Key Points
An N411K mutation in the external gate of the proton-coupled folate transporter within the aqueous channel results in impaired function.
The N411K mutation produces a substrate-specific defect in transport, resulting in hereditary folate malabsorption.
Abstract
Hereditary folate malabsorption (HFM) is an autosomal recessive disorder characterized by impaired intestinal folate absorption and impaired folate transport across the choroid plexus due to loss of function of the proton-coupled folate transporter (PCFT-SLC46A1). We report a novel mutation, causing HFM, affecting a residue located in the 11th transmembrane helix within the external gate. The mutant N411K-PCFT was stable, trafficked to the cell membrane, and had sufficient residual activity to characterize the transport defect and the structural requirements at this site for gate function. The influx Vmax of the N411K mutant was markedly decreased, as was the affinity for most, but not all, folate/antifolate substrates. The greatest loss of activity was for 5-methyltetrahydrofolate. Substitutions with positive charged residues resulted in a loss of activity (arginine > lysine > histidine). Function was retained for the negative charged aspartate, but not the larger glutamate substitutions, whereas the bulky hydrophobic (leucine), or polar (glutamine) substitutions, were tolerated. Homology models of PCFT, in the inward and outward open conformations, based upon the mammalian Glut5 fructose transporter structures, localize Asn411 protruding into the aqueous pathway. This is most prominent when the carrier is in the inward open conformation when the external gate is closed. Mutations at this site likely result in highly specific steric and electrostatic interactions between the Asn411-substituted, and other, residues in the gate region that impede carrier function. The substrate specificity of the N411K mutant may be due to alterations of substrate flows through the external gate, downstream allosteric alterations in the folate-binding pocket, or both.
Introduction
Hereditary folate malabsorption (HFM) is an autosomal recessive disorder in which there is impaired intestinal folate absorption and impaired transport of folates across the choroid plexus into the cerebrospinal fluid. This results in severe systemic and cerebral folate deficiency.1-4 The disorder is due to mutations in the proton-coupled folate transporter (PCFT, SLC46A1) gene that result most commonly in the loss of, or in an unstable, protein.2-4 In only a few instances has a stable mutant protein that traffics to the cell membrane been identified, allowing characterization of the functional defect.5,6 The topology of this transporter has been defined and is typical of the solute carrier (SLC) superfamily of transporters with 12 transmembrane helices, 6 on either side of a large internal loop, with N- and C-termini located within the cytoplasm.7-10 The exofacial regions at the membrane-aqueous interface of transmembrane helices 1, 2, 7, and 11 were recently shown to come together to form a gate at the extracellular entrance to the aqueous translocation pathway,11 as has been reported for other solute transporters.12,13 The proximity of residues in these helices was established by cross-linkages between cysteine-substituted residues within this region of the gate.11 One of those residues was Asn411. Subsequent to this report, this laboratory learned of an infant diagnosed with HFM with a point mutation in PCFT resulting in the substitution of lysine at Asn411. The mutant protein was expressed, stable, and localized to the plasma membrane. Although there was a marked decrease in PCFT function, there was sufficient residual activity to characterize the nature of the defect and the functional consequences and to elucidate the structural requirements at this site within the external gate region.
Methods
Chemicals
[3H]methotrexate (MTX), [3H]pemetrexed, [3H]folic acid, [3H]5-formyltetrahydrofolic acid (5-formylTHF), and [3H]5-methyltetrahydrofolate (5-methylTHF) were purchased from Moravek Biochemicals (Brea, CA). Nonlabeled pemetrexed was purchased from LC Laboratories (Woburn, MA). Nonlabeled MTX and folic acid were purchased from Sigma-Aldrich (St. Louis, MO); 5-formylTHF and 5-methylTHF were purchased from Schircks Laboratories (Jona, Switzerland). Sulfosuccin-imidyl-6-(biotinamido) hexanoate (EZ-Link sulfo-NHS-LC biotin) and streptavidinagarose beads were obtained from Fischer Scientific (Pittsburgh, PA), and protease inhibitor cocktail was obtained from Roche Applied Science (Mannheim, Germany). The sulfhydryl reactive reagents N-biotinylaminoethyl methanethiosulfonate (MTSEA-biotin), methanethiosulfonate-ethyltrimethylammonium (MTSET) and methanethiosulfonate-ethylsulfonate (MTSES) were obtained from Biotium (Hayward, CA).
Cell lines and culture conditions
The PCFT and reduced folate carrier (RFC) null cell line, HeLa R1-11, used for these studies14,15 was maintained in RPMI 1640 medium containing 10% fetal bovine serum (Gemini Bio-Products, Irvine, CA), 100 U/mL penicillin, and 100 µg streptomycin under 5% CO2. Cells (3 × 105) were seeded in 17-mm glass vials for transport measurements; 5 × 105 cells/well were seeded for western blot analyses. After 48 h, cells were transfected with 0.8 µg of DNA/vial of wild-type PCFT (PCFT-WT) and mutants for transport studies, and 1.6 µg of DNA/well for western blot analyses. Lipofectamine 2000 was the transfection reagent (Invitrogen, Carlsbad, CA) in antibiotic-free RPMI 1640.
