Key Points
Improved assays to detect intron 22 and intron 1 inversions in the F8 gene have been developed.
These assays can efficiently detect or rule out the most common genetic mutations resulting in hemophilia A.
Abstract
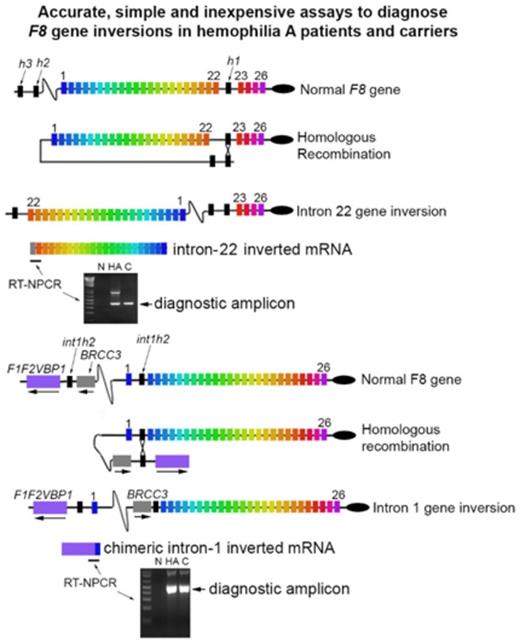
The most frequent mutations resulting in hemophilia A are an intron 22 or intron 1 gene inversion, which together cause ∼50% of severe hemophilia A cases. We report a simple and accurate RNA-based assay to detect these mutations in patients and heterozygous carriers. The assays do not require specialized equipment or expensive reagents; therefore, they may provide useful and economic protocols that could be standardized for central laboratory testing. RNA is purified from a blood sample, and reverse transcription nested polymerase chain reaction (RT-NPCR) reactions amplify DNA fragments with the F8 sequence spanning the exon 22 to 23 splice site (intron 22 inversion test) or the exon 1 to 2 splice site (intron 1 inversion test). These sequences will be amplified only from F8 RNA without an intron 22 or intron 1 inversion mutation, respectively. Additional RT-NPCR reactions are then carried out to amplify the inverted sequences extending from F8 exon 19 to the first in-frame stop codon within intron 22 or a chimeric transcript containing F8 exon 1 and the VBP1 gene. These latter 2 products are produced only by individuals with an intron 22 or intron 1 inversion mutation, respectively. The intron 22 inversion mutations may be further classified (eg, as type 1 or type 2, reflecting the specific homologous recombination sites) by the standard DNA-based “inverse-shifting” PCR assay if desired. Efficient Bcl I and T4 DNA ligase enzymes that cleave and ligate DNA in minutes were used, which is a substantial improvement over previous protocols that required overnight incubations. These protocols can accurately detect F8 inversion mutations via same-day testing of patient samples.
Introduction
Hemophilia A (HA) is an X-linked bleeding disorder that occurs in 1 of every 5000 males. The disease is caused by mutations of the factor VIII gene, F8, which is located in the Xq28 region and consists of 26 exons and 25 introns.1-3 The most frequent HA-causing mutations are either an intron 22 gene inversion (Inv22),4,5 which is responsible for ∼45% of severe HA cases, or an intron 1 gene inversion (Inv1),6 which is responsible for 2% to 3% of severe HA cases. In Inv22, the first 22 F8 exons become separated from exons 23 to 26 due to a recombination event during meiosis in germ cells.7 A 9.5-kb region in intron 22 (int22h1) undergoes homologous recombination with either of 2 extragenic homologs, int22h2 (proximal) or int22h3 (distal), which are located ∼500 kb and 600 kb upstream of the F8 gene, respectively8 (Figure 1). These 2 recombination events are referred to as type 1 and type 2 Inv22 mutations, respectively.9 In Inv1, an intragenic region in intron 1 (int1h1) of the F8 gene undergoes homologous recombination with int1h2, which is located ∼100 kb in the telomeric direction, between the genes VBP1 and BRCC3.10 Additional rare mutations involve partial F8 gene duplications combined with an inversion.11,12 Consistent with recommendations of Jenkins et al in 1994, hemophilia genotyping centers worldwide determine intron 22 inversion status as a first-line test for patients with severe HA and family members that may be carriers of or affected by the disorder.13 Determination of the HA-causing mutation (ie, “hemophilia genotyping”) is useful for the clinical management of patients and their families, helping both patients and providers determine appropriate clinical courses.
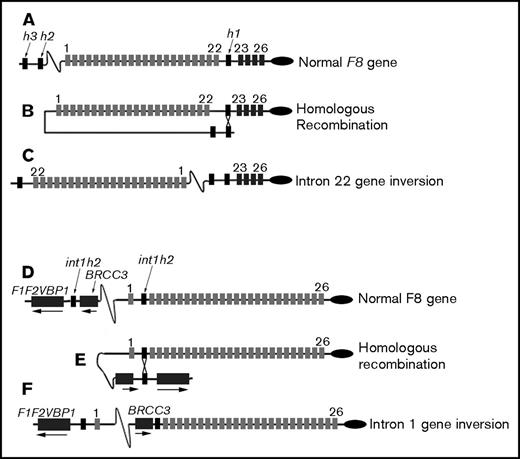
Schematic of F8 intron 22 and intron 1 inversion mutations. (A-C) Light gray rectangles indicate exons 1 to 22, dark gray rectangles indicate exons 23 to 26, and black rectangles indicate the homologous sequences int22h1, int22h2, and int22h3, which are abbreviated h1, h2, and h3, respectively. Introns and extragenic DNA are indicated by black lines. Exons and introns are not drawn to scale. (A) The normal F8 gene. (B) Homologous recombination between int22h1 and either int22h2 or int22h3 separates exons 1 to 22 from exons 23 to 26. (C) The inverted F8 gene. (D-F) Light gray rectangles indicate exons 1 to 26, dark gray rectangles indicate the facultative exons F1 and F2 and VBP1 and BRCC3 genes, and black rectangles indicate the homologous sequences int1h1 and int1h2. Introns and extragenic DNA are indicated by black lines.
