Key Points
Human HSPCs expressing CD34 exhibit E-selectin binding activity, whereas those lacking CD34 do not.
CD34 is a unique E- and P-selectin ligand on human HSPCs that binds with kinetics comparable to other known selectin ligands.
Abstract
CD34 is routinely used to identify and isolate human hematopoietic stem/progenitor cells (HSPCs) for use clinically in bone marrow transplantation, but its function on these cells remains elusive. Glycoprotein ligands on HSPCs help guide their migration to specialized microvascular beds in the bone marrow that express vascular selectins (E- and P-selectin). Here, we show that HSPC-enriched fractions from human hematopoietic tissue expressing CD34 (CD34pos) bound selectins, whereas those lacking CD34 (CD34neg) did not. An unbiased proteomics screen identified potential glycoprotein ligands on CD34pos cells revealing CD34 itself as a major vascular selectin ligand. Biochemical and CD34 knockdown analyses highlight a key role for CD34 in the first prerequisite step of cell migration, suggesting that it is not just a marker on these cells. Our results also entice future potential strategies to investigate the glycoforms of CD34 that discriminate normal HSPCs from leukemic cells and to manipulate CD34neg HSPC-enriched bone marrow or cord blood populations as a source of stem cells for clinical use.
Introduction
Hematopoietic stem cells (HSCs) are rare cells that are maintained throughout life (self-renewing). They produce hematopoietic progenitor cells that differentiate into every type of mature blood cell within a well-defined hierarchy. Among hematopoietic stem/progenitor cell (HSPC) markers, CD34 is well known for its unique expression on HSPCs. For this reason, it is used to enrich donor bone marrow (BM) with HSPCs prior to BM transplantation.1 Although the role of CD34 as a marker of HSCs is under debate,2,3 recent studies suggest the existence of a population of dormant human HSCs that are CD34 negative (CD34neg) but become positive (CD34pos) just prior to cell division.4-8 Studying this negative population is challenging because a defined marker for its enrichment is still lacking and also because it demonstrates extremely poor homing and engraftment capabilities compared with its CD34pos counterpart.9-11 Studies of gene expression comparing lineage negative fractions of human peripheral blood HSPCs that either express the CD34 antigen or not imply that CD34neg HSPC subsets are more kinetically and functionally dormant, whereas CD34 expression in CD34pos HSPCs is related to cell cycle entry, metabolic activation, and HSPC mobilization and homing.12-15 However, a detailed explanation of how CD34 contributes to CD34pos HSPC engraftment into the BM remains unknown.
To date, the functional role of CD34 in migration has most clearly been understood in the context of recruitment of lymphocytes to specialized high endothelial venules16-18 that line the secondary lymphoid organs. Naive T cells home to these lymphoid organs in a multistep process that involves initial tethering and rolling interactions with CD34 (along with other ligands with restricted expression to high endothelial venules, often referred to as peripheral node addressins) mediated by the L-selectin expressed on the migrating T cells.16,17 In fact, ectopic expression of CD34 in murine T cells promoted their binding to human (but not mouse) BM stromal cells, suggesting that CD34 may bind a counterreceptor expressed on human BM endothelial cells to promote their homing.10 In support of this hypothesis, studies using CD34 knockout mice indicate that CD34 increases trafficking and migration of hematopoietic cells11,19 ; however, the precise mechanism is still not fully understood.
Studies in both humans and mice indicate that E-selectin and P-selectin are constitutively expressed on BM endothelial cells,20-22 and intravital studies have revealed that migration of HSPCs to BM occurs at specialized microvascular beds where E-selectin is expressed.23 In another study, P-selectin–coated devices were shown to exhibit a sixfold enrichment of human CD34pos HSPCs over anti-CD34 antibody-coated devices, implying the importance of P-selectin for binding HSPCs.24,25 BM transplantation studies into lethally irradiated mice lacking both endothelial selectins revealed that these mice exhibited a substantial defect in HSPC homing and a reduced survival that was rescued following the expression of either E- or P-selectin.26 These and several other independent lines of evidence have highlighted vascular-selectin–dependent interactions as central to the recruitment of HSPC to BM.26-29 In the current study, we determine the link between CD34 expression and the concurrent hematopoietic activation that leads to its improved homing and whether these vascular selectins can explain the gap in our understanding of this process. We revealed a more defined role for CD34 as a vascular selectin ligand and showed that it has comparable affinity and functional performance to other selectin ligands on human HSPCs. These new findings add to our preexisting understanding of selectin ligand contributions toward hematopoietic cell migration in therapeutic settings.
Methods
Mass spectrometry analysis of cognate E-selectin ligands expressed on human HSPC-enriched cells
Recombinant E-selectin chimeric protein (E-Ig) was used to immunoprecipitate ligands from a lysate of HSPC-enriched cells (as described in the supplemental Materials and methods), and the resulting samples were separated on 4% to 20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis; protein bands were visualized by SimplyBlue SafeStain (Invitrogen). These bands were then reduced, alkylated, and digested in the gel with sequencing grade modified trypsin (Promega); the resultant peptides were extracted using buffer containing (5% acetonitrile [VWR], 95% water, and 0.1% formic acid [Sigma-Aldrich]). Following extraction, peptides were dried to ∼1 μL sample volumes using a speed vacuum, fractionated by nanoflow liquid chromatography, and analyzed using a LTQ Orbitrap mass spectrometer (all acquired from Thermo Scientific). Raw data were converted to Mascot generic format files and searched using the online Mascot database.
Parallel plate rolling assay of CD34 knockdown cells
CD34 small interfering RNA (siRNA) was predesigned by Ambion silencer select (Applied Bioscience). For each nucleofection, 1 × 107 cells were pretreated with bromelain (500 µg/mL, 20 minutes at 37°C), washed with phosphate-buffered saline, gently resuspended in SE buffer mix (Amaxa) containing 500 nM of CD34-targeting (referred to as CD34-knockdown [KD]) or nontargeting control (scrambled), and then pulsed with the program EO-100 using a 4D-Nucleofector system (Amaxa). CD34 expression in CD34-KD and scrambled nucleofected cells were monitored routinely after 48 to 72 hours to be eligible for experimental use. For the cell binding assay, 1 × 106 CD34-KD or scrambled cells were suspended in Hanks balanced salt solution with 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and 2 mM CaCl2 and then perfused over an 80% confluent monolayer of Chinese hamster ovary cells expressing E-selectin (CHO-E) at 0.3 dyne/cm2 for 60 seconds followed by stepwise increases every 15 seconds to a maximum of 4.2 dynes/cm2, as previously described in Wiese et al.30
Hydrodynamic flow analysis of selectin and ligand binding by surface plasmon resonance
Surface plasmon resonance (SPR) binding experiments were performed using the Biacore T-100 system (GE Healthcare) at 25°C. Immobilization of monoclonal antibodies (mAbs) on a carboxy-methylated-5 dextran sensor chip was performed using an amine coupling kit (BIAcore) as described in supplemental Materials and methods to achieve immobilization level of mAbs between 4000 and 8000 response units (RU). Binding experiments were performed at a flow rate of 20 µL/min in running buffer (1% Triton X-100, 50 mM NaCl, 50 mM Tris-base, and 1 mM CaCl2) for E-selectin or running buffer (0.05% P20, 150 mM NaCl, 2 mM CaCl2, 50 mM Tris-base pH 8.0) for P-selectin, unless otherwise stated. Whole cell lysates were prepared in buffer containing (1% Triton X-100 in 250 mM NaCl, 50 mM Tris-HCI; pH 7.4, 20 μg/mL phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablet [Roche]) as described in the supplemental Materials and methods. These lysates were perfused over immobilized mAb, washed with the respective running buffer for at least 3 minutes to remove multiligand complex contamination, and then a standard concentration of E-Ig (177 nM; diluted in the running buffer) was injected for 130 seconds, unless otherwise specified. To correct for the buffer bulk refractive index and nonspecific interactions with the surface and with the mAb, a control flow cell immobilized with an equal amount of the mAb-corresponding isotype was used. For more details about the surface regeneration procedure, glycoprotease experiments, and calculations used to determine the dissociation binding constant, KD, the dissociation rate constant (koff) of the mAb·captured-ligand complex, the apparent dissociation rate constant (koff-apparent) of the interaction of mAb·captured-ligand with E-Ig from a single concentration-type binding study, or the apparent association rate constant from multiple concentration-type binding study (kon-apparent), please refer to the supplemental Materials and methods.
Statistical analysis
Data are expressed as the means ± standard error. Statistical significance of differences between means was determined by 1-way analysis of variance. If means were shown to be significantly different, multiple comparisons were performed post hoc by the Tukey t test. Statistical significance was defined as P < .05.
Additional materials and methods and details of the cells, enzymes, and reagents used are outlined in supplemental Materials and methods.