Site-directed mutagenesis
All Asn411 amino acid substitutions were individually generated using the Quickchange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). PCFT-WT, cloned in pcDNA3.1(+) and containing a C-terminus HA (hemagglutinin) tag, was used as the template.
Influx measurements
Transport was studied 2 d posttransfection using a variety of PCFT substrates. [3H]MTX is used for these studies because of its stability, low cost, robust transport, and sustained initial uptake rates.16 [3H]pemetrexed is a very-high-affinity PCFT substrate especially useful for studying mutant forms of PCFT with decreased transport activity and because its binding properties differ in some respects from natural folates and MTX.5,6,17 Influx measurements were made over 1 min at 37°C, as has been previously described.18,19 Most experiments were performed at the optimal pH for this transporter, 5.5, in MBS buffer [20 mM 2-(N-morpholino)ethanesulfonic acid, 140 mM NaCl, 5 mM KCL, 2 mM MgCl2, and 5 mM dextrose]. To assess pH-dependence, we adjusted (i) the MBS buffer to pH 5.5, 6.0, and 6.5 and (ii) the HBS buffer [20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 140 mM NaCl, 5 mM KCL, 2 mM MgCl2, and 5 mM dextrose] to pH 7.0 and 7.4. The cells were then processed for protein and radioactivity, as has been previously described.18,19
Analysis of PCFT protein expression at the cell surface
Cell surface expression was determined by biotinylation of accessible PCFT lysine residues with EZ-Link sulfo-NHS-LC, as has been previously described in detail.10,11 Briefly, washed cells were treated with 1 mg/mL of EZ-Link sulfo-NHS-LC for 30 min at room temperature (RT). All subsequent steps were at 4°C. After washing with HBS, we added hypotonic buffer (0.5 mM NaHPO4 and 0.1 mM EDTA, pH 7.0, containing protease inhibitors). After removal of cells from the plates and centrifugation, the cell pellet was resuspended in lysis buffer and equal portions mixed with 2X Laemmli buffer for analysis of PCFT expression in the crude membrane fraction. The remaining suspension was then mixed with prewashed streptavidin-agarose beads to pull-down PCFT at the cell surface, following which equal portions of 2X Laemmli buffer containing dithiothreitol were added, and the suspension heated to release the protein from the beads for western blot analyses. Protein samples were resolved on 4% to 12% polyacrylamide gels (BioRad), electroblotted onto polyvinylidine difluoride membranes (Millipore), and followed by blocking with 10% nonfat milk in Tris-buffered saline, as has been previously described.10,11 The blots were probed with anti-HA primary antibody (Sigma, St. Louis, MO) at RT, followed by treatment with HRP-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA). Actin, the internal loading control, was probed with monoclonal anti-β-actin antibody (Sigma, St. Louis, MO) and HRP conjugated anti-mouse antibody (Cell Signaling Technology, Danvers, MA). Blots were developed with ECL Plus reagent (Perkin Elmer, Shelton, CT).
Homology models
Homology models of PCFT were developed, as has been described in detail,18 on the basis of the recently reported structures of the bovine (inward open) and rat (outward open) Glut5 fructose transporters as templates (Protein Data Bank codes 4YB9 and 4YBQ, respectively).20 These Glut5 transporters share 88% sequence identity with each other and 13% sequence identity to PCFT, similar to the level of identity of the glycerol-3-phosphate proton symporter previously used for homology modeling of PCFT.11,21 The resulting models were found to have comparable quality. The optimal alignment between PCFT and the Glut5 template structures and the subsequent molecular model was obtained from multiple mapping method alignment optimization and comparative modeling.22-24 The quality of the model was verified through energetic analysis using statistical pair potentials implemented in Prosa.25 Optimal superposition of the 2 models was obtained using the Align3D program of Modeler.26
Statistical analysis
Statistical analyses were performed with the GraphPad Prism 7 2-tailed paired Student t test.