Schematic of F8 intron 22 and intron 1 inversion mutations. (A-C) Light gray rectangles indicate exons 1 to 22, dark gray rectangles indicate exons 23 to 26, and black rectangles indicate the homologous sequences int22h1, int22h2, and int22h3, which are abbreviated h1, h2, and h3, respectively. Introns and extragenic DNA are indicated by black lines. Exons and introns are not drawn to scale. (A) The normal F8 gene. (B) Homologous recombination between int22h1 and either int22h2 or int22h3 separates exons 1 to 22 from exons 23 to 26. (C) The inverted F8 gene. (D-F) Light gray rectangles indicate exons 1 to 26, dark gray rectangles indicate the facultative exons F1 and F2 and VBP1 and BRCC3 genes, and black rectangles indicate the homologous sequences int1h1 and int1h2. Introns and extragenic DNA are indicated by black lines.
For many years, intron 22 inversion status was determined by Southern blotting,4,5 which, although accurate, is labor and time intensive and must be carried out in specialized laboratories. Modern testing utilizes inverse-shifting polymerase chain reaction (IS-PCR)14 and, less frequently, long-range PCR15,16 on DNA isolated from blood samples to determine patients’ intron 22 or intron 1 inversion status. These methods, especially when carried out in an accredited laboratory, are accurate and they represent the current standard of care in developed countries. Although widely accepted as diagnostic tests, they are time consuming and have proven somewhat difficult to standardize; therefore, there are still some inter- and intralaboratory variations. Next-generation sequencing may be used to detect inversion mutations; however, it is not yet used for routine clinical assays. RNA-based methods to detect Inv22 and Inv1 mutations10,17 have been used in research and have also been proposed for clinical genotyping of HA patients.18 These methods have been based on the presence or absence of PCR-amplified complementary DNA products spanning the normal exon 22 to 23 or exon 1 to 2 splice site, respectively.
The present study introduces a simple protocol in which RNA isolated from a whole blood sample or from peripheral blood mononuclear cells (PBMCs) is subjected to reverse transcription nested PCR (RT-NPCR) to detect specific amplicons indicating the subject does or does not have an Inv22 or Inv1 mutation. We also describe an improved IS-PCR assay that may be performed to diagnose an inversion mutation or as a second-line test if knowledge of the specific homologous recombination site for an Inv22 mutation is desired.5 Either or both of these assays, the first RNA based and the second DNA based, may be used for same-day testing of blood samples to identify inversion mutations in HA patients and carriers. The RNA-based assay requires only PCR amplification and the ability to run and visualize a stained agarose gel or minigel.
Materials and methods
This study was approved by institutional review boards at the University of Pittsburgh, Bloodworks NW, University of Washington, and the Uniformed Services University of the Health Sciences.
Human subjects
All subjects provided written, informed consent according to the Principles of Helsinki. PBMCs were from subjects enrolled in the “INHIBIT” feasibility study (National Heart, Lung, and Blood Institute [NHLBI] HL114674, M.V.R., principal investigator [PI]), the Grifols-funded study “Mechanisms of Immune Tolerance to factor VIII” (K.P.P., PI), or NHLBI 1RC2 HL101851 (T. Howard and K.P.P., PIs). Normal control buffy-coat samples were from the National Institutes of Health blood bank. PBMCs from 8 HA-Inv22, 4 carrier-Inv22, 2 HA-Inv1, 1 carrier-Inv1, and 16 non-HA control subjects were used as sources of RNA and DNA. RNA was also isolated from whole blood from 2 HA-Inv22, 1 carrier-Inv22, and 1 non-HA control subject.
Primer design
Primers for amplification of RNA (isolated from PBMCs or whole blood) were designed using DNASTAR software (DNASTAR, Inc., Madison, WI). The F8 RNA transcript sequence (National Center for Biotechnology Information reference sequence: NM_000132.3) was analyzed using the DNASTAR SeqBuilder program. All primer sequences were checked for specificity to their target sequences using BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast). For the Inv22 test, primers exon 22 forward 1 (exon 22 FWD1) and exon 24 reverse (Exon 24 REV) were designed to amplify a region spanning F8 exons 22 to 24, generating an expected 428-bp product. Internal primers exon 22 forward 2 (exon 22 FWD2) and exon 23 reverse (exon 23 REV) were designed to amplify a shorter F8 sequence spanning exons 22 to 23, generating an expected 225-bp product. To test for the presence of the 3′ end of the truncated F8 mRNA product (containing exons 1-22 plus additional bases within the intron 22 sequence) that is expressed only in individuals with an Inv22 mutation, primers exon 19 forward 1 (exon 19 FWD1) and intron 22 reverse (INT22 REV) were designed to amplify a F8 sequence spanning the exon 22 to intron 22 junction, generating an expected 390-bp product. Internal primers exon 19 forward 2 (exon 19 FWD2) and INT22 REV were designed to amplify a shorter F8 sequence spanning this same junction to generate an expected 378-bp product.
For the Inv1 RT-NPCR test, primers signal peptide forward (SP FWD) and exon 3 reverse (exon 3 REV) were designed to amplify a region spanning the F8 signal peptide sequence and exon 3, generating an expected 509-bp product. Internal primers SP FWD and exon 2 reverse (exon 2 REV) were designed to amplify a shorter sequence that also spans the F8 exon 1 to 2 junction, generating an expected 413-bp product. To test for the presence of the chimeric F8-VBP1 chimeric mRNA product (containing the F8 signal peptide sequence, 2 facultative exons, F8 Exon 1 and VBP1 gene exon sequences), which is expressed only in individuals with an Inv1 mutation, primer sets from Bagnall et al10 were used. Primer names and sequences, expected PCR products, and their diagnostic indications are listed in Table 1. Table 2 lists primers for the IS-PCR assays.
