a distinct difference between patient and control individuals, in that there is higher MFI ratio for γH2AX in both T cells (8.61) and NK cells (9.59), indicating a defect in DNA repair (Figure 1A). This is also apparent when evaluating the relative frequencies of T and NK cells expressing γH2AX after low-dose irradiation (Figure 1B). The patient, the experimental controls, and the pediatric reference range cohort show comparable frequencies of T cells and NK cells for γH2AX at 1 hour postirradiation (Figure 1B). At 24 hours postirradiation, however, T cells from the patient demonstrate a clear bimodal population (Figure 1C), with 1 subset (38% T cells) showing dephosphorylation comparable to the controls and a second subset (62% T cells) positive for γH2AX, albeit decreased in amount (8.61 vs 28.47) compared with the 1-hour point (Figure 1A,C). In the NK cell subset, at 24 hours postirradiation, a significant proportion (96.9%) of cells demonstrated γH2AX persistence (Figure 1B-C) compared with the pediatric reference cohort (35.54%) and experimental controls (8.52% and 7.55%, respectively, for HC1 and HC2; Figure 1B); however, the MFI ratio in the patient was decreased relative to 1 hour (Figure 1A,C), although it was still substantially higher than either control type (reference range or experimental controls; Figure 1A).
(A) Assessment of histone H2AX phosphorylation (γH2AX) at 1 and 24 hours postirradiation (2 Gy) by MFI ratio. The mode ratio of the MFI of γH2AX (irradiated [IR] to unirradiated [non-IR]) in T-cell and NK cell subsets is shown. (B) Frequencies of T and NK cells expressing histone H2AX phosphorylation (γH2AX) 1 and 24 hours postirradiation (2 Gy). The frequency of NK cells with dephosphorylated H2AX at 24 hours remains essentially unchanged (but there is a reduced MFI ratio). (C) Visual depiction of flow cytometric analysis showing γH2AX at 1 and 2 hours postirradiation in a representative control and patient. Patient T cells show 2 peaks for CD3+ T cells at 1 hour postirradiation (absent at 24 hours). Approximately two-thirds of T cells still have γH2AX at 24 hours (reduced MFI). About a third of T cells show complete dephosphorylation at 24 hours. The majority of NK cells still have γH2AX, although there is evidence of partial dephosphorylation.
(A) Assessment of histone H2AX phosphorylation (γH2AX) at 1 and 24 hours postirradiation (2 Gy) by MFI ratio. The mode ratio of the MFI of γH2AX (irradiated [IR] to unirradiated [non-IR]) in T-cell and NK cell subsets is shown. (B) Frequencies of T and NK cells expressing histone H2AX phosphorylation (γH2AX) 1 and 24 hours postirradiation (2 Gy). The frequency of NK cells with dephosphorylated H2AX at 24 hours remains essentially unchanged (but there is a reduced MFI ratio). (C) Visual depiction of flow cytometric analysis showing γH2AX at 1 and 2 hours postirradiation in a representative control and patient. Patient T cells show 2 peaks for CD3+ T cells at 1 hour postirradiation (absent at 24 hours). Approximately two-thirds of T cells still have γH2AX at 24 hours (reduced MFI). About a third of T cells show complete dephosphorylation at 24 hours. The majority of NK cells still have γH2AX, although there is evidence of partial dephosphorylation.
The flow data parallel the findings of γH2AX foci counting assay, as depicted in supplemental Figure 1, in which the maximal difference between the patient and control fibroblasts was seen at 24 hours postirradiation during dephosphorylation.
Discussion
The flow cytometric assay permitted ex vivo evaluation of the DNA repair pathway in individual lymphocyte subsets (lineage-specific analysis), if present, which is not possible when using fibroblast cultures or Epstein-Barr virus cell lines (typically restricted to 1 specific lymphocyte subset). In addition, we observed differences in phosphorylation and dephosphorylation kinetics among specific T-cell subpopulations, based on relative expression of CD3 on the cell surface. T cells from the patient show 2 peaks (based on relative intensities) for CD3+ T cells, as depicted in Figure 1C. This bimodal expression of CD3 disappears 24 hours postirradiation. In the T-cell subset, approximately two-thirds of T cells (62%) still have γH2AX, although the MFI is reduced in this subset at 24 hours compared with 1 hour postirradiation. Approximately one-third of T cells show complete dephosphorylation at 24 hours postirradiation (Figure 1C). Also of particular importance is the ability to assess the DNA repair pathway specifically in NK cells, as most radiosensitive SCID conditions manifest with severe T- and B-cell lymphopenia, but the normal presence of NK cells. The majority of the NK cells in the patient showed relatively incomplete dephosphorylation at 24 hours postirradiation, suggesting the DNA repair pathway is defective.
In summary, flow-based analysis of γH2AX, and specifically the magnitude of dephosphorylation, is a useful marker for the diagnosis of LIG4-SCID and may be useful in other forms of RS-SCID. Moreover, these analyses permit the characterization of γH2AX dephosphorylation in individual lymphocyte subsets using only a relatively small amount of blood (5 cc sodium heparin) with a rapid assessment (3-4 days). Clinical research with flow cytometric characterization of other RS-SCID defects will help expand and clarify the role of this diagnostic tool and potential application in the clinical laboratory.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patient and her family for participating in our research studies. Patient blood samples and biopsy material were obtained after provision of informed consent.