Results
CD34pos HSPC-enriched fractions from human BM express more E-selLs than CD34neg HSPC-enriched fractions
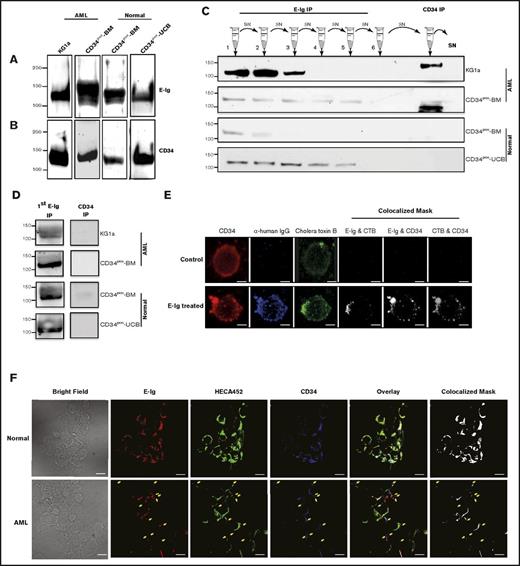
Given the requisite expression of E-selectin on BM endothelial cells for HSPC migration and trafficking,23 we sought to compare the ability of a recombinant E-selectin chimeric protein (E-Ig) to bind HSPC-enriched fractions either expressing CD34 (CD34pos) or not (CD34neg) that were isolated from human BM that was first depleted of lineage committed cells, including those cells expressing CD38 (Linneg CD38neg). As expected, we found more CD34neg than CD34pos cells (15% vs 1%, respectively), which is in accordance with previous studies5,31 (Figure 1A). Flow cytometric analysis comparing the LinnegCD38negCD34pos to LinnegCD38negCD34neg fractions showed higher E-Ig staining to the CD34pos population than to the CD34neg population (Figure 1B left panel). The positive population displayed a higher amount of E-selectin ligands (E-selLs) relative to the negative population after normalizing the slight differences in the amount of β-actin loaded as a control (Figure 1B middle panel). Note that CD34pos-BM cells (isolated from mononuclear cells [MNCs] of human BM using only anti-CD34 microbeads alone; ie, not Linneg CD38neg) express a range of E-selLs from ∼100 to 250 kDa, whereas the LinnegCD38negCD34pos cells express ligands at a molecular weight (MW) just over 100 kDa primarily (Figure 1B middle panel). The protein concentration of each extract was normalized using a bicinchoninic acid assay and β-actin staining (Figure 1B middle panel). To determine whether the difference in E-Ig staining between CD34pos and CD34neg was due to differential expression of E-selectin protein ligands, the expression of the common E-selLs expressed on HSPCs, including CD44, PSGL-1, and CD43,32-34 was analyzed using flow cytometry. As shown in Figure 1B right panel, CD44 was expressed on both populations. Western blot analysis of the whole-cell lysates further confirmed that although CD44 expression in the CD34neg fraction was higher, it did not display HECA-452 reactivity (supplemental Figure 1) or E-selL activity (Figure 1B middle panel). Thus, the CD44 expressed on the CD34neg fraction is not hematopoietic cell E-/L-selectin ligand (HCELL) because it does not display HECA-452 reactive CD44 that binds E-selectin, whereas that expressed on the CD34pos fraction did exhibit HCELL characteristics (supplemental Figure 1).32,35 Other known E-selLs (CD43 and PSGL-1) were expressed at low levels or absent (20% ± 4% vs 66.5% ± 24% for CD43 and 13% ± 4% vs 51% ± 11% for PSGL-1 positive cells in CD34neg and CD34pos populations, respectively; P < .05) (Figure 1B right panel). Overall, these results support that E-selectin binding is more pronounced on the CD34pos fraction than on the CD34neg fraction in part due to the differential expression of E-selLs between these 2 populations.
Differential expression of E-selLs on CD34pos and CD34neg subsets isolated from the Linneg CD38neg fraction of human BM. (A) Overview of the gating strategy used to isolate CD34negCD38neg and CD34posCD38neg fractions by fluorescence-activated cell sorting of lineage-depleted (Linneg) BM MNCs. Left panel, dot plot represents the cell surface expression of a lineage marker cocktail (including CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD123, CD235a, and CD7). Cells residing in the negative fraction (R1) were further gated for CD38-negative cells (R2) (middle panel) and then subdivided into 2 subpopulations based on CD34 expression, CD34pos and CD34neg residing in R3 and R4 gates, respectively (right panel). Data shown are representative of n = 4 experiments. (B) Left panel, representative E-Ig staining profile of CD34pos and CD34neg subpopulations isolated as depicted in panel A. The shaded curve shows EDTA control (20 mM; on the LinnegCD38negCD34pos subset), whereas dotted red and solid blue curves show the specific binding of CD34neg and CD34pos subsets, respectively (n = 4). Middle panel, lysates of CD34pos BM cells (CD34pos-BM), LinnegCD38negCD34pos, and LinnegCD38negCD34neg populations isolated from human BM were normalized for total protein level and subjected to western blot analysis. Membranes were blotted with E-Ig, CD44, or β-actin followed by isotype-matched HRP-conjugated mAb for visualization. This is representative of n = 4 independent experiments. Supplemental Figure 1 shows western blots where CD44 was immunoprecipitated from these cell populations and blotted with E-Ig, CD44, and HECA-452. Right panel, flow cytometric analysis of E-selLs expressed on the 2 subpopulations isolated as in panel A is shown as the average percent of expression (above the isotype control) of n = 3 independent experiments. *P < .05 relative to CD34neg subpopulation. NIH Image J was used to quantify the intensity of western blot bands using the gel analyzer tool; the number displayed represents the density of each band related to the LinnegCD38negCD34neg band as a standard. FSC, forward scatter.
Differential expression of E-selLs on CD34pos and CD34neg subsets isolated from the Linneg CD38neg fraction of human BM. (A) Overview of the gating strategy used to isolate CD34negCD38neg and CD34posCD38neg fractions by fluorescence-activated cell sorting of lineage-depleted (Linneg) BM MNCs. Left panel, dot plot represents the cell surface expression of a lineage marker cocktail (including CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD123, CD235a, and CD7). Cells residing in the negative fraction (R1) were further gated for CD38-negative cells (R2) (middle panel) and then subdivided into 2 subpopulations based on CD34 expression, CD34pos and CD34neg residing in R3 and R4 gates, respectively (right panel). Data shown are representative of n = 4 experiments. (B) Left panel, representative E-Ig staining profile of CD34pos and CD34neg subpopulations isolated as depicted in panel A. The shaded curve shows EDTA control (20 mM; on the LinnegCD38negCD34pos subset), whereas dotted red and solid blue curves show the specific binding of CD34neg and CD34pos subsets, respectively (n = 4). Middle panel, lysates of CD34pos BM cells (CD34pos-BM), LinnegCD38negCD34pos, and LinnegCD38negCD34neg populations isolated from human BM were normalized for total protein level and subjected to western blot analysis. Membranes were blotted with E-Ig, CD44, or β-actin followed by isotype-matched HRP-conjugated mAb for visualization. This is representative of n = 4 independent experiments. Supplemental Figure 1 shows western blots where CD44 was immunoprecipitated from these cell populations and blotted with E-Ig, CD44, and HECA-452. Right panel, flow cytometric analysis of E-selLs expressed on the 2 subpopulations isolated as in panel A is shown as the average percent of expression (above the isotype control) of n = 3 independent experiments. *P < .05 relative to CD34neg subpopulation. NIH Image J was used to quantify the intensity of western blot bands using the gel analyzer tool; the number displayed represents the density of each band related to the LinnegCD38negCD34neg band as a standard. FSC, forward scatter.
E-selectin binds CD34 expressed on human HSPC-enriched
To fully elucidate all noncanonical E-selLs expressed on CD34pos HSPC-enriched cells from the BM (Figure 1B middle panel), we used a mass spectrometry (MS)–based proteomics approach. Among the several hundred ligands recognized by MS with a P value < .01, our dataset verified the presence of CD44, CD43, and PSGL-1, 3 well-known HSPC E-selLs (Table 1).32-34 Furthermore, MS data analysis identified CD34, a ligand previously identified on KG1a cells to bind E-selectin in a parallel plate flow setting,17 as a potential ligand on HSPCs. To validate the binding activity of CD34 to E-selectin, CD34 immunoprecipitates were prepared from lysates of CD34pos fractions from normal umbilical cord blood (CD34pos-UCB) and BM (CD34pos-BM) as well as from acute myeloid leukemic (AML) cells (KG1a cell line and BM from AML patient [CD34pos-AML]), all of which had been normalized for protein concentration. Western blots of these immunoprecipitates probed with E-Ig (1 µg/mL, n = 3) revealed a 120-kDa band in all samples tested, confirming that CD34 isolated from HSPC-enriched cells bound E-selectin (Figure 2A). Consistently, results from the reverse experiment verified that CD34 bound E-selectin (Figure 2B). MS analysis of CD34 immunoprecipitates from lysates of KG1a cells and CD34pos human BM cells confirmed that the primary protein immunoprecipitated with the CD34 antibody is indeed CD34 without any contamination of other proteins (supplemental Table 1; supplemental Figure 2). Furthermore, an exhaustive immunoprecipitation with E-Ig (6 rounds) was performed on each of these cell types. Following the clearance of E-Ig reactive bands, the residual lysate was immunoprecipitated with the anti-CD34 mAb, QBend-10, to remove any leftover CD34 protein that was not removed by the E-Ig immunoprecipitations. Western blots were stained for CD34 and revealed the presence of a CD34 glycoform that was not bound by E-Ig in lysates from AML samples (Figure 2C); this glycoform was not present in lysates from normal cells. In addition, this glycoform lacked expression of sialyl Lewis x (sLex), which is essential for E-selectin binding (Figure 2D). Finally, confocal microscopy was used to further confirm the interaction of CD34 (CD34-mAb; red) and E-selectin (E-Ig; blue) on KG1a cells that were either pretreated with E-Ig for 1 hour or left untreated (Figure 2E). Using the Imaris Coloc analysis tool, we observed that CD34 appeared to colocalize with E-Ig (Figure 2E). Interestingly, CD34 appears to colocalize into clusters following a short exposure to E-Ig, suggesting that CD34 may be recruited to lipid rafts following exposure to E-Ig. Indeed, staining with lipid raft markers (CTB, green) indicated that E-Ig induced lipid raft formation and recruited CD34 to lipid rafts after E-Ig binding (Figure 2E). It should be noted that incubation of cells with E-Ig resulted in increased aggregation of cells (supplemental Figure 3). Furthermore, a confocal analysis following staining of CD34posCD38neg lineage-depleted BM cells from AML and normal BM with E-Ig (E-selectin), HECA-452 (sLex), and 8G12 (CD34 protein) revealed that CD34pos AML cells from the BM (Figure 2F lower panels) express a CD34 glycoform that does not bind E-selectin or express sLex/a epitopes, whereas CD34pos from normal BM (Figure 2F upper panels) counterparts do not; this is indicated by the yellow arrows in lower panels of Figure 2F. These data reinforce the data presented in Figure 2C-D, indicating that a unique glycoform of CD34 exists in AML samples that does not appear to be present in normal samples.