Oligonucleotide primer sequences and expected RT-NPCR amplicons
| Primer name | Sequence (5′-3′) | Nested PCR reaction (product size) | Diagnostic for |
| To test F8 exon 22-23 integrity and detect inverted F8 exon 22 to intron 22 mRNA product | |||
| Exon 22 FWD1 | GTGGATCTGTTGGCACCAATG | First PCR | Exon 22-23 junction |
| Exon 24 REV | CTCCCTTGGAGGTGAAGTCG | (428 bp) | |
| Exon 22 FWD2 | ACCAATGATTATTCACGGCATCAAGA | Second PCR | Exon 22-23 junction |
| Exon 23 REV | TGCAAACGGATGTATCGAGCAATAA | (225 bp) | |
| Exon 19 FWD1 | TCCAAAGCTGGAATTTGGCG | First PCR | Intron 22 inverted RNA |
| INT22 REV | CAATTCTTTCCATTTTCCAAGACACCGTG | (428 bp) | |
| Exon 19 FWD2 | TGCTGGGATGAGCACACTTTT | Second PCR | Intron 22 inverted RNA |
| INT22 REV | CAATTCTTTCCATTTTCCAAGACACCGTG | (378 bp) | |
| To test F8 Exon1-2 integrity and detect inverted F8 exon1-VBP1 chimeric mRNA product | |||
| SP FWD | GCACATCCAGTGGGTAAAGTTC | First PCR | Exon 1-2 junction |
| Exon 3 REV | AGACTGACAGGATGGGAAGC | (509 bp) | |
| SP FWD | GCACATCCAGTGGGTAAAGTTC | Second PCR | Exon 1-2 junction |
| Exon 2 REV | GGCCTTGGCTTAGCGATGTT | (413 bp) | |
| Exon 1 FWD1 | TGGGAGCTAAAGATATTTTAGAGAA | First PCR | F8-VBP1 chimeric RNA |
| VBP1 REV1 | ACTTATACTTCTGGTACTGTTCATCCAGC | (590 bp) | |
| Exon 1 FWD2 | GAATTAACCTTTTGCTTCTCCAGTTGAAC | Second PCR | F8-VBP1 chimeric RNA |
| VBP1 REV2 | TGTTTCATGAAGGAATCTAC | (500 bp) |
| Primer name | Sequence (5′-3′) | Nested PCR reaction (product size) | Diagnostic for |
| To test F8 exon 22-23 integrity and detect inverted F8 exon 22 to intron 22 mRNA product | |||
| Exon 22 FWD1 | GTGGATCTGTTGGCACCAATG | First PCR | Exon 22-23 junction |
| Exon 24 REV | CTCCCTTGGAGGTGAAGTCG | (428 bp) | |
| Exon 22 FWD2 | ACCAATGATTATTCACGGCATCAAGA | Second PCR | Exon 22-23 junction |
| Exon 23 REV | TGCAAACGGATGTATCGAGCAATAA | (225 bp) | |
| Exon 19 FWD1 | TCCAAAGCTGGAATTTGGCG | First PCR | Intron 22 inverted RNA |
| INT22 REV | CAATTCTTTCCATTTTCCAAGACACCGTG | (428 bp) | |
| Exon 19 FWD2 | TGCTGGGATGAGCACACTTTT | Second PCR | Intron 22 inverted RNA |
| INT22 REV | CAATTCTTTCCATTTTCCAAGACACCGTG | (378 bp) | |
| To test F8 Exon1-2 integrity and detect inverted F8 exon1-VBP1 chimeric mRNA product | |||
| SP FWD | GCACATCCAGTGGGTAAAGTTC | First PCR | Exon 1-2 junction |
| Exon 3 REV | AGACTGACAGGATGGGAAGC | (509 bp) | |
| SP FWD | GCACATCCAGTGGGTAAAGTTC | Second PCR | Exon 1-2 junction |
| Exon 2 REV | GGCCTTGGCTTAGCGATGTT | (413 bp) | |
| Exon 1 FWD1 | TGGGAGCTAAAGATATTTTAGAGAA | First PCR | F8-VBP1 chimeric RNA |
| VBP1 REV1 | ACTTATACTTCTGGTACTGTTCATCCAGC | (590 bp) | |
| Exon 1 FWD2 | GAATTAACCTTTTGCTTCTCCAGTTGAAC | Second PCR | F8-VBP1 chimeric RNA |
| VBP1 REV2 | TGTTTCATGAAGGAATCTAC | (500 bp) |
The protocol for both the reverse transcription PCR and the nested PCR steps of the Inv22 assay was as follows: 50°C, 30 min; 95°C, 15 min; 30 cycles of 94°C (30 s), 50°C (45 s), and 72°C (60 s) followed by a 10-min incubation at 72°C. The protocol for both the reverse transcription PCR and the nested PCR steps of the Inv1 assay was as follows: 50°C, 30 min; 95°C, 15 min; 30 cycles of 94°C (30 s), 59°C (45 s), and 72°C (60 s) followed by a 10-min incubation at 72°C.
Primers for IS-PCR tests to detect Inv1 and Inv22 mutations
| Primer name . | Sequence (5′-3′) . |
|---|---|
| 1U | CCTTTCAACTCCATCTCCAT |
| 2U | ACGTGTCTTTTGGAGAAGTC |
| 3U | CTCACATTGTGTTCTTGTAGTC |
| 1D | ACATACGGTTTAGTCACAAGT |
| ED | TCCAGTCACTTAGGCTCAG |
| 1-IU | GCCGATTGCTTATTTATATC |
| 1-ID | TCTGCAACTGGTACTCATC |
| 1-ED | GCCTTTACAATCCAACACT |
| Primer name . | Sequence (5′-3′) . |
|---|---|
| 1U | CCTTTCAACTCCATCTCCAT |
| 2U | ACGTGTCTTTTGGAGAAGTC |
| 3U | CTCACATTGTGTTCTTGTAGTC |
| 1D | ACATACGGTTTAGTCACAAGT |
| ED | TCCAGTCACTTAGGCTCAG |
| 1-IU | GCCGATTGCTTATTTATATC |
| 1-ID | TCTGCAACTGGTACTCATC |
| 1-ED | GCCTTTACAATCCAACACT |
PBMCs
PBMCs were harvested from blood samples by Ficoll-Paque PLUS underlay (GE Healthcare, Pittsburg, PA) and used immediately or frozen in 0.4% dimethyl sulfoxide Hybrid-Max (Sigma, St. Louis, MO) in 1mL of heat-inactivated fetal bovine serum (FBS; Gibco BRL, Green Island, NY) in liquid nitrogen. RNA yields from frozen cells could optionally be improved by culturing thawed cells for 24 hours before RNA isolation, as follows: Vials containing ∼10 million PBMCs were thawed at 37°C and then transferred into a 15-mL tube. Next, 2 mL of 50% heat-inactivated FBS in RPMI 1640 (Gibco BRL) containing Pen-strep (Gibco BRL) and 2 mM glutamate (Gibco BRL) was added drop-wise while gently mixing the cells. Then, 7 mL RPMI 1640 was slowly added to this mix and centrifuged at 329g for 8 min with the brakes off. After aspiration of the supernatant, the cells were resuspended in 10 mL of freshly prepared 5% FBS in RPMI 1640. Cells were centrifuged and resuspended in 10% FBS in RPMI 1640 and then counted using 0.45% Trypan blue (Gibco BRL) and the cell concentration was adjusted to 1 million cells per milliliter. Finally, 10 million PBMCs in 5 mL of 10% FBS in RPMI 1640 were seeded in 2 wells of a 6-well plate and cultured for 24 hours at 37°C in a 5% CO2 incubator for 24 hours.