Results
Case history
The male patient, with consanguineous parents of Moroccan ethnicity, had a history of failure to thrive with recurrent diarrhea, stomatitis, and anemia. Seizures began at age 10 months. Oral folic acid was begun at 14 months of age when the plasma folate was noted to be <1.5 nM. At age 15 months he developed status epilepticus, which was treated with levetiracetam, and was referred to the Department of Pediatrics, Academic Medical Center, University Hospital of Amsterdam. At that time the patient was febrile, dehydrated, with bullous stomatitis and mild-to-moderate global developmental delays (growth parameters −2.5 SD). The patient’s Hb was 7.7 g/dL, mean corpuscular volume was 103, white blood cell count was 5.9 ×109/L (14% neutrophils, 44% lymphocytes, 42% monocytes), and platelets were 529 × 109/L. Plasma immunoglobulins (Ig) were normal (IgG 9.0 g/L [reference, 3.3-11.6], IgM 0.82 g/L [reference, 0.4-1.7], IgA 0.3 g/L [reference, 0.1-1.0]). Plasma folate was 6.3 nM (reference, 7-39 nM). A magnetic resonance image of the brain showed cortical and occipital calcifications. Whole-exome sequencing of PCFT identified a c.1233C>G mutation, resulting in an N411K substitution. Both parents were heterozygotes for this mutation. An older brother was not genotyped but was considered normal. The patient was hydrated and treated with trimethoprim-sulfamethoxazole for Pneumocystis prophylaxis, and the antiseizure medication was continued. The folic acid was stopped, and the patient was started on oral leucovorin (d,l-5-formylTHF), which was gradually increased to 28 mg/kg per day; at that dose the cerebrospinal fluid (CSF) 5-methylTHF level was 11.5 nM (reference for age: 8-84 nM), and the peak folate blood level was 68 nM. The regimen was then changed to intramuscular sodium-leucovorin at 2 mg/kg per day. This resulted in a CSF 5-methylTHF level of 43 nM, and the peak folate blood level was 2240 nM. At age 2 years, the patient’s hemogram was normal, and he was seizure-free on levetiracetam; his growth and psychomotor development had normalized.
Expression and functional impact of the N411K PCFT mutation
The patient-derived N411K mutation was generated in a wild-type hemaglutini-tagged PCFT template. Function was assessed after transfection into a HeLa cell line, which lacks endogenous PCFT or RFC expression.14,15 Under these conditions, the background activity is negligible, so even very low levels of residual mutant PCFT activity can be detected and quantitated. Transport activity based upon influx measurements was assessed using as a folate surrogate 0.5 µM [3H]MTX at pH 5.5, the pH optimum of this transporter. As is indicated in Figure 1A, the N411K mutation resulted in a marked (∼90%) loss of transport activity; however, the level of protein in the crude membrane preparation and at the cell surface was comparable to that of PCFT-WT (Figure 1B). Hence, there was an intrinsic defect in the function of the mutant protein.
Expression and function of the N411K PCFT mutant. (A) Influx of 0.5 μM [3H] MTX was assessed over 1 min at 37°C in RFC-/PCFT-null HeLa R1-11 cells transfected with wild-type PCFT, the N411K mutant, or vector control. Data are indicated as percentage of PCFT-WT activity and are the average of 3 independent experiments ± SEM. (B) Expression at the cell surface is depicted in the upper row. PCFT expression in the crude membrane preparation is depicted in the lower row. The western blot is representative of 3 independent experiments.
Expression and function of the N411K PCFT mutant. (A) Influx of 0.5 μM [3H] MTX was assessed over 1 min at 37°C in RFC-/PCFT-null HeLa R1-11 cells transfected with wild-type PCFT, the N411K mutant, or vector control. Data are indicated as percentage of PCFT-WT activity and are the average of 3 independent experiments ± SEM. (B) Expression at the cell surface is depicted in the upper row. PCFT expression in the crude membrane preparation is depicted in the lower row. The western blot is representative of 3 independent experiments.
Structural requirements at the PCFT Asn411 residue
To understand the structural requirements at the Asn411 position, we generated a spectrum of amino acid substitutions, following which expression and function were assessed. As is indicated in Figure 2A, all the mutant proteins were expressed and trafficked to the plasma membrane. For studies of function, influx was assessed at 0.5 µM MTX, a level far below the influx Kt (the concentration at which influx is one half of maximum) and at 50 µM MTX, a saturating concentration an order of magnitude higher than the influx Kt, at which influx approximates the maximum rate (Vmax). As is illustrated in Figure 2B, substitutions with the like-charged arginine and histidine resulted in a loss of function slightly more than and similar to, respectively, that of N411K. Although transport function was fully retained with the negatively charged N411D substitution, it was substantially decreased with the addition of a single carbon to the side-chain with the N411E substitution. Yet function was preserved with the glutamine (polar) substitution with a similar bulk. Activity was also preserved with residues with comparable (leucine) or lesser (alanine) bulk. The loss of activity of the lysine, arginine, histidine, and glutamate mutants was greater at the low concentration than at the high concentration. Because a decrease in Vmax should produce the same proportional decrease in transport irrespective of the substrate concentration, the data indicated that these substitutions also resulted in an increase in the MTX influx Kt. For instance, for the N411K mutant, there was a 5-fold increase in the MTX influx Kt and a 2.5-fold decrease in Vmax. Likewise, the essentially unchanged influx at 0.5 µM MTX for N411A and N411Q, accompanying the increase in Vmax, is consistent with a modest increase in the MTX influx Kt. The unchanged rate at the saturating concentration for the N411T mutant along with a decrease in transport at the low concentration is consistent with a modest increase in the influx Kt, alone, for this substitution. Hence, the type of change in influx kinetics with substitutions at the Asn411 position depended on the properties of the specific replacement residue.
Structural requirement at the Asn411 residue. (A) Expression at the cell surface (upper row), and in the crude membrane fraction (middle row), of the Asn411 mutants. β-Actin was the internal control (lower row). The blot is representative of 3 independent experiments. (B) Influx of 0.5 and 50 μM [3H] MTX was measured in HeLa R1-11 cells transfected with a variety of substitutions at the Asn411 residue. Data are the averages ± SEM of 3 independent experiments. *P < .01; **P < .0001, in comparison with PCFT-WT activity.