Summary of potential E-selLs expressed on CD34pos-BM cells identified by MS
| Description . | Probability . | Coverage of peptide sequence, % . | Peptide sequence . |
|---|---|---|---|
| CD34 | 1 | 9.6 | LGILDFTEQDVASHQSYSQK,TSSC[160]AEFKK,LGILDFTEQDVASHQSYSQK, TSSC[160]AEFK, DRGEGLAR |
| Galectin-9B | .9997 | 7.3 | FEDGGYVVC[160]NTR SIILSGTVLPSAQR |
| Galectin-9 | .9998 | 7 | FEDGGYVVC[160]NTR |
| CD44/HCELL | 1 | 5.8 | LVINSGNGAVEDR SQEM[147]VHLVNK NLQNVDM[147]K |
| Galectin-3 | 1 | 5.6 | ASHEEVEGLVEK LADGGATNQGR AVDTWSWGER |
| CD43 | .9996 | 4.5 | TGALVLSR |
| Integrin β-2 | 1 | 3.9 | TTEGC[160]LNPR YNGQVC[160]GGPGR SSQELEGSC[160]R |
| PSGL-1 | .9907 | 2.2 | SPGLTPEPR |
| Fms-related tyrosine kinase 3 ligand cytokine receptor | .9977 | 1.6 | EMDLGLLSPQAQVEDS |
| Integrin α-L | .9337 | 0.9 | HGGLSPQPSQR |
| Description . | Probability . | Coverage of peptide sequence, % . | Peptide sequence . |
|---|---|---|---|
| CD34 | 1 | 9.6 | LGILDFTEQDVASHQSYSQK,TSSC[160]AEFKK,LGILDFTEQDVASHQSYSQK, TSSC[160]AEFK, DRGEGLAR |
| Galectin-9B | .9997 | 7.3 | FEDGGYVVC[160]NTR SIILSGTVLPSAQR |
| Galectin-9 | .9998 | 7 | FEDGGYVVC[160]NTR |
| CD44/HCELL | 1 | 5.8 | LVINSGNGAVEDR SQEM[147]VHLVNK NLQNVDM[147]K |
| Galectin-3 | 1 | 5.6 | ASHEEVEGLVEK LADGGATNQGR AVDTWSWGER |
| CD43 | .9996 | 4.5 | TGALVLSR |
| Integrin β-2 | 1 | 3.9 | TTEGC[160]LNPR YNGQVC[160]GGPGR SSQELEGSC[160]R |
| PSGL-1 | .9907 | 2.2 | SPGLTPEPR |
| Fms-related tyrosine kinase 3 ligand cytokine receptor | .9977 | 1.6 | EMDLGLLSPQAQVEDS |
| Integrin α-L | .9337 | 0.9 | HGGLSPQPSQR |
The sialomucin CD34 is a novel ligand for E-selectin. (A) CD34 was immunoprecipitated from HSPC-enriched lysates of CD34pos cells isolated from normal UCB cells (CD34pos-UCB), normal BM cells (CD34pos-BM), AML BM cells (CD34pos-AML), or KG1a cells (n = 3 patient samples and KG1a cells). Lysates were subjected to western blot analysis with E-Ig chimera. This is a representative blot of n = 3 experiments. (B) The reciprocal immunoprecipitation assay was performed where E-Ig chimera was used first for immunoprecipitation prior to western blot analysis using CD34 QBend-10 mAb (n = 3). (C-D) AML cells express a unique form of CD34 that does not function as an E-selL. Multiple rounds of E-Ig immunoprecipitations were performed on both normal and AML sample cell lysates, and following the clearance of E-Ig reactive bands, the residual lysates were immunoprecipitated using QBend-10 (CD34-mAb that recognizes all classes of CD34) and subjected to western blot analysis for CD34 (QBend-10) (C) and anti-sLex (HECA-452) (D). SN = supernatant. For panel D, as described in the supplemental Materials and methods, only the first elution after E-Ig immunoprecipitation and the CD34 immunoprecipitation are shown. These blots are representative of n = 4 separate experiments. (E) KG1a cells were pretreated with E-Ig chimera or left untreated prior to lipid raft staining with choleratoxin-β (CTB)-AF-594 (green). Fixed cells were then stained with CD34 (Cy5; red) and AlexaFluor-488 streptavidin (blue) toward biotinylated anti-human-Ig to detect E-Ig. The colocalized mask was analyzed using Imaris Coloc software. Cell surface labeling with an isotype control or a secondary antibody alone served as background controls (data not shown). Results are representative of n = 3 independent experiments, 7 fields per experiments; >5 cells per field. Scale bar is 5 μm. (F) CD34pos cells from AML BM (lower panels) express a CD34 glycoform that does not bind E-selectin, whereas CD34pos from normal BM (upper panels) counterparts does not. CD34posCD38neg lineage-depleted BM cells from either AML or normal BM were prepared for confocal analysis and stained for E-selectin chimera (E-Ig; red), sLex expression (HECA-452; green), and CD34 (8G12; blue). Bright field images are also shown. Colocalization analysis was performed using Imaris 7 software to construct the colocalization mask (white). Yellow arrows in the AML images (lower panels) point to spots where CD34 expression is not overlaid with E-selectin binding or sLex expression. Results are representative of n = 3 independent experiments. Scale bar is 10 μm. For clarity, panels A-D were performed on CD34pos samples from normal or AML BM wherever stated and panel F was performed on LinnegCD38negCD34pos cells from normal or AML BM. IP, immunoprecipitation.
The sialomucin CD34 is a novel ligand for E-selectin. (A) CD34 was immunoprecipitated from HSPC-enriched lysates of CD34pos cells isolated from normal UCB cells (CD34pos-UCB), normal BM cells (CD34pos-BM), AML BM cells (CD34pos-AML), or KG1a cells (n = 3 patient samples and KG1a cells). Lysates were subjected to western blot analysis with E-Ig chimera. This is a representative blot of n = 3 experiments. (B) The reciprocal immunoprecipitation assay was performed where E-Ig chimera was used first for immunoprecipitation prior to western blot analysis using CD34 QBend-10 mAb (n = 3). (C-D) AML cells express a unique form of CD34 that does not function as an E-selL. Multiple rounds of E-Ig immunoprecipitations were performed on both normal and AML sample cell lysates, and following the clearance of E-Ig reactive bands, the residual lysates were immunoprecipitated using QBend-10 (CD34-mAb that recognizes all classes of CD34) and subjected to western blot analysis for CD34 (QBend-10) (C) and anti-sLex (HECA-452) (D). SN = supernatant. For panel D, as described in the supplemental Materials and methods, only the first elution after E-Ig immunoprecipitation and the CD34 immunoprecipitation are shown. These blots are representative of n = 4 separate experiments. (E) KG1a cells were pretreated with E-Ig chimera or left untreated prior to lipid raft staining with choleratoxin-β (CTB)-AF-594 (green). Fixed cells were then stained with CD34 (Cy5; red) and AlexaFluor-488 streptavidin (blue) toward biotinylated anti-human-Ig to detect E-Ig. The colocalized mask was analyzed using Imaris Coloc software. Cell surface labeling with an isotype control or a secondary antibody alone served as background controls (data not shown). Results are representative of n = 3 independent experiments, 7 fields per experiments; >5 cells per field. Scale bar is 5 μm. (F) CD34pos cells from AML BM (lower panels) express a CD34 glycoform that does not bind E-selectin, whereas CD34pos from normal BM (upper panels) counterparts does not. CD34posCD38neg lineage-depleted BM cells from either AML or normal BM were prepared for confocal analysis and stained for E-selectin chimera (E-Ig; red), sLex expression (HECA-452; green), and CD34 (8G12; blue). Bright field images are also shown. Colocalization analysis was performed using Imaris 7 software to construct the colocalization mask (white). Yellow arrows in the AML images (lower panels) point to spots where CD34 expression is not overlaid with E-selectin binding or sLex expression. Results are representative of n = 3 independent experiments. Scale bar is 10 μm. For clarity, panels A-D were performed on CD34pos samples from normal or AML BM wherever stated and panel F was performed on LinnegCD38negCD34pos cells from normal or AML BM. IP, immunoprecipitation.
CD34 is an E-selL under flow conditions
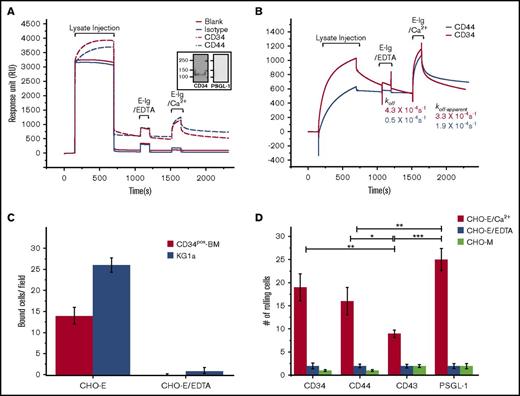
To examine whether native CD34 on human HSPC-enriched populations displayed functional E-selL activity in flow-based assays, we employed 3 approaches: the SPR-based binding assay developed in our laboratory,36 the Stamper-Woodruff assay,37 and the blot-rolling assay.38,39 We compared CD34 binding to the well-established E-selL, CD44 (ie, HCELL).32,35 The real-time binding feature of our SPR assay enabled us to isolate endogenously expressed CD34 and CD44 from human HSPC-enriched lysate and then measure their direct binding to recombinant E-Ig. Ligand-specific mAbs (4H11 for CD34 and Hermes-3 for CD44) or isotype controls (mouse immunoglobulin [MsIgG]) were first immobilized on a carboxy-methylated-5 sensor chip. Following the injection of the HSPC-enriched lysate, significant RU accumulated in the CD34 and CD44 flow cells, whereas only residual RUs were detected in the control flow cells that were either left blank (no mAb) or were immobilized with isotype control (MsIgG) (Figure 3A). It should be noted that the CD44 mAb used here captured a mixture of CD44 glycoforms36 of which only a fraction bound E-selectin. Furthermore, the purity of the captured CD34 was assessed as described previously.36 Briefly, following lysate injection, a 5-minute buffer-washing step was introduced in order to eliminate potential multiligand complexes. Captured proteins were recovered and then subjected to western blot analysis as detailed in supplemental Materials and methods. Immunostaining with CD34-mAb verified the presence of CD34 (Figure 3A inset), whereas immunostaining for the presence of PSGL-1, a highly expressed E-selL on hematopoietic cells,33,34,36 verified its absence (Figure 3A inset). As estimated from Equation 1 in supplemental Materials and methods, we observed low ligand-capturing efficiency, likely attributed to the immobilization of the mAb rendering its binding site inaccessible, with only 9.5% and 20.2% for CD34 and CD44, respectively. The values used in these calculations for the maximum RU reached at the end of the lysate injection (RUmax) for CD34 and CD44 were 546 and 559, respectively (Figure 3A), and the apparent MWs for mAb, CD34, and CD44 were nearly 150, 120, and 80 kDa, respectively. Next, we examined the binding of CD34·4H11-mAb and CD44·Hermes-3-mAb complexes with E-selectin. Results in Figure 3A represent the raw data before subtracting the buffer bulk refractive index and residual nonspecific interactions using a flow cell with the isotype control, whereas Figure 3B shows the subtracted sensorgrams that were subsequently normalized based on the difference in the ratio of the captured CD34 and CD44 just before E-Ig injection. We observed an 8.6-fold faster dissociation rate constant (koff) during the washing step for captured CD34 relative to CD44 from the mAb complex (Figure 3B). To assess the specificity of this interaction, we injected E-Ig (177 nM) in the presence of EDTA (5 mM) and confirmed that no interaction occurred in either complex. A marked increase in RU was detected when E-Ig was injected in the presence of Ca2+ (Figure 3B) with no or minimal binding to either isotype or blank controls (Figure 3A). The apparent dissociation rate constant (koff-apparent), which consists of the dissociation of CD34 or CD34·E-Ig from 4H11-mAb as well as the dissociation of E-Ig from CD34·4H11-mAb, was 3.3 × 10−4 s−1 (Figure 3B).