RNA and DNA isolation from whole blood or PBMCs
Total cellular RNA from 2.5 mL whole blood cryopreserved in PAXgene RNA tubes (Applied Biosystems, Foster City, CA) or from PBMCs was purified using the RNeasy Plus Mini Kit (Qiagen, Gaithersburg, MD), with removal of contaminating genomic DNA using an RNase-free DNase Set (Qiagen) following the manufacturer’s protocols. Genomic DNA from whole blood or PBMCs was purified using the PureLink Genomic DNA mini kit (Invitrogen, Carlsbad, CA) as per the instruction manual. The purified nucleic acid concentrations were determined using a NanoPhotometer P-Class (Implen, Westlake Village, CA).
RT-NPCR
PCR runs were carried out on a GeneAmp PCR System 9700 (Applied Biosystems). DNase- and RNase-free distilled water was used for all reactions. The PCR mix, which was freshly made before each PCR run, consisted of 2 µL reverse transcriptase, 10 µL reaction buffer, and 2 µL nucleotide mix. For the first (outer) PCR reaction of each nested PCR experiment, 0.4 to 0.5 μg template RNA and the volumes of each outer primer needed for a final concentration of 1 µM were added to the PCR mix, and the total volume was brought to 50 µL with distilled water. For the second (inner) PCR reaction, 5 µL of the first PCR reaction product was added to a new PCR mix plus the volumes of each outer primer needed for a final concentration of 1 µM, and the total volume was brought to 50 µL with distilled water. Primers used for each RT-NPCR reaction are summarized in Table 1.
Agarose gel electrophoresis and complementary DNA sequencing
A total of 20 µL of each RT-NPCR product was run on a 2% agarose/tris acetate-EDTA gel (LE Agarose, BioExpress (Kaysville, UT) using a Smart mini gel electrophoresis system 1 (Luminous Biosciences, Rockville, MD). DNA bands were visualized by ethidium bromide (Fisher Scientific, Fair Lawn, NJ) staining, cut from the gel and purified using a QIAquick PCR purification kit (Qiagen). Sequencing was performed in the Uniformed Services University of the Health Sciences Biomedical Instrument Center Genomic Core facility.
Simplified IS-PCR
IS-PCR was performed as described elsewhere,14 with the following modifications. Digestion of 1 to 2 µg genomic DNA was carried out using a FastDigest Bcl I kit (Thermo Fisher Scientific) according to the supplier’s specifications for 5 min at 37°C. This step replaced the overnight incubation that was required using the earlier version of the Bcl I enzyme. The enzyme was then inactivated at 80°C for 20 min. DNA fragments were circularized using a Rapid DNA Ligation kit (Thermo Fisher Scientific) with T4 DNA ligase at 22°C for 15 min (DNA preconcentration by phenol-chloroform/ethanol precipitation was not required). A total of 5 µL of the circularized DNA product was subjected to PCR using polymerase from a One-Step RT-PCR Kit (Qiagen) with 1 µM concentration of each primer as shown in Tables 3 and 4. Then, 1U, 2U, 3U, 1D, and ED primers were used for Inv22 screening, while 1-IU, 1-ID, and 1-ED primers were used for Inv1 screening, as described previously.14 PCR cycling steps were as follows: 95°C (15 min); 35 cycles of (94°C [30 s], 56°C [1 min], and 72°C [1.5 min]) followed by a 2-min incubation at 94°C and 5-min incubation at 72°C. The IS-PCR amplified product solutions were loaded on 2% to 2.15% agarose gels and electrophoresed at 100 mV for 25 min using a Smart minigel electrophoresis system (Luminous Biosciences), and gel images were obtained on a FluorChem imager (ProteinSimple, San Jose, CA).
Expected molecular weights (bp) of amplicons from IS-PCR and RT-NPCR assays to detect intron 22 inversions
| F8 allele . | IS-PCR diagnostic Inv22 . | IS-PCR complementary Inv22 . | First RT-NPCR . | Second RT-NPCR . |
|---|---|---|---|---|
| Male | ||||
| N | 487 | 457; 405 | 225 | — |
| Inv22-1 | 333 | 559; 457 | — | 378 |
| Inv22-2 | 385 | 559; 405 | — | 378 |
| Inv22-3* | NR | NR | — | 378 |
| Dup 1-22* | 487 | 559; 457; 405 | 225 | 378 |
| Del 22-1* | 333 | 457 | — | — |
| Del 22-2* | 385 | 405 | — | — |
| Female (or male with >1 F8 gene or Xq28 segment) | ||||
| N/N | 487 | 457; 405 | 225 | — |
| N/Inv22-1 | 487; 333 | 559; 457; 405 | 225 | 378 |
| N/Inv22-2 | 487; 385 | 559; 457; 405 | 225 | 378 |
| N/Inv22-3* | NR | NR | 225 | 378 |
| N/Dup 1-22* | 487 | 559; 457; 405 | 225 | 378 |
| N/Del 22-1* | 487; 333 | 457; 405 | 225 | — |
| N/Del 22-2* | 487; 385 | 457; 405 | 225 | — |
| F8 allele . | IS-PCR diagnostic Inv22 . | IS-PCR complementary Inv22 . | First RT-NPCR . | Second RT-NPCR . |
|---|---|---|---|---|
| Male | ||||
| N | 487 | 457; 405 | 225 | — |
| Inv22-1 | 333 | 559; 457 | — | 378 |
| Inv22-2 | 385 | 559; 405 | — | 378 |
| Inv22-3* | NR | NR | — | 378 |
| Dup 1-22* | 487 | 559; 457; 405 | 225 | 378 |
| Del 22-1* | 333 | 457 | — | — |
| Del 22-2* | 385 | 405 | — | — |
| Female (or male with >1 F8 gene or Xq28 segment) | ||||
| N/N | 487 | 457; 405 | 225 | — |
| N/Inv22-1 | 487; 333 | 559; 457; 405 | 225 | 378 |
| N/Inv22-2 | 487; 385 | 559; 457; 405 | 225 | 378 |
| N/Inv22-3* | NR | NR | 225 | 378 |
| N/Dup 1-22* | 487 | 559; 457; 405 | 225 | 378 |
| N/Del 22-1* | 487; 333 | 457; 405 | 225 | — |
| N/Del 22-2* | 487; 385 | 457; 405 | 225 | — |
Del22-1 and Del 22-2, F8 exon 1-22 deletion involving 0.5 Mb from int22h-1 and the most centromeric of the homologs int22h-2, int22h-3. Dup 1-22, tandem duplication(s) of an F8 gene segment spanning int22h-1 to the most centromeric copy of int22h (int22h-2 or int22h-3). Inv1, intron 1 inversion; Inv22, intron 22 inversion; Inv22-3, type 3 inversions11,12 involve homologous recombination of int22h-1 with a duplicated int22h sequence (a rare genetic event, <1% of F8 inversion mutations); N, normal F8 gene; NR, not reported here, as this family of rare mutations would produce amplicons of various lengths.