Structural requirement at the Asn411 residue. (A) Expression at the cell surface (upper row), and in the crude membrane fraction (middle row), of the Asn411 mutants. β-Actin was the internal control (lower row). The blot is representative of 3 independent experiments. (B) Influx of 0.5 and 50 μM [3H] MTX was measured in HeLa R1-11 cells transfected with a variety of substitutions at the Asn411 residue. Data are the averages ± SEM of 3 independent experiments. *P < .01; **P < .0001, in comparison with PCFT-WT activity.
Impact of the addition of a bulky group to the N411C-PCFT mutant
To determine the impact of the addition of a bulky group at the 411 position, we generated an N411C mutant, which did not alter PCFT function. The substituted-Cys sulfhydryl group was then modified with MTSEA-biotin (molecular weight [MW] = 382, uncharged), MTSET (MW = 278, positive charge), or MTSES (MW = 242, negative charge). As is indicated in Figure 3, all the sulfhydryl additions markedly inhibited function, with somewhat greater inhibition by MTSET and MTSEA-biotin. Hence, the addition of a bulky group of either charge, or without a charge, at N411C impaired transport activity.
Effects of sulfhydryl modifications of the N411C PCFT mutant. Cells harboring the N411C PCFT mutation were treated with 3 mM MTSES, 3 mM MTSET, or 0.5 mM MTSEA-biotin for 30 min at pH 7.4 and RT followed by assessment of [3H]MTX influx. Data are percentages of untreated cells ± SEM, from the average of 3 independent experiments.
Effects of sulfhydryl modifications of the N411C PCFT mutant. Cells harboring the N411C PCFT mutation were treated with 3 mM MTSES, 3 mM MTSET, or 0.5 mM MTSEA-biotin for 30 min at pH 7.4 and RT followed by assessment of [3H]MTX influx. Data are percentages of untreated cells ± SEM, from the average of 3 independent experiments.
pH dependence of the N411K-PCFT mutant
PCFT-mediated transport is proton-coupled, and residues involved in proton binding to the protein and proton coupling have been identified. The PCFT mutation that decreased proton binding resulted primarily in an increase in influx Kt,27 whereas the mutation that impaired proton coupling resulted primarily in a decrease in influx Vmax.28 Influx over a pH range of from 5.5 to 7.4 for the N411K mutant was comparable to that of PCFT-WT, as is indicated in Figure 4 with the data normalized to the peak activity at pH 5.5 for both the N411K and wild-type transporters. Hence, mutation of Asn411 to Lys411 does not alter the pH dependence of the transporter excluding a role for this residue in either proton binding or proton coupling.
pH-dependence of [3H]MTX influx. Activities for the PCFT-WT and N411K PCFT mutant are normalized to the rate at pH 5.5, indicated as 100%. Data are the means ± SEM from 3 independent experiments.
pH-dependence of [3H]MTX influx. Activities for the PCFT-WT and N411K PCFT mutant are normalized to the rate at pH 5.5, indicated as 100%. Data are the means ± SEM from 3 independent experiments.
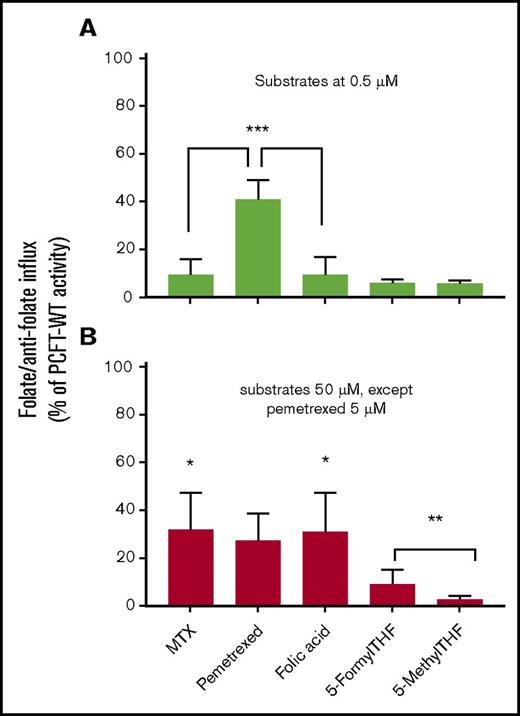
Substrate specificity of the N411K-PCFT mutant
To determine the extent to which the loss of activity of the N411K mutant might be substrate specific and might inform both treatment and the structural requirement at this site, we evaluated the transport activities of several folate compounds. These included 5-methylTHF, the physiological blood folate; 5-formylTHF, the folate-cofactor available for parenteral administration; and folic acid. The antifolates evaluated were MTX, a dihydrofolate reductase inhibitor, and pemetrexed, which in its polyglutamyl forms is an inhibitor of thymidylate synthase and, to a lesser extent, the enzymes required for 1-carbon additions to the purine ring.29 Influx was assessed at substrate concentrations below and far above the influx Kt for these substrates. As is indicated in Figure 5, at the high concentration, transport activity was comparable and best preserved for MTX, pemetrexed, and folic acid in relation to PCFT-WT. At the low concentration, a substantial portion of pemetrexed transport was preserved. However, transport of MTX and folic acid was very low, consistent with a high influx of Kt for these 2 substrates as is indicated for MTX in Figure 2B. Transport of 5-methylTHF was negligible at both concentrations. Although there was slightly greater preservation of transport activity at the high concentration for 5-formylTHF, it was still only 10% of PCFT-WT. Hence, the transport phenotype with mutations at this residue depend not only on the nature of the substituted residue but on the specific transport substrate as well.