Flow-based binding assays confirm that CD34 is a functional E-selL. (A) Raw data depicting the capturing of E-selectin ligand from KG1a lysate on surface-immobilized antibodies and their subsequent binding to E-selectin by SPR. The lanes of the flow cell represent blank (red line), immobilized CD34-mAb (4H11) (7360 RU; red dashed line), immobilized CD44-mAb (Hermes3) (5200 RU; blue dashed line), and immobilized isotype control (4377 RU; blue line). The mAb immobilization (step 1) is not shown. Lysate injection, arrows mark the start and end of the lysate injection, which is then followed by a buffer washing step. The sensorgrams represent the raw data of the uncorrected RU for the buffer bulk refractive index and nonspecific interactions. Inset, western blot analysis and staining for either CD34 (left panel) or PSGL-1 (right panel) after eluting them from the chip confirming specificity of their capturing by SPR. E-Ig/EDTA injection, the injection involves E-Ig (177 nM) in the presence of EDTA (5 mM) as a control to show specificity of E-Ig to its ligands. E-Ig/Ca2+ injection, the injection involves Ca2+ (1 mM) to support E-Ig (177 nM) binding to its ligands. (B) Corrected sensorgrams for the buffer refractive index and nonspecific interactions. Data were presented as described in panel A but after subtracting the RU of the buffer refractive index and nonspecific interactions from the standard reference flow cell that contains the isotype control. Furthermore, normalization was applied to even out the difference in the level of mAb-captured CD34 and CD44 as described in the supplemental Materials and methods. koff is the dissociation rate constant for CD34 and CD44 from their respective mAb, and koff-apparent is the apparent dissociation rate constant for E-Ig, CD34/E-Ig, or CD44/E-Ig from their respective mAb as well as E-Ig from the complexes CD44·Hermes-3-mAb or CD34·4H11-mAb (n = 3 independent experiments). (C) Immunoprecipitations of CD34 were prepared from lysates of CD34pos-BM and KG1a cells and spotted on glass slides to test for CHO-E binding using a Stamper-Woodruff assay. Adherent CHO-E cells were counted by light microscopy using an ocular grid under magnification ×20. The data are representative of 1 experiment, and the error bars indicate the standard error of the mean (SEM) of 7 fields per slide on 2 slides for each experiment (n = 3 independent experiments). (D) Blot-rolling assays were performed on western blots of CD34, CD44, CD43, and PSGL-1 immunoprecipitates from KG1a cells stained for HECA-452. CHO-E cells were subsequently perfused over immunoprecipitated glycoproteins at 0.25 dyne/cm2. After cell perfusion, the numbers of rolling cells per field were counted (red bars). As a control, CHO-E cells incubated with EDTA (blue bars) or mock-transfected CHO cells (CHO-M) (light green bars) were used. Results shown reflect the average number of rolling cells over the HECA-452 blots of n = 7 membrane preparations from 4 distinct fields of view each. Data are mean ± SEM (error bars). *P < .05; **P < .01; ***P < .001.
Flow-based binding assays confirm that CD34 is a functional E-selL. (A) Raw data depicting the capturing of E-selectin ligand from KG1a lysate on surface-immobilized antibodies and their subsequent binding to E-selectin by SPR. The lanes of the flow cell represent blank (red line), immobilized CD34-mAb (4H11) (7360 RU; red dashed line), immobilized CD44-mAb (Hermes3) (5200 RU; blue dashed line), and immobilized isotype control (4377 RU; blue line). The mAb immobilization (step 1) is not shown. Lysate injection, arrows mark the start and end of the lysate injection, which is then followed by a buffer washing step. The sensorgrams represent the raw data of the uncorrected RU for the buffer bulk refractive index and nonspecific interactions. Inset, western blot analysis and staining for either CD34 (left panel) or PSGL-1 (right panel) after eluting them from the chip confirming specificity of their capturing by SPR. E-Ig/EDTA injection, the injection involves E-Ig (177 nM) in the presence of EDTA (5 mM) as a control to show specificity of E-Ig to its ligands. E-Ig/Ca2+ injection, the injection involves Ca2+ (1 mM) to support E-Ig (177 nM) binding to its ligands. (B) Corrected sensorgrams for the buffer refractive index and nonspecific interactions. Data were presented as described in panel A but after subtracting the RU of the buffer refractive index and nonspecific interactions from the standard reference flow cell that contains the isotype control. Furthermore, normalization was applied to even out the difference in the level of mAb-captured CD34 and CD44 as described in the supplemental Materials and methods. koff is the dissociation rate constant for CD34 and CD44 from their respective mAb, and koff-apparent is the apparent dissociation rate constant for E-Ig, CD34/E-Ig, or CD44/E-Ig from their respective mAb as well as E-Ig from the complexes CD44·Hermes-3-mAb or CD34·4H11-mAb (n = 3 independent experiments). (C) Immunoprecipitations of CD34 were prepared from lysates of CD34pos-BM and KG1a cells and spotted on glass slides to test for CHO-E binding using a Stamper-Woodruff assay. Adherent CHO-E cells were counted by light microscopy using an ocular grid under magnification ×20. The data are representative of 1 experiment, and the error bars indicate the standard error of the mean (SEM) of 7 fields per slide on 2 slides for each experiment (n = 3 independent experiments). (D) Blot-rolling assays were performed on western blots of CD34, CD44, CD43, and PSGL-1 immunoprecipitates from KG1a cells stained for HECA-452. CHO-E cells were subsequently perfused over immunoprecipitated glycoproteins at 0.25 dyne/cm2. After cell perfusion, the numbers of rolling cells per field were counted (red bars). As a control, CHO-E cells incubated with EDTA (blue bars) or mock-transfected CHO cells (CHO-M) (light green bars) were used. Results shown reflect the average number of rolling cells over the HECA-452 blots of n = 7 membrane preparations from 4 distinct fields of view each. Data are mean ± SEM (error bars). *P < .05; **P < .01; ***P < .001.
Immunoprecipitations of CD34 from both CD34pos-BM cells and KG1a cells also supported the presence of adhesive interactions observed with CHO-E cells in Stamper-Woodruff assays displaying 14 ± 2 bound cells to CD34 isolated from CD34pos-BM and 26 ± 2 bound cells to CD34 isolated from KG1a; no binding was observed in the presence of EDTA (Figure 3C). Moreover, using blot-rolling assays of HECA-452 stained blots of E-selL immunoprecipitates, we observed that CD34 supported significant rolling of CHO-E cells under physiological shear stress in addition to the well-known E-selLs (CD44, CD43, and PSGL-1) immunoprecipitated from the human KG1a cell lysate (Figure 3D). CD43 supported the least amount of CHO-E rolling of all the ligands tested. Specificity for E-selectin binding was confirmed by the elimination of binding in the presence of EDTA or by using mock-transfected CHO cells (CHO-M) (Figure 3D). Overall, these multiple binding studies revealed that CD34 is a relevant and functional E-selL that may cooperate with other E-selLs in directing human HSPCs to E-selectin expressing sites.
The equilibrium dissociation binding constants of E-selectin to CD44, CD34, and PSGL-1 were relatively similar but varied in their binding stoichiometry
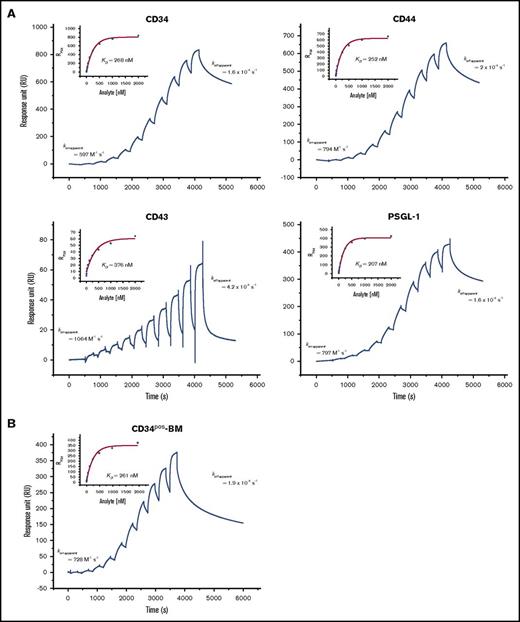
Next, we directly compared the binding affinities of E-selLs (CD44, CD34, CD43, and PSGL-1) expressed on KG1a cells by consecutive E-Ig injection at a physiological NaCl concentration (150 mM) using the SPR-based immunoprecipitation assay. During 5 minutes of washing at 20 µL/min, captured CD34 and CD43 continuously dissociated from their mAbs such that the amount of protein collected decreased by 20% to 25% (Figure 3B). This created an unreliable estimate of the dissociation binding constant (KD). Therefore, the CD34 563-mAb and CD43·polyclonal-Ab complexes were covalently linked by including another run of amine coupling that comprised injecting a 1:1 mix of N-hydroxysuccinimide:1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (NHS:EDC) for 350 seconds followed by deactivation with ethanolamine for 400 seconds at 5 µL/min. Using a steady-state model, KD, koff-apparent, and the apparent association rate constant (kon-apparent) were calculated for E-selLs (Figure 4A; Table 2). We observed that CD34, CD44, and PSGL-1 bound to E-Ig similarly with KD of 236.7 ± 38, 233 ± 9, and 259 ± 34 nM, respectively, values that are nearly half of that for CD43 (442 ± 47 nM) (Table 2). These results are consistent with the results of the blot-rolling assay, where the numbers of CHO-E cells rolling over CD43 immunoprecipitates were significantly reduced (P < .05; Figure 3D).
Determination of the dissociation binding constant (KD) for the binding of E-selLs from HSPC-enriched lysates to E-Ig. The sensorgrams show binding of consecutive injections of E-Ig at 15 μL/min for 240 seconds each at concentrations of 3.9, 7.8, 15.6, 31.3, 62.5, 125, 250, 500, 1000, and 2000 nM that are each spaced by a 60-second buffer washing step (A) over captured E-selLs (CD34, CD44, CD43, and PSGL-1) expressed in KG1a cell lysate (150 mM NaCl) and (B) over captured CD34 from CD34pos-BM lysate (50 mM NaCl). 563-mAb (10 800 RU for KG1a lysate or 6952 RU for CD34pos-BM lysate) or MsIgG1 isotype control (8320 RU for KG1a and 7500 RU for CD34pos-BM lysate) was immobilized to capture CD34 (left upper panel). Hermes-3-mAb (9300 RU) or MsIgG2a isotype control (7700 RU) was immobilized to capture CD44 (right upper panel). KPL-1-mAb (11 400 RU) or MsIgG1 isotype control (8090 RU) was immobilized to capture PSGL-1 (right lower panel). A polyclonal CD43 Ab (15 800 RU) or Goat isotype control (14450 RU) was immobilized to capture CD43 (left lower panel). The sensorgrams presented are corrected for the bulk refractive index and nonspecific interactions using the isotype controls. KD was determined by fitting the binding isotherm using a steady-state model and the RUmax values just prior to the start of the buffer injection where steady-state conditions were nearly met (inset) and koff-apparent as described in Figure 3B. Data are representative of n = 3 independent experiments.