Funding sources include the Mayo Clinic Department of Laboratory Medicine and Pathology, Jeffrey Modell Foundation for Primary Immunodeficiencies for their support of the Mayo Clinic Diagnostic and Research Center for PIDs (R.S.A.) and California Institute of Regenerative Medicine (TR3-05535, CLIN1-08363) (M.J.C. and M.K.) and National Institutes of Health, National Center for Advancing Translational Science, and National Institute of Allergy and Infectious Diseases (U54-AI 082973) (M.J.C.).
Authorship
Contribution: All authors helped draft and approved the submitted manuscript; D.B. and R.S.A. coordinated investigation of the patient’s immunological analyses and creation of the manuscript; D.B. diagnosed and treated the patient’s immune disorder; M.J.S., M.K., M.J.C., and R.S.A. helped characterize the immunological disturbance; M.J.S., M.J.C., and R.S.A. helped review the literature and illustrate the manuscript; M.K. and J.S.B. provided laboratory support for fibroblast culture and associated analyses; and D.B. recognized the patient’s enigmatic presentation, initiated diagnostic studies, and made possible her multi-institutional investigation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Buchbinder, Department of Pediatric Hematology, CHOC Children’s Hospital, 1201 W. La Veta Ave, Orange, CA 92868; e-mail: dbuchbinder@choc.org.

![(A) Assessment of histone H2AX phosphorylation (γH2AX) at 1 and 24 hours postirradiation (2 Gy) by MFI ratio. The mode ratio of the MFI of γH2AX (irradiated [IR] to unirradiated [non-IR]) in T-cell and NK cell subsets is shown. (B) Frequencies of T and NK cells expressing histone H2AX phosphorylation (γH2AX) 1 and 24 hours postirradiation (2 Gy). The frequency of NK cells with dephosphorylated H2AX at 24 hours remains essentially unchanged (but there is a reduced MFI ratio). (C) Visual depiction of flow cytometric analysis showing γH2AX at 1 and 2 hours postirradiation in a representative control and patient. Patient T cells show 2 peaks for CD3+ T cells at 1 hour postirradiation (absent at 24 hours). Approximately two-thirds of T cells still have γH2AX at 24 hours (reduced MFI). About a third of T cells show complete dephosphorylation at 24 hours. The majority of NK cells still have γH2AX, although there is evidence of partial dephosphorylation.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/15/10.1182_bloodadvances.2018016113/3/m_advances016113f1-1.jpeg?Expires=1764968690&Signature=HqOO~HfPKWrgpLX1UOr3ZCLuOcqB0YYxhuHaQVKqUbpzrspKi0HVA2g10TUl9drjRng4CqnuC~vXZLBX5Nlhr1~B~maDEEHj~6y1-NQIryI1m63elEZB6nz6sxNPCEQKax-l5zMPjS72fgCP6N5s-5kVGpw8EdybpyXljCviHht~ISW6Y73JiOqmx3Z~IyLnH5pcqI0Rs2~rRkk35cYTWuDys-iioRnVHwQIyEYLOowkArRd0WsKu5nRim8~GukEvEZLGheICM~uSmssFzy280A6gYLUnhJDBcM1fd9N8kdF-ttuPKFDbMM5F-3u31Kpc4JSdztRFIPNNBaJApBQow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![(A) Assessment of histone H2AX phosphorylation (γH2AX) at 1 and 24 hours postirradiation (2 Gy) by MFI ratio. The mode ratio of the MFI of γH2AX (irradiated [IR] to unirradiated [non-IR]) in T-cell and NK cell subsets is shown. (B) Frequencies of T and NK cells expressing histone H2AX phosphorylation (γH2AX) 1 and 24 hours postirradiation (2 Gy). The frequency of NK cells with dephosphorylated H2AX at 24 hours remains essentially unchanged (but there is a reduced MFI ratio). (C) Visual depiction of flow cytometric analysis showing γH2AX at 1 and 2 hours postirradiation in a representative control and patient. Patient T cells show 2 peaks for CD3+ T cells at 1 hour postirradiation (absent at 24 hours). Approximately two-thirds of T cells still have γH2AX at 24 hours (reduced MFI). About a third of T cells show complete dephosphorylation at 24 hours. The majority of NK cells still have γH2AX, although there is evidence of partial dephosphorylation.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/15/10.1182_bloodadvances.2018016113/3/m_advances016113f1-2.jpeg?Expires=1764968690&Signature=jOmWWurvfxd9pJPA0QqGGcs57eX6w2jLP3G4lOp6s8hYWQm4gx6TYDFVC-O~FK5oVEdiMmpgFs02A5NPI2xWILWVsTvjsRzns2qJQ~qusz8xGFKoC0bl7GWXw4U3TAecklvzAHyipdu7~nohq6oEZQRBNqsVn9T6xFby03rdHGY73btAmCpwaizzy4cLqTOwnzWrG~V5ZAx3QfTXQrLZM7Fl2INL0YvXiolXX3jmEYKp6v1BWfaAvHgAEAM2e3jvyluew4GgRsk07GDEEeK~26c2Tu4GUg4kFiIFk12alrN4p6bDr0rMqoKZJ7P9k7xQ5EygVGvE4~bcJYFeau6okw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)