Substrate specificity of transport mediated by the N411K PCFT mutant. Influx of tritiated PCFT substrates was assessed at a concentration far below the influx Kt for PCFT-WT, 0.5 µM (A) and at least an order of magnitude higher than the influx Kt for PCFT-WT, 50 µM (5 µM for pemetrexed) (B). Transport at the high concentration reflects the influx Vmax. Since a change in Vmax should result in a comparable proportional change in influx, irrespective of the substrate concentration, a discrepancy in the percentage change between the low and high substrate concentrations reflects an additional change in the influx Kt. The data shown are percentages of influx activity in relation to PCFT-WT ± SEM from 3 independent experiments. *Comparing the differences between the percentage reduction in transport of MTX (P < .01), pemetrexed (not significant), and folic acid (P <. 01) at the low vs high concentrations. **Comparing the difference between 5-formylTHF and 5-methylTHF influx at the high concentration (P < .05). ***Comparing influx of MTX (P < .005) and folic acid (P < .005) with transport of pemetrexed at the low substrate concentration.
Substrate specificity of transport mediated by the N411K PCFT mutant. Influx of tritiated PCFT substrates was assessed at a concentration far below the influx Kt for PCFT-WT, 0.5 µM (A) and at least an order of magnitude higher than the influx Kt for PCFT-WT, 50 µM (5 µM for pemetrexed) (B). Transport at the high concentration reflects the influx Vmax. Since a change in Vmax should result in a comparable proportional change in influx, irrespective of the substrate concentration, a discrepancy in the percentage change between the low and high substrate concentrations reflects an additional change in the influx Kt. The data shown are percentages of influx activity in relation to PCFT-WT ± SEM from 3 independent experiments. *Comparing the differences between the percentage reduction in transport of MTX (P < .01), pemetrexed (not significant), and folic acid (P <. 01) at the low vs high concentrations. **Comparing the difference between 5-formylTHF and 5-methylTHF influx at the high concentration (P < .05). ***Comparing influx of MTX (P < .005) and folic acid (P < .005) with transport of pemetrexed at the low substrate concentration.
Pemetrexed influx kinetics for the N411K-PCFT mutant
The observation that the retention of transport activity for pemetrexed was at least as great at the low concentration as at the high concentration suggested that the low Vmax was not accompanied by an increase in Kt. To assess this further, we documented influx kinetics for pemetrexed. This drug has a very low influx Kt, reflecting a very high affinity for PCFT-WT. This maximizes activity at low substrate concentrations and allows saturation of the carrier even under conditions at which the influx Kt of the mutant protein is substantially increased. As is indicated in Figure 6, though there was a ∼38% decrease in the influx Kt that did not reach statistical significance, there was a 7.5-fold decrease in the influx Vmax. Hence, with pemetrexed as substrate, the major alteration in carrier function was a marked decrease in the rate of oscillation of the carrier between its conformational states.
Pemetrexed influx kinetics. Influx of 0.05 to 5.0 µM [3H]pemetrexed was assessed in cells transfected with either PCFT-WT or the N411K mutant. Data are best fit to the Michaelis-Menten equation V = Vmax[S]/(Kt + [S]), where [S] is extracellular substrate concentration, Vmax is the maximum transport rate, and Kt is the concentration at which influx is one half of maximum. Data shown are the means ± SEM from 3 independent experiments.
Pemetrexed influx kinetics. Influx of 0.05 to 5.0 µM [3H]pemetrexed was assessed in cells transfected with either PCFT-WT or the N411K mutant. Data are best fit to the Michaelis-Menten equation V = Vmax[S]/(Kt + [S]), where [S] is extracellular substrate concentration, Vmax is the maximum transport rate, and Kt is the concentration at which influx is one half of maximum. Data shown are the means ± SEM from 3 independent experiments.