Determination of the dissociation binding constant (KD) for the binding of E-selLs from HSPC-enriched lysates to E-Ig. The sensorgrams show binding of consecutive injections of E-Ig at 15 μL/min for 240 seconds each at concentrations of 3.9, 7.8, 15.6, 31.3, 62.5, 125, 250, 500, 1000, and 2000 nM that are each spaced by a 60-second buffer washing step (A) over captured E-selLs (CD34, CD44, CD43, and PSGL-1) expressed in KG1a cell lysate (150 mM NaCl) and (B) over captured CD34 from CD34pos-BM lysate (50 mM NaCl). 563-mAb (10 800 RU for KG1a lysate or 6952 RU for CD34pos-BM lysate) or MsIgG1 isotype control (8320 RU for KG1a and 7500 RU for CD34pos-BM lysate) was immobilized to capture CD34 (left upper panel). Hermes-3-mAb (9300 RU) or MsIgG2a isotype control (7700 RU) was immobilized to capture CD44 (right upper panel). KPL-1-mAb (11 400 RU) or MsIgG1 isotype control (8090 RU) was immobilized to capture PSGL-1 (right lower panel). A polyclonal CD43 Ab (15 800 RU) or Goat isotype control (14450 RU) was immobilized to capture CD43 (left lower panel). The sensorgrams presented are corrected for the bulk refractive index and nonspecific interactions using the isotype controls. KD was determined by fitting the binding isotherm using a steady-state model and the RUmax values just prior to the start of the buffer injection where steady-state conditions were nearly met (inset) and koff-apparent as described in Figure 3B. Data are representative of n = 3 independent experiments.
Summary of affinity and kinetic values for E-Ig binding to E-selLs: CD34, CD44, CD43, and PSGL-1 using a SPR-based assay
| . | CD34 . | CD44 . | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st . | 2nd . | 3rd . | Mean ± SEM . | 1st . | 2nd . | 3rd . | Mean ± SEM . | |
| KD, nM | 145 | 268 | 297 | 236.7 ± 38 | 234 | 252 | 214 | 233 ± 9 |
| koff-apparent, s−1 | 2.90E−04 | 1.60E−04 | 3.31E−04 | 2.6E−4 ± 0.4E−4 | 1.10E−04 | 2.00E−04 | 2.73E−04 | 1.9E−4 ± 0.4E−4 |
| kon-apparent, M−1 s−1 | 2030 | 597 | 1054 | 1230 ± 346 | 641 | 794 | 1280 | 903 ± 156 |
| CD43 | PSGL-1 | |||||||
| 1st | 2nd | Mean ± SEM | 1st | 2nd | 3rd | Mean ± SEM | ||
| KD, nM | 508.7 | 376 | 442 ± 47 | 342.5 | 207 | 229 | 259 ± 34 | |
| koff-apparent, s−1 | 4.80E−04 | 4.20E−04 | 4.5E−4 ± 0.2E−5 | 1.50E-04 | 1.65E−04 | 2.62E−04 | 1.9E−4 ± 0.3E−4 | |
| kon-apparent, M−1 s−1 | 944 | 1063.8 | 1004 ± 43 | 438 | 797 | 1144 | 793 ± 166 | |
| . | CD34 . | CD44 . | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st . | 2nd . | 3rd . | Mean ± SEM . | 1st . | 2nd . | 3rd . | Mean ± SEM . | |
| KD, nM | 145 | 268 | 297 | 236.7 ± 38 | 234 | 252 | 214 | 233 ± 9 |
| koff-apparent, s−1 | 2.90E−04 | 1.60E−04 | 3.31E−04 | 2.6E−4 ± 0.4E−4 | 1.10E−04 | 2.00E−04 | 2.73E−04 | 1.9E−4 ± 0.4E−4 |
| kon-apparent, M−1 s−1 | 2030 | 597 | 1054 | 1230 ± 346 | 641 | 794 | 1280 | 903 ± 156 |
| CD43 | PSGL-1 | |||||||
| 1st | 2nd | Mean ± SEM | 1st | 2nd | 3rd | Mean ± SEM | ||
| KD, nM | 508.7 | 376 | 442 ± 47 | 342.5 | 207 | 229 | 259 ± 34 | |
| koff-apparent, s−1 | 4.80E−04 | 4.20E−04 | 4.5E−4 ± 0.2E−5 | 1.50E-04 | 1.65E−04 | 2.62E−04 | 1.9E−4 ± 0.3E−4 | |
| kon-apparent, M−1 s−1 | 944 | 1063.8 | 1004 ± 43 | 438 | 797 | 1144 | 793 ± 166 | |
Assuming 1:1 stoichiometry, the percentage of active E-selLs that bound E-Ig was derived from the binding isotherms of their interactions (Figure 4A; Table 2) and was found to be 54% ± 17%, 23.0% ± 0.3%, 19% ± 2%, and 75% ± 3% for CD34, CD44, CD43, and PSGL-1, respectively. These percentages could, however, be upper estimates because we did not rule out the possibility that each ligand could bind >1 E-Ig molecule. Although E-Ig bound E-selLs with similar intrinsic binding behavior (comparable kon-apparent and koff-apparent), PSGL-1 and CD34 were found to express a significantly greater number of E-Ig binding sites than did CD44 or CD43, which could explain the rolling behavior of CHO-E over these ligands compared with rolling over PSGL-1 (P < .05 and P < .001, respectively; Figure 3D). These data suggest that PSGL-1 displayed the highest number of ligand active sites (P < .001 for CD44 and P < .01 for CD43) followed by CD34, CD44, and then CD43.
Further analysis of the E-selectin binding kinetics of CD34 extracted from primary cells was performed using CD34pos-BM lysate. Because of the relatively low protein concentration in these cell lysates, limited amounts of CD34 (172 RU, data not shown) were captured compared with that from the KG1a cell lysate. However, E-Ig binding still displayed a dose-dependent response with a KD of 261 nM (Figure 4B). After the last E-Ig injection, koff-apparent was estimated to be 1.9 × 10−4 s−1, which was very similar to that of the CD34 extracted from the KG1a lysate. Thus, kon-apparent of 728 M−1 s−1 was considered a reasonable estimate for CD34. Overall, these results demonstrated relative similarities between the binding affinities of CD34 with cells isolated from either primary CD34pos-BM or KG1a cell lysates, displaying slow on-/slow off-rate kinetics.
CD34 silencing resulted in markedly faster rolling velocities at higher shear stresses likely due to reduction in microvilli
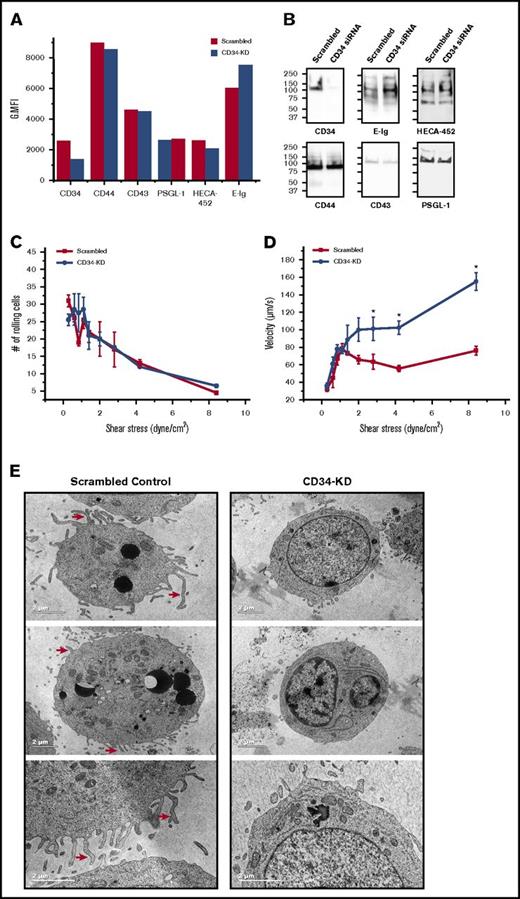
We used a parallel plate-rolling assay to directly measure the relative contribution of CD34 to the overall rolling behavior of HSPCs. Using a knockdown approach, we compared the ability of cells lacking CD34 (CD34-KD) and control cells (scrambled) to tether and roll over E-selectin-expressing cells. Changes in the phenotype and loss of the sLex epitope that are associated with native HSPCs knockdown cells34 made it favorable to use the HSPC-like cell line KG1a. As measured by flow cytometry, siRNA nucleofection of KG1a cells resulted in a 50% reduction in the surface expression of CD34 relative to scrambled cells (geometric mean fluorescent intensity) (Figure 5A). Meanwhile, flow cytometry indicated that there were no changes in the expression of other E-selLs or in HECA-452 reactivity of CD34-KD cells compared with scrambled control, but a slight increase in E-Ig staining was observed in CD34-KD cells (Figure 5A). Western blots of equal amounts of loaded proteins revealed that CD34 expression was substantially reduced, correlating with a slight increase in both HECA-542 and E-Ig staining, especially at ∼100 to 150 kDa; this observation was not due to an increase in the expression of any of the measured E-selLs (Figure 5B). We observed that although the number of rolling cells after perfusing scrambled control or CD34-KD over CHO-E cell monolayers did not vary under any shear stresses tested (Figure 5C), CD34-KD cells rolled markedly faster than did the control, especially at or above 3 dyne/cm2 (postcapillary venule shear; P < .05; Figure 5D). Specificity for E-selectin binding was confirmed by treatment of CHO-E cells with a function-blocking mAb (data not shown). Interestingly, transmission electron microscopic (TEM) images comparing CD34-KD and scrambled control KG1a cells revealed a profound reduction in microvilli type structures following CD34 knockdown (Figure 5E). Moreover, the cells showing this reduction in microvilli and plasma membrane convolutions did not present with any other changes in their ultrastructures: the endoplasmic reticulum was intact and not swollen; the mitochondria were comparable to those of control cells; and the nuclear envelope was intact. These results indicate that CD34 plays a significant role in slowing down the rate at which KG1a cells roll, although it does not affect the number of interactions between KG1a cells and CHO-E cells and suggests that this may be due, in part, to its role in regulating microvilli structures.