Additional F8 gene mutations/rearrangements resulting from alternative pairings among the int22h homologs int22h-1, int22h-2, and int22h-3.14,24
Expected molecular weights (bp) of amplicons from IS-PCR and RT-NPCR assays to detect intron 1 inversions
| F8 allele . | IS-PCR diagnostic Inv1 . | First RT-NPCR . | Second RT-NPCR . |
|---|---|---|---|
| Male | |||
| N | 304 | 413 | — |
| Inv1 | 224 | — | 500 |
| Del 1-2 | — | — | — |
| Female (or male with >1 F8 gene or Xq28 segment) | |||
| N/N | 304 | 413 | — |
| N/Inv1 | 304; 224 | 413 | 500 |
| N/Del 1-2 | 304 | 413 | — |
| F8 allele . | IS-PCR diagnostic Inv1 . | First RT-NPCR . | Second RT-NPCR . |
|---|---|---|---|
| Male | |||
| N | 304 | 413 | — |
| Inv1 | 224 | — | 500 |
| Del 1-2 | — | — | — |
| Female (or male with >1 F8 gene or Xq28 segment) | |||
| N/N | 304 | 413 | — |
| N/Inv1 | 304; 224 | 413 | 500 |
| N/Del 1-2 | 304 | 413 | — |
Del 1-2, any F8 gene deletion that significantly alters or removes the exon 1 to 2 splice site sequences and that does not produce a chimeric F8 exon 1/VBP1 sequence. Other abbreviations are explained in Table 3.
Results
RT-NPCR
RNA was analyzed immediately or stored as 0.5-μg aliquots at −80°C. For all of the RNA samples, the absorbance (λ) = 260/280 nm ratio was between 2 and 2.2 and the λ = 260/230 nm ratio was between 2 and 2.2. Thus, highly pure total RNA was obtained. RT-NPCR assays were carried out for all subjects. Figure 2 shows representative results of Inv22 diagnostic assays. The 225-bp product indicating an intact exon 22 to 23 junction was amplified from all non-HA samples and from the 4 carrier-Inv22 samples, but not from 9 of the 10 HA-Inv22 samples. Interestingly, RNA from 1 HA-Inv22 subject amplified both the 225-bp and the 378-bp bands (not shown); DNA sequencing and IS-PCR indicated a partial F8 gene duplication in addition to an Inv22 mutation. The 378-bp amplicon produced only from F8 mRNA in which intron 22 was not spliced and removed was amplified from all HA-Inv22 and carrier-Inv22 samples, but not from any of the non-HA samples. DNA sequencing of the bands confirmed that they were indeed the expected amplicons. Figure 3 shows representative results of Inv1 diagnostic assays. The 413-bp product indicating an intact exon 1 to 2 junction was amplified from all carrier-Inv1 and non-HA control samples, but not from the 2 HA-Inv1 samples. The 500-bp amplicon produced only from F8 mRNA in which intron 1 was not spliced and removed was amplified from the 2 HA-Inv1 samples and the carrier-Inv1 sample, but not from the non-HA control samples. DNA sequencing of these bands confirmed that they were indeed the expected amplicons.
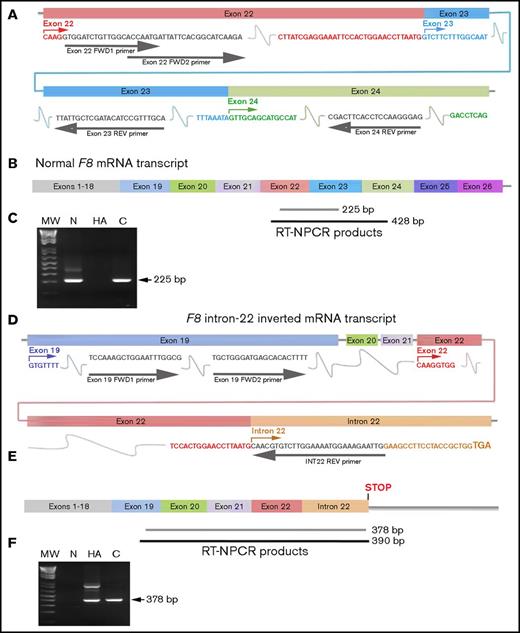
RT-NPCR to detect F8 intron 22 inversion mutations. (A) Schematic representation of exons 22 to 24 in WT F8 mRNA. Primers (gray arrows and font) were designed to hybridize within F8 exons 22 (pink), 23 (turquoise), and 24 (green). (B) WT-F8 mRNA transcript. RT-NPCR produces 428-bp (black line, often not visible on the gel) and 225-bp (gray bar) bands that are both diagnostic of an intact F8 exon 22 to 23 junction sequence. (C) RT-NPCR Inv22 test 1, representative result. MW, 1 kb plus DNA ladder (Invitrogen); N, HA, C, normal control, HA-Inv22, and carrier-Inv22 subjects, respectively. The 225-bp band is amplified from the N and C samples. (The weak ∼350-bp band seen in lane N is due to nonspecific binding of the primers.) (D) Schematic representation of F8 exons 19 to 22 plus part of the transcribed intron 22 sequence in F8 mRNA from an individual with an Inv22 mutation. Primers were designed to hybridize within F8 exon 19 (blue) and the F8 intron 22 sequence (orange). Note that 51 bases 3′ to the end of the exon 22 sequence, terminating in a TGA stop codon within intron 22, are transcribed as a consequence of the inversion mutation. (E) RT-NPCR produces 390-bp (black line, often not visible on the gel) and 378-bp (gray bar) bands that are both diagnostic of an unspliced F8 exon 22 to intron 22 sequence. (F) RT-NPCR Inv22 test 2, representative result. MW, molecular weight ladder; N, HA, C, normal control, HA-Inv22, and carrier-Inv22 subjects, respectively. The 378-bp band is amplified from the HA and C samples. (The weaker ∼750-bp band is due to nonspecific binding of the primers.)