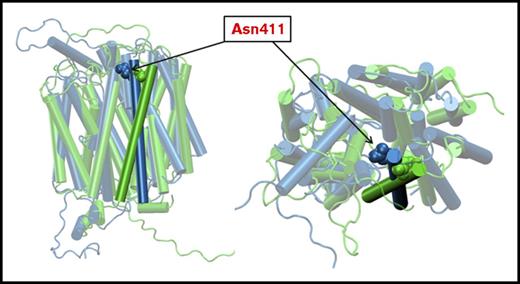
Analysis of a homology model of PCFT in the inward and outward open conformations
Recently, the structures of the GLUT5 fructose transporters were obtained in both the inward and outward conformations.20 On the basis of these structures, we built a homology model for PCFT in these 2 conformations.18 In Figure 7, the 11th transmembrane helix in the inward open conformation, along with the Asn411 residue, are highlighted in blue. The position of the helix in the outward open conformation, and the Asn411 residue, are highlighted in green. It can be seen that when the protein is outward open, Asn411 points into the aqueous pathway, but there is a large shift further into the pathway when the protein is in the inward open conformation. This displaces Asn411 toward other helices and residues that could result in hydrophobic or charge interactions that impair or enhance PCFT function. For instance, in the inward open conformation, a contact analysis predicts potential Asn411 interactions with 6 proximal residues on other helices. While in the outward open conformation, Asn411 interacts with only 2 residues in other helices. Hence, replacement of Asn411 with other residues provides a spectrum of potential new interactions, or interruption of interactions, depending on the properties of the replacing residue. Beyond this, it is possible that alterations in interactions between the Asn411-substituted residues in the same helix could affect function.
Homology modeling of PCFT. Homology models of PCFT based on the reported structures of the bovine and rat fructose transporters, Glut5, in the inward open and outward open conformations, respectively.18,20 The 11th TMD with the Asn411 residue is highlighted. The predicted structure in the inward open configuration is indicated in blue. The predicted structure in the outward open conformation is indicated in green. (Left) A planar view of the protein. (Right) A view from the extracellular compartment into the aqueous translocation pathway. The Asn411 residue protrudes into the aqueous pathway and shifts further into the pathway when the protein is in the inward open conformation.
Homology modeling of PCFT. Homology models of PCFT based on the reported structures of the bovine and rat fructose transporters, Glut5, in the inward open and outward open conformations, respectively.18,20 The 11th TMD with the Asn411 residue is highlighted. The predicted structure in the inward open configuration is indicated in blue. The predicted structure in the outward open conformation is indicated in green. (Left) A planar view of the protein. (Right) A view from the extracellular compartment into the aqueous translocation pathway. The Asn411 residue protrudes into the aqueous pathway and shifts further into the pathway when the protein is in the inward open conformation.
Discussion
The identification of subjects with HFM is based on the presence of a mutation in the PCFT gene that produces a defect in protein expression, function, or both, sufficiently severe that the ensuing degree of folate deficiency leads to clinical presentation and diagnosis. The presence of only 1 functional PCFT allele does not result in clinical signs of the disorder.1-4 In the current case, many types of substitutions at the Asn411 position were tolerated. None affected protein stability or trafficking to the cell membrane, and there were only a few mutations at this site that produced sufficient functional impairment to result in clinical signs of this disorder. Beyond the lack of tolerability of all positive charged and a negative charged substitutions (N411E), activity was largely preserved irrespective of whether the residue was polar, hydrophobic, small, or bulky, or small with a strong negative charge (N411D). However, the addition of a bulky sulfhydryl-linked group to the N411C mutant impaired function, irrespective of the charge or size of the added moiety. Taken together, the structural requirement at the Asn411 site is complex and difficult to categorize.
The Asn411 residue, per se, is not required for function. Rather, it is located in a region that can be perturbed due to steric and electrostatic effects that are very specific to the substituted residue. The location of this residue in the 11th transmembrane helix, which undergoes rapid, large shifts as the external gate opens and closes, might explain the complexity of the impact of structural changes at this site. These effects can perturb access to the flow of folate substrates through the aqueous pathway and impede the flexibility of the protein as it oscillates between its conformational states. Likewise, the substrate specificity of the N411K mutant may be related to allosteric alterations in the folate-binding pocket when the conformation of the external gate is distorted. It is of interest that the affinity of the protein for all folate/antifolate substrates tested was decreased, except for pemetrexed, for which it was unchanged. This pattern has been observed for other PCFT mutants, one of which, P425R, was detected in a subject with HFM.5 In that case, the influx Kt was increased and the Vmax decreased for MTX while both parameters were decreased for pemetrexed, consistent with a substantial difference in how MTX and pemetrexed bind to the protein.