Silencing CD34 leads to a decrease in microvilli and higher rolling velocities with increasing shear stress. (A) Flow cytometric analysis of E-selL expression and E-Ig and HECA-452 binding of scrambled and CD34 siRNA-nucleofected KG1a cells (CD34-KD). This is a representative figure of n = 4 independent experiments depicting the geometric mean fluorescent intensity (G.MFI). (B) Equivalent amounts of scrambled and CD34-KD cell lysates were subjected to western blot analysis and stained for CD34, CD44, CD43, PSGL-1, sLex (HECA-452), and E-Ig. Blots are representative of n = 4 independent experiments. (C) Scrambled or CD34-KD KG1a cells were each perfused over CHO-E cell monolayers for 1 minute at 0.28 dyne/cm2, and then detachment assays were employed by increasing the shear stress stepwise every 15 seconds. The average number of rolling cells in 4 distinct fields of view for each experiment (n = 4) was counted. (D) Single-cell tracking with Imaris V7.6.4 software was used to calculate KG1a rolling velocity over CHO-E cells from (C) at each shear stress depicted as described. The adhesion histogram is representative of n = 4 independent experiments, and data are reported as the mean ± SEM (error bars). *P < .05. (E) KG1a cells were either transfected with scrambled control siRNA (scrambled control) or with CD34 siRNA (CD34-KD), and 48 hours later, cells were fixed with glutaraldehyde and prepared for TEM analysis as outlined in supplemental Materials and methods. Red arrows point out some of the microvilli-type structures that are evident in the scrambled control but not in the CD34-KD images. These are representative cells of over 30 different cells imaged under each condition.
Silencing CD34 leads to a decrease in microvilli and higher rolling velocities with increasing shear stress. (A) Flow cytometric analysis of E-selL expression and E-Ig and HECA-452 binding of scrambled and CD34 siRNA-nucleofected KG1a cells (CD34-KD). This is a representative figure of n = 4 independent experiments depicting the geometric mean fluorescent intensity (G.MFI). (B) Equivalent amounts of scrambled and CD34-KD cell lysates were subjected to western blot analysis and stained for CD34, CD44, CD43, PSGL-1, sLex (HECA-452), and E-Ig. Blots are representative of n = 4 independent experiments. (C) Scrambled or CD34-KD KG1a cells were each perfused over CHO-E cell monolayers for 1 minute at 0.28 dyne/cm2, and then detachment assays were employed by increasing the shear stress stepwise every 15 seconds. The average number of rolling cells in 4 distinct fields of view for each experiment (n = 4) was counted. (D) Single-cell tracking with Imaris V7.6.4 software was used to calculate KG1a rolling velocity over CHO-E cells from (C) at each shear stress depicted as described. The adhesion histogram is representative of n = 4 independent experiments, and data are reported as the mean ± SEM (error bars). *P < .05. (E) KG1a cells were either transfected with scrambled control siRNA (scrambled control) or with CD34 siRNA (CD34-KD), and 48 hours later, cells were fixed with glutaraldehyde and prepared for TEM analysis as outlined in supplemental Materials and methods. Red arrows point out some of the microvilli-type structures that are evident in the scrambled control but not in the CD34-KD images. These are representative cells of over 30 different cells imaged under each condition.
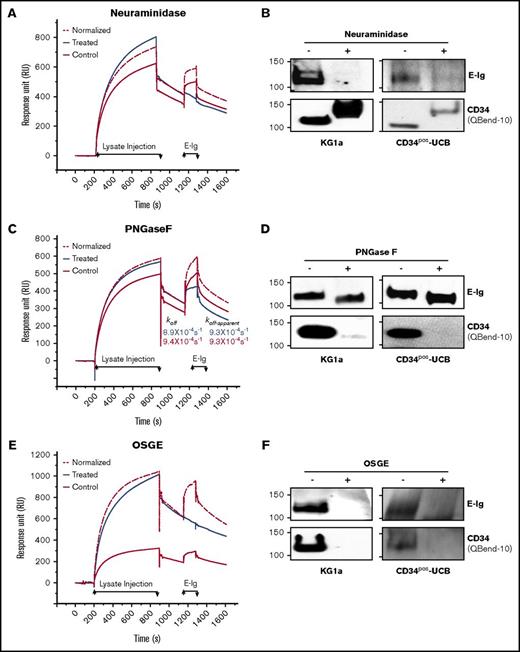
CD34 binding to E-selectin is dependent on sialofucosylated O-glycans
L-selectin is well known to bind sulfated sLex (6-sulfo-sLex) capped O- and N-glycans of the CD34 core protein.40 We performed both SPR-based immunoprecipitation assay and western blot analysis to determine the glycan modifications necessary for CD34 to bind E-selectin following treatment with the glycosidases: O-sialoglycoprotein endopeptidase (OSGE; to remove O-glycans), Peptide:N-Glycosidase F (PNGase F; to remove N-glycans), or neuraminidase (to remove sialic acid). To perform a quantitative comparative analysis, we normalized the amount of mAb-captured ligand in the control to that in the treated based on their RUs prior to E-Ig injection, as described in the supplemental Materials and methods. Sensorgrams of E-Ig (354 nM) binding to antibody captured CD34 (CD34·4H11-mAb) from either neuraminidase-treated or control lysates showed that binding is eliminated following sialic acid digestion (Figure 6A). Western blot analysis of treated CD34 immunoprecipitates from KG1a or CD34pos-UCB lysates verified SPR results (Figure 6B top panels). We evaluated the efficiency of neuraminidase to digest sialic acid using western blots and probing with the QBend-10 antibody (Class 2 mAb) to track the shift in the MW of CD34 from ∼120 kDa to 150 kDa (Figure 6B lower panels).36,41 To determine whether the E-selectin binding epitope resides on N- and/or O-glycans, we treated the KG1a lysate with PNGase F or OSGE, respectively. We found that removing the N-glycans resulted in only a small reduction in RUmax (1.4-fold) and a similar dissociation constant (koff-apparent) as the control lysate (Figure 6C). By contrast, O-glycan removal completely abolished the interaction of E-Ig with the CD34·4H11-mAb complex (Figure 6E); results were verified by western blot analysis (Figure 6D,F top panels). The glycoprotease-sensitive CD34-mAb, QBend-10, is sensitive to the removal of O- or N-linked glycans on CD34 and is therefore routinely used to detect the efficiency of glycan removal (Figure 6D,F lower panels).36 These findings indicate that, in contrast to CD44 (ie, HCELL), which displays E-selL determinants on N-glycans and O-glycans,32,36 CD34 presents sLex decorations mainly on O-glycans similar to CD43 and PSGL-1.34,42
E-selectin binds predominantly to O-glycans on CD34. (A) 4H11-mAb (4964 RU) or its isotype control (4036 RU) were immobilized to capture CD34 from KG1a lysates that were treated with neuraminidase (Treated; blue line) or left untreated but subjected to the same buffer-treatment conditions, incubation times, and temperatures (Control; red line). Following CD34 capture, E-Ig was injected at 354 nM. The same surface was used for both the treated and the control binding studies with a surface regeneration step between the 2 runs. The normalized (red dashed line) sensorgram is the same as the control but normalized to the treated sensorgram based on the ratio of accumulated CD34 RU prior to E-Ig injection. (B) For western blot analysis, equivalent amounts of CD34 immunoprecipitates from HSPC-enriched lysates (KG1a or CD34pos-UCB) were either treated with neuraminidase (+) or not (−) and blotted with either E-Ig (top panel) or CD34 (QBend-10, lower panel). Note that the apparent increase in MW of CD34 is attributed to the loss of the negatively charged sialic acid. (C) SPR analysis of the PNGase F treatment was performed as in panel A using 4H11-mAb (6500 RU) or its isotype control (5810 RU). koff and koff-apparent were calculated as described in Figure 3B. (D) CD34 immunoprecipitates were treated with PNGase F and subjected to western blotting for E-Ig (top panel) or CD34 (lower panel) as in panel B. (E) SPR analyses of OSGE treatment were performed as in panels A and C using 4H11-mAb (9000 RU) or its isotype control (6500 RU). (F) Western blot analysis of the treated CD34 immunoprecipitates were performed as in panels B and D. CD34 (Qbend-10) was used as an internal control to confirm N- and O-glycan removal. The sensorgrams presented are corrected for the bulk refractive index and nonspecific interactions using the isotype controls. All results are representative of n = 3 independent experiments.
E-selectin binds predominantly to O-glycans on CD34. (A) 4H11-mAb (4964 RU) or its isotype control (4036 RU) were immobilized to capture CD34 from KG1a lysates that were treated with neuraminidase (Treated; blue line) or left untreated but subjected to the same buffer-treatment conditions, incubation times, and temperatures (Control; red line). Following CD34 capture, E-Ig was injected at 354 nM. The same surface was used for both the treated and the control binding studies with a surface regeneration step between the 2 runs. The normalized (red dashed line) sensorgram is the same as the control but normalized to the treated sensorgram based on the ratio of accumulated CD34 RU prior to E-Ig injection. (B) For western blot analysis, equivalent amounts of CD34 immunoprecipitates from HSPC-enriched lysates (KG1a or CD34pos-UCB) were either treated with neuraminidase (+) or not (−) and blotted with either E-Ig (top panel) or CD34 (QBend-10, lower panel). Note that the apparent increase in MW of CD34 is attributed to the loss of the negatively charged sialic acid. (C) SPR analysis of the PNGase F treatment was performed as in panel A using 4H11-mAb (6500 RU) or its isotype control (5810 RU). koff and koff-apparent were calculated as described in Figure 3B. (D) CD34 immunoprecipitates were treated with PNGase F and subjected to western blotting for E-Ig (top panel) or CD34 (lower panel) as in panel B. (E) SPR analyses of OSGE treatment were performed as in panels A and C using 4H11-mAb (9000 RU) or its isotype control (6500 RU). (F) Western blot analysis of the treated CD34 immunoprecipitates were performed as in panels B and D. CD34 (Qbend-10) was used as an internal control to confirm N- and O-glycan removal. The sensorgrams presented are corrected for the bulk refractive index and nonspecific interactions using the isotype controls. All results are representative of n = 3 independent experiments.