RT-NPCR to detect F8 intron 22 inversion mutations. (A) Schematic representation of exons 22 to 24 in WT F8 mRNA. Primers (gray arrows and font) were designed to hybridize within F8 exons 22 (pink), 23 (turquoise), and 24 (green). (B) WT-F8 mRNA transcript. RT-NPCR produces 428-bp (black line, often not visible on the gel) and 225-bp (gray bar) bands that are both diagnostic of an intact F8 exon 22 to 23 junction sequence. (C) RT-NPCR Inv22 test 1, representative result. MW, 1 kb plus DNA ladder (Invitrogen); N, HA, C, normal control, HA-Inv22, and carrier-Inv22 subjects, respectively. The 225-bp band is amplified from the N and C samples. (The weak ∼350-bp band seen in lane N is due to nonspecific binding of the primers.) (D) Schematic representation of F8 exons 19 to 22 plus part of the transcribed intron 22 sequence in F8 mRNA from an individual with an Inv22 mutation. Primers were designed to hybridize within F8 exon 19 (blue) and the F8 intron 22 sequence (orange). Note that 51 bases 3′ to the end of the exon 22 sequence, terminating in a TGA stop codon within intron 22, are transcribed as a consequence of the inversion mutation. (E) RT-NPCR produces 390-bp (black line, often not visible on the gel) and 378-bp (gray bar) bands that are both diagnostic of an unspliced F8 exon 22 to intron 22 sequence. (F) RT-NPCR Inv22 test 2, representative result. MW, molecular weight ladder; N, HA, C, normal control, HA-Inv22, and carrier-Inv22 subjects, respectively. The 378-bp band is amplified from the HA and C samples. (The weaker ∼750-bp band is due to nonspecific binding of the primers.)
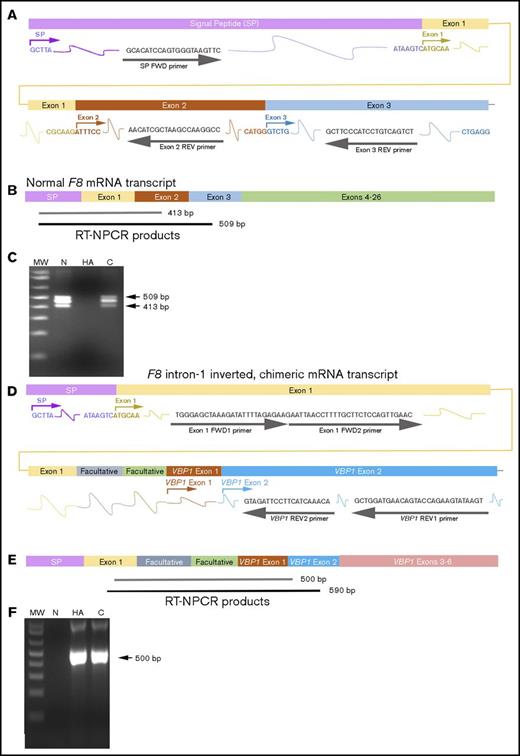
RT-NPCR to detect F8 intron-1 inversion mutations. (A) Schematic representation of wild-type F8 (WT-F8) mRNA from the signal peptide (SP) sequence through exon 3. Primers (gray arrows and font) were designed to hybridize within F8 signal peptide (SP) sequence (violet) and F8 exons 1 (yellow), 2 (brown), and 3 (light blue). (B) WT-F8 mRNA transcript. RT-NPCR produces 509-bp (black line, often not visible on the gel) and 413-bp (gray bar) bands that are both diagnostic of an intact F8 exon 1 to 2 junction sequence. (C) RT-NPCR Inv1 test 1, representative result. MW, molecular weight ladder; N, HA, C, normal control, HA-Inv1, and carrier-Inv1 subjects, respectively. The 413-bp band is amplified from the N and C samples. (The 509-bp outer RT-NPCR product is also seen on this gel.) (D) Schematic representation of the chimeric F8-VBP1 mRNA from an individual with an Inv1 mutation, showing the transcript from the F8 SP sequence to the 5′ region of the VBP1 gene. Primers were designed to hybridize within F8 exon 1 (yellow), and VBP1 exon 2 (turquoise). Note that 2 facultative exons between F8 exon 1 and VBP1 exon 1 are also contained in this chimeric transcript formed as a consequence of the inversion mutation. Outer primers exon 1 FWD and VBP1 REV1 were designed to amplify a 590-bp product. Inner primers exon 1 FWD2 and VBP1 REV2 were designed to amplify a 500-bp product. (E) RT-NPCR produces 590-bp (black line, often not visible on the gel) and 500-bp (gray bar) bands that are both diagnostic of the chimeric RNA resulting from an Inv1 mutation. (F) RT-NPCR Inv1 test 2, representative result. MW, molecular weight ladder; N, HA, C, normal control, HA-Inv1, and carrier-Inv1 subjects, respectively. The 500-bp band is amplified from the HA and C samples.