Because the mutated PCFT in this case had residual function, it was possible to assess the substrate specificity of the mutant for the possibility that this might inform the most optimal treatment. Interestingly, folic acid retained about 30% of wild-type transport activity at a saturating concentration (orders of magnitude higher than could be achieved clinically), whereas 5-formylTHF was a very poor substrate. The use of folic acid for the treatment of HFM has been discouraged because this agent binds tightly to folate receptors and might interfere with 5-methylTHF transport across the choroid plexus, which is a folate receptor-α- and PCFT- dependent process.2,30,31 Hence, mutations of folate receptor-α, which result in loss of function of the protein, result in severe cerebral folate deficiency, but unlike that in HFM, intestinal folate absorption and folate blood levels are normal.32,33 Although 5-methylTHF is the major blood and tissue folate, and a formulation is available for oral use, it was the poorest substrate for the N411K mutant. At this point, 5-formylTHF administered parenterally and, preferably, in the active isomer form if available (PCFT is stereospecific), is considered to be the optimal folate for treatment.2,3 The intestinal absorption of 5-formylTHF in this patient, at the high doses administered, likely mediated by the reduced folate carrier or another process, was sufficient to achieve only slightly increased folate blood levels in this patient. The challenge, irrespective of the folate type and mode of administration, is achieving adequate CSF folate levels.1-3,34
HFM is a very rare disorder, and its presentation is often confusing. Hence, the diagnosis of HFM and its treatment are often delayed,3 as was the case with this child. Perhaps an element in this child’s ability to sustain this degree of folate deficiency, beyond the neurological and developmental consequences, was the absence of hypoimmunoglobulinemia. This adds a life-threatening infectious element that often accompanies HFM, predisposing in particular to Pneumocytis jerovici pneumonia, usually after treatment with folate is initiated. Trimethoprim-sulfamethoxazole is administered to prevent this complication under these conditions.1-3,35 This child did present with neutropenia, an unusual consequence of this disorder, but this was not, apparently, clinically significant in this case.
The very low CSF 5-methylTHF levels despite the achievement of very high blood folate levels is typical of this disorder; rarely do CSF levels go beyond 40 to 50 nM, irrespective of the folate blood level.34 In this case, even when the peak blood folate level was 2242 nM, the CSF 5-methyltHF concentration was 1/50th at 43 nM. Although CSF 5-methylTHF levels of 40 to 50 nM are sufficient in adults, this is far lower than the much higher CSF 5-methylTHF levels in heathy infants and toddlers.36,37 Hence, the choroid plexus represents a very tight barrier for folates in the absence of either PCFT or FRα. Despite the achievement of very high blood levels, arterial delivery via the vascular-blood-brain barrier to the apical ventricular and the inner subventricular zones, where neural progenitor cells are located,38 is inadequate to achieve folate sufficiency in this region of the brain and generate appreciable levels of CSF folate. Folate starvation in this region is likely the cause of the neurological complications of this disorder, which can ultimately result in seizures for which antiepileptic drugs are required. Although some antiepileptic drugs appear to impair intestinal folate absorption,39 others do not, such as the levetiracetam used to treat this patient. This is not a factor when patients are treated with parenteral folates.
Acknowledgments
The authors acknowledge the contributions that Karen van den Hurk, Department of Clinical Chemistry, Onze Lieve Vrouwe Gasthuis General Hospital, Amsterdam, The Netherlands, and Clara van Karnebeek, Department of Pediatrics, Division of Metabolic Disorders, Academic Medical Centre, University Hospital of Amsterdam, made in the diagnosis and management of this patient.
This work was supported by the National Institutes of Health, National Cancer Institute grant CA082621, and National Institutes of Health, National Institute of General Medical Sciences grant GM118709.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.A., R.Z., A.F., and I.D.G. designed the experiments, interpreted the results, and prepared the manuscript; S.A. performed the experiments; A.F. performed the homology modeling; C.L. provided medical care to the patient and contributed to the clinical component of the manuscript; S.M.I.G. was instrumental in performing, and the interpretation of the studies that led to the diagnosis of hereditary folate malabsorption in this patient. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. David Goldman, Medicine and Molecular Pharmacology, Charin Building, Room 209, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: i.david.goldman@einstein.yu.edu.