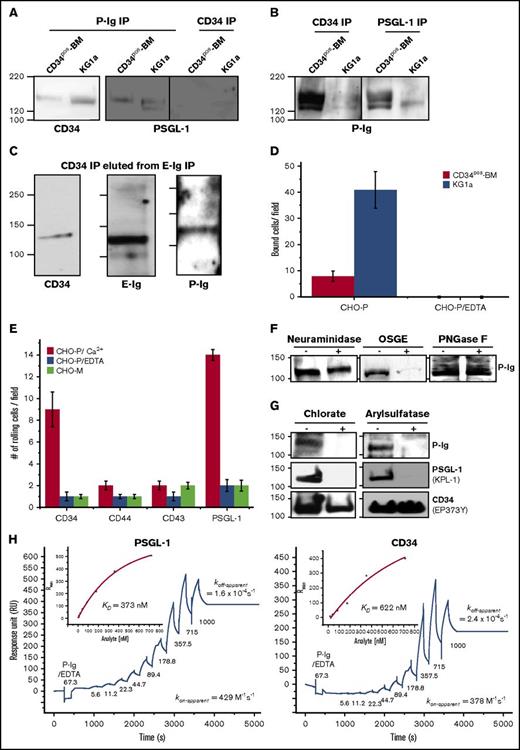
P-selectin binds CD34 on sulfated O-glycans expressed on human HSPC-enriched cells
E- and P-selectin are expressed on human BM endothelial cells.20,21 To date, the only ligand known to bind all 3 selectins (E-/P- and L-selectin) on HSPCs is PSGL-1.33,43,44 In order to determine if CD34 on HSPCs is a P-selectin ligand, recombinant P-selectin human immunoglobulin chimeric protein (P-Ig) was used to immunoprecipitate P-selectin ligands from KG1a or CD34pos-BM lysates, and subsequently western blots were stained for CD34 (EP373Y and QBend-10-mAb) or for PSGL-1 (KPL-1-mAb). CD34 and PSGL-1 were pulled out using P-Ig, and no PSGL-1 was found within the CD34 immunoprecipitate (Figure 7A). In addition, reciprocal studies also confirmed that CD34 and PSGL-1 immunoprecipitated from lysates of KG1a and CD34pos-BM bound P-selectin (Figure 7B). Intriguingly, 2 MW forms (between 120 kDa and 130 kDa) of CD34 appeared to be able to bind P-selectin in the HSPC-enriched lysates (Figure 7B); therefore, to determine whether E-selectin recognizes the same CD34 glycoform as P-selectin, we eluted E-selLs following E-Ig immunoprecipitation of KG1a lysate using EDTA and subsequently immunoprecipitated this elution with CD34-mAb. As shown in Figure 7C, the higher MW form of CD34 (∼130-kDa) is both an E- and a P-selectin ligand, denoting this MW glycoform of CD34 as a ligand that is able to bind both vascular selectins on HSPC-enriched cells. Furthermore, CD34 immunoprecipitated from CD34pos-BM and KG1a cells supported CHO-P cell rolling (8 ± 2, compare with 41 ± 7 bound cells per field, respectively) using Stamper-Woodruff assays, whereas no binding was observed with the EDTA control (Figure 7D). Moreover, among the other E-selLs (CD34, CD44, CD43, and PSGL-1), only CD34 and PSGL-1 exhibited functional binding activity to CHO-P in blot-rolling assays (Figure 7E). Glycoprotease treatment of CD34 immunoprecipitates from KG1a cells revealed neither treatment with PNGase F nor with neuraminidase inhibited the binding of P-Ig to CD34, suggesting that N-glycans and sialic acid are not critical for P-selectin binding. Alternatively, OSGE treatment significantly reduced P-Ig staining, suggesting that O-glycans are essential for binding. To determine whether sulfation of CD34 is critical for P-selectin binding, we cultured KG1a cells in the presence or absence of chlorate, a metabolic inhibitor of the main sulfate donor on both tyrosine residues and glycoconjugates45 prior to the preparation of cell lysates. As shown in Figure 7G, CD34 immunoprecipitates after chlorate treatment were not able to bind P-selectin compared with the control (left panel). Furthermore, CD34 immunoprecipitates treated with arylsulfatase, an enzyme that releases sulfates from tyrosine residues but not carbohydrates,46,47 abrogated P-selectin binding to CD34 compared with the control (treated with buffer alone) (Figure 7G right panel). Complete cleavage of sulfated tyrosine residues was confirmed by the loss of anti-PSGL-1-mAb, KPL-1epitope (Figure 7G middle panels), which recognizes the tyrosine-sulfated motif without affecting the overall amount of PSGL-1 protein recognized by PL-2 (data not shown) or the CD34 protein level recognized by QBend-10-mAb following treatment (Figure 7D lower panels).46 In summary, these data imply that unlike PSGL-1 binding to P-selectin, which requires sialylated glycans,47,48 the binding of CD34 to P-selectin is not dependent on sialylation but does require O-glycans and tyrosine sulfation.
A novel CD34 glycoform acts as a P-selectin ligand and its interaction is dependent on O-glycans and tyrosine sulfation. (A) P-Ig was used to immunoprecipitate potential ligands from KG1a and CD34pos-BM lysates, and the resultant proteins were analyzed by western blot for CD34 (QBend-10 and EP373Y mAb) or PSGL-1 (KPL-1-mAb). Note that CD34 immunoprecipitates were free from any PSGL-1 contamination (n = 3 independent experiments). (B) CD34 and PSGL-1 were immunoprecipitated from KG1a or CD34pos-BM lysates and then analyzed by western blot for P-Ig binding (n = 3 independent experiments). (C) E-Ig was used to immunoprecipitate E-selLs from the KG1a lysate and resultant proteins were eluted with 30 mM EDTA. The eluate was then immunoprecipitated with CD34 mAbs (clones 4H11 and 581) prior to western blot analysis for CD34, E-Ig, and P-Ig (n = 3 independent experiments). (D) CD34 immunoprecipitates were prepared from CD34pos-BM and KG1a lysates and spotted on glass slides to test for CHO-P binding using a Stamper-Woodruff assay. Adherent CHO-P cells were counted by light microscopy. The data are a representative experiment, and the error bars indicate the SEM of 7 fields per slide on 2 slides for each experiment (n = 3 independent experiments). (E) Adhesion bar graph representing results obtained for the blot-rolling assay using CHO-P cell rolling (red bars) at 0.25 dyne/cm2 (rolling cells per field) over western blots of immunoprecipitated CD34, CD44, CD43, or PSGL-1 from KG1a cell lysates as in Figure 3D. As controls, CHO-E cells were incubated with EDTA (blue bars) or mock-transfected CHO cells (CHO-M; light green bars) were used. The adhesion bar graph is the average of 4 fields of view per experiment from n = 5 independent experiments, and data are reported as the mean ± SEM (error bars). (F) CD34 immunoprecipitates from KG1a lysates were treated with neuraminidase, OSGE, or PNGase F or no treatment followed by western blot for P-Ig binding. Note that CD34 (QBend-10) was used as an internal control (data not shown) as in Figure 6 (n = 3 independent experiments). (G) To inhibit sulfation, KG1a cells were treated with 150 mM sodium chlorate for 72 hours (+; left panel) while KG1a whole-cell lysates were treated with 5 U/mL arylsulfatase for 3 hours (+; right panel) as described in supplemental Materials and methods. Negative controls consisted of the buffers used for the treatment without the sodium chlorate (−; left panel) while KG1a whole-cell lysates were treated arylsulfatase (−; right panel). Following treatment, CD34 was immunoprecipitated from cell lysates, and the resulting proteins were analyzed by western blot for P-Ig binding (top). Note that both treatments abrogated PSGL-1 binding to the KPL-1-mAb from KG1a lysates (middle; KPL-1 is sensitive to the loss of sulfation on PSGL-1), whereas the treatments did not significantly affect CD34 protein levels as indicated by EP373Y-mAb staining (bottom) or PSGL-1 staining (data not shown). Blots are representative of n = 3 independent experiments. (H) Divalent metal ion dependency and binding of P-Ig to PSGL-1 and CD34 captured from KG1a cell lysate at 150 mM NaCl were measured by injecting P-Ig (67 nM) in the presence of 10 mM EDTA followed by consecutive injection of different concentrations of P-Ig (as indicated) in the presence of 2 mM CaCl2. The experimental conditions are similar to those in Figure 4 with P-Ig injected for 170 seconds interrupted by a 60-second washing buffer step. KPL-1-mAb (11 000 RU) or MsIgG1 isotype control (5000 RU) were immobilized to capture PSGL-1 (left panel). 563-mAb (6527 RU) or MsIgG1 isotype control (5000 RU) were immobilized to capture CD34 (right panel) (n = 3). koff-apparent and KD were determined as described in Figures 3B and 4, respectively. The sensorgrams presented are corrected for the bulk refractive index and nonspecific interactions using the isotype controls.
A novel CD34 glycoform acts as a P-selectin ligand and its interaction is dependent on O-glycans and tyrosine sulfation. (A) P-Ig was used to immunoprecipitate potential ligands from KG1a and CD34pos-BM lysates, and the resultant proteins were analyzed by western blot for CD34 (QBend-10 and EP373Y mAb) or PSGL-1 (KPL-1-mAb). Note that CD34 immunoprecipitates were free from any PSGL-1 contamination (n = 3 independent experiments). (B) CD34 and PSGL-1 were immunoprecipitated from KG1a or CD34pos-BM lysates and then analyzed by western blot for P-Ig binding (n = 3 independent experiments). (C) E-Ig was used to immunoprecipitate E-selLs from the KG1a lysate and resultant proteins were eluted with 30 mM EDTA. The eluate was then immunoprecipitated with CD34 mAbs (clones 4H11 and 581) prior to western blot analysis for CD34, E-Ig, and P-Ig (n = 3 independent experiments). (D) CD34 immunoprecipitates were prepared from CD34pos-BM and KG1a lysates and spotted on glass slides to test for CHO-P binding using a Stamper-Woodruff assay. Adherent CHO-P cells were counted by light microscopy. The data are a representative experiment, and the error bars indicate the SEM of 7 fields per slide on 2 slides for each experiment (n = 3 independent experiments). (E) Adhesion bar graph representing results obtained for the blot-rolling assay using CHO-P cell rolling (red bars) at 0.25 dyne/cm2 (rolling cells per field) over western blots of immunoprecipitated CD34, CD44, CD43, or PSGL-1 from KG1a cell lysates as in Figure 3D. As controls, CHO-E cells were incubated with EDTA (blue bars) or mock-transfected CHO cells (CHO-M; light green bars) were used. The adhesion bar graph is the average of 4 fields of view per experiment from n = 5 independent experiments, and data are reported as the mean ± SEM (error bars). (F) CD34 immunoprecipitates from KG1a lysates were treated with neuraminidase, OSGE, or PNGase F or no treatment followed by western blot for P-Ig binding. Note that CD34 (QBend-10) was used as an internal control (data not shown) as in Figure 6 (n = 3 independent experiments). (G) To inhibit sulfation, KG1a cells were treated with 150 mM sodium chlorate for 72 hours (+; left panel) while KG1a whole-cell lysates were treated with 5 U/mL arylsulfatase for 3 hours (+; right panel) as described in supplemental Materials and methods. Negative controls consisted of the buffers used for the treatment without the sodium chlorate (−; left panel) while KG1a whole-cell lysates were treated arylsulfatase (−; right panel). Following treatment, CD34 was immunoprecipitated from cell lysates, and the resulting proteins were analyzed by western blot for P-Ig binding (top). Note that both treatments abrogated PSGL-1 binding to the KPL-1-mAb from KG1a lysates (middle; KPL-1 is sensitive to the loss of sulfation on PSGL-1), whereas the treatments did not significantly affect CD34 protein levels as indicated by EP373Y-mAb staining (bottom) or PSGL-1 staining (data not shown). Blots are representative of n = 3 independent experiments. (H) Divalent metal ion dependency and binding of P-Ig to PSGL-1 and CD34 captured from KG1a cell lysate at 150 mM NaCl were measured by injecting P-Ig (67 nM) in the presence of 10 mM EDTA followed by consecutive injection of different concentrations of P-Ig (as indicated) in the presence of 2 mM CaCl2. The experimental conditions are similar to those in Figure 4 with P-Ig injected for 170 seconds interrupted by a 60-second washing buffer step. KPL-1-mAb (11 000 RU) or MsIgG1 isotype control (5000 RU) were immobilized to capture PSGL-1 (left panel). 563-mAb (6527 RU) or MsIgG1 isotype control (5000 RU) were immobilized to capture CD34 (right panel) (n = 3). koff-apparent and KD were determined as described in Figures 3B and 4, respectively. The sensorgrams presented are corrected for the bulk refractive index and nonspecific interactions using the isotype controls.