RT-NPCR to detect F8 intron-1 inversion mutations. (A) Schematic representation of wild-type F8 (WT-F8) mRNA from the signal peptide (SP) sequence through exon 3. Primers (gray arrows and font) were designed to hybridize within F8 signal peptide (SP) sequence (violet) and F8 exons 1 (yellow), 2 (brown), and 3 (light blue). (B) WT-F8 mRNA transcript. RT-NPCR produces 509-bp (black line, often not visible on the gel) and 413-bp (gray bar) bands that are both diagnostic of an intact F8 exon 1 to 2 junction sequence. (C) RT-NPCR Inv1 test 1, representative result. MW, molecular weight ladder; N, HA, C, normal control, HA-Inv1, and carrier-Inv1 subjects, respectively. The 413-bp band is amplified from the N and C samples. (The 509-bp outer RT-NPCR product is also seen on this gel.) (D) Schematic representation of the chimeric F8-VBP1 mRNA from an individual with an Inv1 mutation, showing the transcript from the F8 SP sequence to the 5′ region of the VBP1 gene. Primers were designed to hybridize within F8 exon 1 (yellow), and VBP1 exon 2 (turquoise). Note that 2 facultative exons between F8 exon 1 and VBP1 exon 1 are also contained in this chimeric transcript formed as a consequence of the inversion mutation. Outer primers exon 1 FWD and VBP1 REV1 were designed to amplify a 590-bp product. Inner primers exon 1 FWD2 and VBP1 REV2 were designed to amplify a 500-bp product. (E) RT-NPCR produces 590-bp (black line, often not visible on the gel) and 500-bp (gray bar) bands that are both diagnostic of the chimeric RNA resulting from an Inv1 mutation. (F) RT-NPCR Inv1 test 2, representative result. MW, molecular weight ladder; N, HA, C, normal control, HA-Inv1, and carrier-Inv1 subjects, respectively. The 500-bp band is amplified from the HA and C samples.
IS-PCR
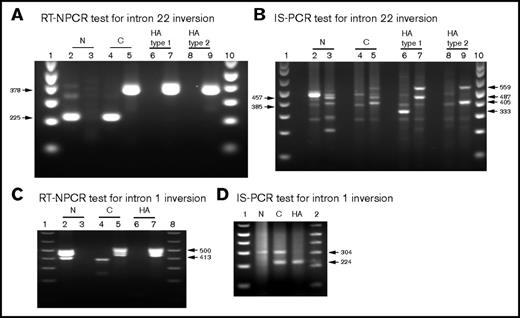
IS-PCR assays were carried out using DNA isolated from 11 of the subjects whose RNA was used for the RT-NPCR assays described above: 4 HA-Inv22 and 3 carrier-Inv22 subjects, 1 HA-Inv1 subject, 1 HA-Inv carrier, and 2 non-HA controls. Results of IS-PCR using the revised protocol described above were comparable to published results of assays using the classic IS-PCR method.14 In each case, the HA genotype determined by IS-PCR was consistent with that obtained using the RT-NPCR assay, indicating the RT-NPCR method is an acceptable alternative or corollary to IS-PCR genotyping to detect F8 gene inversion mutations. Figure 4 shows side-by-side comparisons of RT-NPCR and IS-PCR test results using RNA and DNA, respectively, from normal controls and from HA and carrier subjects with an Inv22 or Inv1 mutation.
Side-by-side comparison of RT-NPCR (RNA test) and IS-PCR (DNA test) results. See Tables 3 and 4 for an explanation of the bands visualized on these agarose gels. (A) Representative RT-NPCR to detect Inv22 mutations. Lane 1, molecular weight (MW) ladder; lanes 2 and 3, normal control (N) subject; lanes 4 and 5, carrier-Inv22 (C) subject with a type 2 Inv22 mutation; lanes 6 and 7, HA subject with a type 1 Inv22 mutation; lanes 8 and 9, HA subject with a type 2 Inv22 mutation. The 225-bp band is amplified from the N and C samples. The 378-bp band is amplified from the C and HA-Inv22 type 1 or type 2 mutation samples. (B) Representative IS-PCR to detect Inv22 mutations using DNA samples from the same subjects shown in panel A. Lanes 2, 4, 6, and 8 show diagnostic IS-PCR results for the N, C, HA-Inv22-type 1, and HA-Inv22-type 2 samples, respectively. Lanes 3, 5, 7, and 9 show complementary IS-PCR tests for the same samples. Lanes 1 and 10, MW ladder. (C) Representative RT-NPCR to detect Inv1 mutations. Lane 1, MW ladder; lanes 2 and 3, normal control (N) subject; lanes 4 and 5, carrier-Inv1 (C) subject; lanes 6 and 7, HA-Inv1 subject; lane 8, MW ladder. The 413-bp band is amplified from the N and C samples. The 500-bp band is amplified from the C and HA-Inv1 samples. (D) Representative IS-PCR to detect Inv1 mutations using DNA samples from the same subjects shown in panel C. Lanes 1 and 2, MW ladder; lanes N, C, and HA show IS-PCR results for the N, C, and HA-Inv1 subjects.
Side-by-side comparison of RT-NPCR (RNA test) and IS-PCR (DNA test) results. See Tables 3 and 4 for an explanation of the bands visualized on these agarose gels. (A) Representative RT-NPCR to detect Inv22 mutations. Lane 1, molecular weight (MW) ladder; lanes 2 and 3, normal control (N) subject; lanes 4 and 5, carrier-Inv22 (C) subject with a type 2 Inv22 mutation; lanes 6 and 7, HA subject with a type 1 Inv22 mutation; lanes 8 and 9, HA subject with a type 2 Inv22 mutation. The 225-bp band is amplified from the N and C samples. The 378-bp band is amplified from the C and HA-Inv22 type 1 or type 2 mutation samples. (B) Representative IS-PCR to detect Inv22 mutations using DNA samples from the same subjects shown in panel A. Lanes 2, 4, 6, and 8 show diagnostic IS-PCR results for the N, C, HA-Inv22-type 1, and HA-Inv22-type 2 samples, respectively. Lanes 3, 5, 7, and 9 show complementary IS-PCR tests for the same samples. Lanes 1 and 10, MW ladder. (C) Representative RT-NPCR to detect Inv1 mutations. Lane 1, MW ladder; lanes 2 and 3, normal control (N) subject; lanes 4 and 5, carrier-Inv1 (C) subject; lanes 6 and 7, HA-Inv1 subject; lane 8, MW ladder. The 413-bp band is amplified from the N and C samples. The 500-bp band is amplified from the C and HA-Inv1 samples. (D) Representative IS-PCR to detect Inv1 mutations using DNA samples from the same subjects shown in panel C. Lanes 1 and 2, MW ladder; lanes N, C, and HA show IS-PCR results for the N, C, and HA-Inv1 subjects.