![Figure 1. Expression and function of the N411K PCFT mutant. (A) Influx of 0.5 μM [3H] MTX was assessed over 1 min at 37°C in RFC-/PCFT-null HeLa R1-11 cells transfected with wild-type PCFT, the N411K mutant, or vector control. Data are indicated as percentage of PCFT-WT activity and are the average of 3 independent experiments ± SEM. (B) Expression at the cell surface is depicted in the upper row. PCFT expression in the crude membrane preparation is depicted in the lower row. The western blot is representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/1/10.1182_bloodadvances.2017012690/3/m_advances012690f1.jpeg?Expires=1765887260&Signature=iCwDtk1AeGG2zH2P9jW1vLyd6EtC4O4vlnEGLBw2vRK61dHLditK4J3-oaWcPNly682UcrCQoKjPUmgsl36vdNDE~SkI-73ITubwPTTDgKGUZQFYUFkHiSuoBTj9djX6bZx6oz2RocQTJ~tvHt~7-Oc-kjwzvswht-~ZZqmXfEblFWs3QnQ6ANfOn9GVRIAxsKAQ85Fz14NDPsZpwx1G9v1rW-1urk6BRzcEXHQbTEzZB2czx2pDtl6lIIZ8C~RukyK0AE7JxE9gAG3cXktzLBB1VbwTfsuKhg~TjLD5K0Ay3KGiaZ9HBO34wh2O0RGO21jVfIefB1pe7smwtr93nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Structural requirement at the Asn411 residue. (A) Expression at the cell surface (upper row), and in the crude membrane fraction (middle row), of the Asn411 mutants. β-Actin was the internal control (lower row). The blot is representative of 3 independent experiments. (B) Influx of 0.5 and 50 μM [3H] MTX was measured in HeLa R1-11 cells transfected with a variety of substitutions at the Asn411 residue. Data are the averages ± SEM of 3 independent experiments. *P < .01; **P < .0001, in comparison with PCFT-WT activity.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/1/10.1182_bloodadvances.2017012690/3/m_advances012690f2.jpeg?Expires=1765887260&Signature=ekZzAtH63gX8f52SFWGt19XxEdAhOSt~wudXaJKgkJvJ23iUbmOrBj3cgqSakLC06OHEUFG-E~y5xfXD7WSjJsR6byyZhr~9Aap9MXiw2oTRSZqeM8tsUXJRELxxHFsrP526zbUTFdVrqWZj~UGKJJZL3fa5Gf-PFrC8nQCDAzFMJ1Z2Rw1o3PLvEiTBIPJnQqJtJelq86Tm5OQMIX7SiMnpJqK0OdGvheioOXJ7RngmA2tN0X3l976lz~WdklhxL8tYBLrj5NPMyfly9xR2okP5Lrf4KizRRqar-q-qIHGmi9I5Eny2R261jjjbfnycQNFZ2y6VzQJtBtZGP2P-qg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Effects of sulfhydryl modifications of the N411C PCFT mutant. Cells harboring the N411C PCFT mutation were treated with 3 mM MTSES, 3 mM MTSET, or 0.5 mM MTSEA-biotin for 30 min at pH 7.4 and RT followed by assessment of [3H]MTX influx. Data are percentages of untreated cells ± SEM, from the average of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/1/10.1182_bloodadvances.2017012690/3/m_advances012690f3.jpeg?Expires=1765887260&Signature=XkZd0lwd25oY3-9oEA4j9J6I2JcZf6PyQMgVOxy0CDDiOJPgYnbOeNmGxBfGOJRFKbrJo5qyrpDC8rnVz5thp7Yc00jwVSBNN0SYfuaeo3oFwMBWiKCZaPDx9HCHR2Z285E5B7wF8~HAc03p7pFJGJ3hoUnDNBZygoYrGxdSFUH6z3fWcqq0NHAgRTJCaNKGy5C9BCKc~lX0s0evGQtJa0nrAin8k5o8Au368mzIURqlbRu6CLb6mM6sTrvG1iHDNWfIeo0ApnaZbN~m75XLe7HFEDbUdXTCsN1Ksym5derCYtiUpd-va1VlsjYr28sUoVzoazbHboFfzPz8rpjdTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. pH-dependence of [3H]MTX influx. Activities for the PCFT-WT and N411K PCFT mutant are normalized to the rate at pH 5.5, indicated as 100%. Data are the means ± SEM from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/1/10.1182_bloodadvances.2017012690/3/m_advances012690f4.jpeg?Expires=1765887260&Signature=r0k9dz-QtoH5sVGMp4Rp1tkZkycldA5wgKk11e8wEHotiFiBCxShyyy7eg3jS68UaYnvU6UG~u223a0NnXnGqKLmgO2CbI5uvI053r8P8o3VdUTaZnP70oXlmqWYIF-w-SOucaLfbjG9FCAxFCsLTTckrQgMDIiLx6lj056jMNaF11AEp5-8PFh22g-Qw1Vxdjwba5M3MYeyHe9R4N30zI6rQ8c1WC3biKt62bPPYf3EhcqUJ7oXjFsa~9KC5DC66BXi3NWmFe6ukHpEFqdye0vbgCWbAcTSeIW785oX9CTgUDaixQntEosVdDNn9mTG7JJ8cNPacgW3ZpH9CfO3dA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Pemetrexed influx kinetics. Influx of 0.05 to 5.0 µM [3H]pemetrexed was assessed in cells transfected with either PCFT-WT or the N411K mutant. Data are best fit to the Michaelis-Menten equation V = Vmax[S]/(Kt + [S]), where [S] is extracellular substrate concentration, Vmax is the maximum transport rate, and Kt is the concentration at which influx is one half of maximum. Data shown are the means ± SEM from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/1/10.1182_bloodadvances.2017012690/3/m_advances012690f6.jpeg?Expires=1765887260&Signature=xap5isOM1nndKv3kDn12sM9-CYxud0g~KuvzjvYz9RfclIDtNlojnCVlwSdFNJPbjmDnUVYHPHtD8D-vZyxVceth8mURULqdaLVgCx4q-qnbnL~G6RPMNc75RH7JRfliA0DXvbdQapwddL3GIQvq-gncM4px9rd1qwAAXmMHMlXks3XKHVD~4pDcZORugK6YrzwScXZCzGL5xk5pvJMagPu5pPb1Ru94Ww~3OTMecnfwuYuFZ9QvxhHV-LifYTLCD6dDsIL3cUY7tNApVDwhJKH2R~n57VFJBXvqTFzaE64EiEd-2AjXv7jIHtoiwmH~-Du599gN5-ERiWZszdpyCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)