Discussion
Many hemophilia patients, especially in developing countries, have limited options for diagnosis and treatment, although the World Federation for Hemophilia and other organizations are working to improve access to appropriate medical care. Genotyping of hemophilia patients and family members (ie, determining the hemophilia-causing mutation) is important to patients and their families, as well as to physicians who provide their clinical care.19,20 Genotyping can aid in clinical management by predicting the potential severity of the bleeding disorder and by indicating (albeit with less accuracy) the relative risk that patients may develop neutralizing anti–factor VIII antibodies.21 Because most HA carriers possess both a normal and a mutated X chromosome, these carriers have a 50% chance of transmitting the mutated copy to their children. Sons who inherit the variant X chromosome will be affected by HA, while daughters of a non-HA father and a carrier mother who inherit the variant gene from their mother will be carriers. Many family members wish to have their carrier status determined through genetic testing. We report here a set of simple and relatively inexpensive assays to detect Inv22 and Inv1 mutations, which are the causative mutation in approximately half of severe HA cases. These assays can provide same-day or next-day results, thereby improving the efficiency of diagnosing inversion mutations.
Most current inversion assays rely on either IS-PCR or long-range PCR of DNA samples. Both methods have been validated in accredited laboratories. However, the substantial time required to conduct these assays, and difficulties with amplifying products for the long-range PCR,14 indicated that diagnostic assays could be improved further. Several recent publications have reported alternative methods of identifying Inv22 mutations. A capillary gel electrophoresis system to detect PCR products has been described, which has sufficient sensitivity to distinguish between a 584-bp product (Inv22 positive) and a 512-bp product (Inv22 negative).22 Kumar et al have reported a quantitative reverse transcription PCR method that is diagnostic for Inv22 in HA patients. However, quantitative reverse transcription PCR without gel electrophoresis cannot be used to diagnose Inv22 carriers.18 Gau et al reported a method that used a different set of primers to amplify an intact exon 22 to 23 splice site PCR product23 ; a potential drawback to using this method is that lack of an exon 22 to 23 diagnostic band could also be caused by improper PCR conditions or other experimental artifacts.
Our RT-NPCR method offers several advantages to improve sensitivity and accuracy, specifically (1) nested PCR using the primers reported here allows reliable amplification of PCR products without requiring specialized equipment or more expensive reagents required for quantitative PCR, and (2) in addition to testing the integrity of exon 22 to 23 and exon 1 to 2 junction regions, the method includes a second set of RT-NPCR reactions to amplify specific products that are seen only in samples from HA patients and carriers who have an intron 22 or intron 1 inversion mutation, respectively. In other words, the diagnosis of an Inv22 or Inv1 mutation requires both the lack of a PCR band corresponding to the normal splice site (RT-NPCR assay 1) and the presence of a specific PCR product indicating the chimeric RNA region resulting from an inversion mutation (RT-NPCR assay 2). RNA from individuals without an inversion mutation will produce specific PCR products in the first, but not the second, of each pair of reactions. RNA from carriers who are heterozygous for the normal and mutated F8 allele will produce both the normal and mutated RT-NPCR products. As can be seen in Figure 4, these optimized RT-NPCR assays produce minimal off-target, nonspecific PCR products, making the analysis of agarose gels straightforward. Taking advantage of the availability of improved Bcl I and T4 DNA ligase enzymes, we also report that the “gold standard” IS-PCR assay may now be accomplished in a significantly shorter time than the previous iteration of this useful method.
Tables 3 and 4 list the expected amplicons produced following IS-PCR and the new RT-NPCR method to analyze DNA or RNA samples, respectively, from HA patients with a hemizygous F8 mutation and from carriers. Table 3 also includes the expected results for F8 gene duplications and deletions resulting from alternative pairings of int22-h1, int22-h2, and int22-h3, some of which can result in severe HA. The Xq28 region that includes F8 is highly polymorphic, with multiple possible duplications and deletions.24 Proper analysis of these rare variants requires DNA-based assays, including IS-PCR and copy-number variation analysis. The RNA-based assays described here can accurately detect the most common HA-causing F8 inversion mutations and some but not all of the alternative, rare int22h-mediated DNA rearrangements. As noted in “Results,” 1 of the 10 HA-Inv22 subjects analyzed here had an atypical Inv22 plus a partial F8 duplication mutation that was detected by RT-NPCR and confirmed by sequencing and IS-PCR. Other previously noted Xq28 rearrangements include an int22h1/int22h2-mediated duplication that is associated with cognitive impairment (X-linked intellectual disability).25,26 This mutation has not been associated with HA, presumably because the normal F8 gene is not directly affected. Similarly, Del22-1 and Del 22-2 mutations have been described in females with skewed X-chromosome inactivation but are not associated with a clinical phenotype, whereas they are embryonic lethal in males.
The RT-NPCR assays to diagnose an Inv22 mutation will produce neither the 225-bp nor the 378-bp amplicon in assays of male HA patients with a major F8 gene deletion that significantly alters or removes the exon 22 to 23 splice site sequences. Such a negative PCR result would suggest, but not prove, the presence of a F8 deletion mutation spanning the exon 22 to 23 junction region (because lack of the diagnostic amplicons could also result from incorrect PCR conditions). Similarly, the RT-NPCR assays to diagnose an Inv1 mutation will produce neither the 413-bp nor the 500-bp amplicon in assays of male HA patients with a major F8 gene deletion that significantly alters or removes the exon 1 to 2 splice site sequences.
We propose that the RT-NPCR method reported here may be used as an accurate and efficient means of screening for F8 gene inversion mutations. IS-PCR may be used as an alternative method or as a confirmatory step (eg, to distinguish between type 1 and type 2 Inv22 mutations). Both assays may be carried out in 1 or 2 days, allowing same-day or next-day diagnosis of patients. The relatively low cost of reagents and the rapidity of these assays should encourage their widespread use in HA genotyping, including in resource-poor environments where current costs may be prohibitive.
Acknowledgments
The authors thank Zhaozhang Li for primers and sequencing data.
This work was funded by National Institutes of Health, National Heart, Lung, and Blood Institute research grants 1R01-HL130448, 1RC2-HL101851, and U34-HL114674, and a hemophilia research grant from Grifols, Inc. The funders had no role in study design, data collection and analysis, or manuscript preparation.
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Authorship
Contribution: D.D. designed and performed experiments, analyzed data, and wrote the paper; K.P.P. designed experiments, analyzed data, wrote the paper; D.G. purified PBMCs and reviewed the paper; and M.V.R. enrolled subjects and reviewed the paper.
Conflict-of-interest disclosure: K.P.P. is an inventor on a patent application related to this study. The remaining authors declare no competing financial interests.
Correspondence: Kathleen P. Pratt, Department of Medicine A3075, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Rd, Bethesda, MD 20814; e-mail: kathleen.pratt@usuhs.edu.