Finally, we sought to validate the binding kinetics of CD34 in comparison with PSGL-1 using our SPR-based immunoprecipitation assay. The amount of detergent and salt was increased in this binding measurement relative to those in E-Ig in order to minimize the nonspecific interactions of P-Ig with the istoype control (see supplemental Materials and methods). As shown in Figure 7H, P-selectin binding to PSGL-1 and CD34 was Ca2+ dependent because binding was abolished when injected in the presence of EDTA. The mean KD of P-Ig binding to PSGL-1 was 372 ± 5 nM, whereas that of P-Ig to CD34 was 621 ± 4 nM (Figure 7H). This difference is attributed to a difference in their dissociation rates, koff-apparent for CD34 = 3 × 10−4 ± 0.1 × 10−4 s−1 and for PSGL-1 = 2 × 10−4 ± 0.3 × 10−4 s−1.
Discussion
For almost 30 years, the cell-surface sialomucin CD34 has been used as a marker to identify and enrich HSPCs in preparation for BM transplantation.1 However, studies have revealed that the CD34neg fraction of normal human BM is capable of differentiating into CD34pos subsets that possess a more activated phenotype.4-6,15 However, the CD34neg fraction suffers from a profound impairment in migration after IV transplantation compared with the CD34pos fraction.5,7,8,11,13,49-52 Our flow cytometric and western blot data revealed a more pronounced E-selL activity on the CD34pos subset of the LinnegCD38neg fraction than on the CD34neg subset, suggesting that the enhanced migratory ability of CD34pos cells is due to its possession of a specific set of functional E-selLs, required for proper migration and homing of HSPCs. In agreement with these results, a previous study illustrated that BM and fetal liver–derived CD34pos subsets rolled over immobilized E-selectin with higher efficiency than CD34neg subsets.53 Furthermore, microarray data analysis of CD34pos vs CD34neg subsets revealed exclusive expression of PSGL-1 and CD43 in the positive subset, suggesting that these ligands may be responsible for mediating interactions with E-selectin in this subset.12 Note that the lack of the HCELL glycoform found here indicates that the higher expression of CD44 in the CD34neg subset does not confer E-selectin binding activity, similar to that suggested previously.32 A recent study also implied that distinct differences in other molecules critical to homing are also differentially expressed between these 2 populations of cells and may account for the inefficiencies in homing observed with CD34neg subsets.13 These results also elicit the question of whether the appropriate glycosyltransferases/glycosidases and/or appropriate substrates for these enzymes are differentially expressed in these subpopulations of HSPCs. Such an understanding of this valuable population of cells will allow researchers to harness technologies34,54-57 that improve the migration capacity of these cells with the ultimate goal of using these cells in clinical settings.
Among several E-selL candidates suggested by our MS analysis, CD34 surfaced as an attractive ligand. CD34 is a heavily sialyated O-glycosylated type 1 transmembrane glycoprotein that is negatively charged and is speculated to behave in an antiadhesive manner, much like its relative mucin CD43.19,58,59 Using a number of biochemical and functional assays, we provide evidence that CD34 from human HSPCs binds E-selectin similar to the other well-described E-selLs, CD44 (ie, HCELL) and PSGL-1. However, unlike CD34, CD43 has been shown to contribute only modestly to the E-selectin interaction.34,60 Here, we document individual E-selL (CD34, CD44, and PSGL-1) binding affinities expressed on human HSPCs in their native form and show that all ligands display similar dissociation binding constants (KD) with slow on- and off-rate kinetics consistent with previously reported data from our laboratory.36,60 Blot-rolling assay results confirmed that CD43 has a weaker binding KD by 1.8- to 2.0-fold because of the higher rate by which CD43 dissociates from E-Ig. Furthermore, using a CD34 knockdown approach, CD34 was found to play a crucial role in slowing down the velocity of rolling cells at shear stresses ≥3 dyne/cm2. Interestingly, although an increase in E-Ig staining was observed in cell lysates from CD34 knockdown cells, this did not result in an increase in the number of rolling cells. This may be explained based on the TEM imaging data of KG1a cells outlined in this study. Knockdown of CD34 conclusively showed that microvilli structures were dramatically decreased on KG1a cells, implicating the importance of CD34 and CD34 family members in microvilli formation.18,59,61,62 Interestingly, many studies suggest that microvilli protrusions are critical for mediating interactions between KG1a cells in flow and selectins (or endothelial cells). The microvilli undergo extensions and may form tethers,63-69 so if these structures are eliminated by the knockdown of CD34, this would likely explain the effect on cell rolling. Furthermore, these data are in agreement with previous flow-based studies that showed that CD34 isolated from lysates of KG1a cells bound CHO-E cells17 and that mouse thymocytes ectopically expressing CD34 specifically bound to human BM endothelial cells where control thymocytes not expressing CD34 did not.10
Both vascular selectins are required for human CD34pos cell rolling and homing on BM microvessels, whereby defective rolling is only observed in E-/P-selectin double-knockout nonobese/severe combined immunodeficiency mice.27 Indeed, P-selectin was found to significantly purify committed human HSPCs (CD34posCD38neg cells) from total BM MNCs.24,25,70 Our data show that CD34 on HSPCs can function as an alternative P-selectin ligand. Analysis of the glycan requirements of CD34 binding to P-selectin underscore similar characteristic modifications of PSGL-1 binding to P-selectin with 1 key difference47,48 : both CD34 and PSGL-1 depend on O-glycosylation and tyrosine sulfation but only PSGL-1 requires sialylation to mediate binding.71 However, the specific glycosylation profile needed for P-selectin recognition remains unknown. For example, P-selectin may bind to sialylated and nonsialylated forms of Lex/a structures.72 Also, binding of TIM-1 (T-cell immunoglobulin and mucin domain 1), a major P-selectin ligand that controls the rolling of activated T cells, requires α1-3 fucosylation and tyrosine sulfation for efficient binding but not sialylation.73 On a similar note, CD24, a sialoglycoprotein highly expressed in neutrophils as well as at early stages of B-cell development, does not display the sLex epitope but does carry a HNK-1 sulfate-containing epitope and the O-glycans that are required for such binding.73-75 In addition to the modifications for P-selectin binding discussed above, CD34 also stained positive for the HNK-1 epitope, suggesting that it may also be important in mediating the binding of CD34 to P-selectin (data not shown). The dissociation binding constants measured here for dimeric PSGL-1 and CD34 were 372 ± 5 and 621 ± 4 nM, respectively, which are in accordance with previous studies that reported a KD of 320 ± 20 nM (with a koff = 1.4 ± 0.1 s−1 and kon = 4.4 × 106 M−1 s−1) for monomeric P-selectin binding to PSGL-1 (isolated from human neutrophils).76 The injection of membrane-derived P-selectin, mainly composed of dimeric and oligomeric forms of P-selectin, resulted in slower on and off rates compared with monomeric P-selectin.76 Our data therefore have a characteristically similar KD but with a slow apparent dissociation rate constant (koff-apparent ∼10 000-fold increase) and a slow apparent association rate constant (kon-apparent ∼100-fold reduction). This difference in binding kinetics of monomeric and dimeric forms of P-selectin is supported by previous studies.76,77
Our results show that treatment of KG1a cells with E-selectin resulted in their aggregation (supplemental Figure 3), and CD34 appeared to cluster toward lipid rafts, as indicated by an enhanced CTB staining pattern and intensity following E-selectin treatment. A number of studies have suggested that, prior to activation, CD34 is homogeneously distributed over the entire cell surface of HSPCs. However, following activation of these cells (via fibronectin or ligation using anti-CD34 antibodies), CD34 redistributes to lipid rafts and in some cases is even polarized toward the uropod, suggesting a role in enhanced homotypic adhesion.78-81 In fact, studies comparing full-length CD34, a truncated form of CD34 (where most of the cytoplasmic domain is removed), and a chimeric molecule where the cytoplasmic domain of CD34 is fused with the extracellular domain of a cytokine receptor that is not believed to have a role in adhesion, implicate the cytoplasmic domain in the mediation of signaling events causing increased adhesion.4,79 This role of CD34 in homotypic cell adhesion is significantly abrogated through the tyrosine kinase inhibitor and integrin mAb blockers for LFA-1 and ICAM-1, suggesting a concomitant activation of the LFA-1/ICAM-1 pathway.79 Thus, CD34 clustering following E-Ig treatment could enhance adhesion, downstream of selectin binding by activating LFA-1/ICAM-1 integrins and/or unmasking adhesiveness of integrins to one another or toward the endothelium.19,79,82
The CD34pos population in AML is of special interest because the leukemic stem cells likely reside in this population.83-85 Our work also reveals that a HECA-452 nonreactive form of CD34 that does not bind E-selectin was uniquely expressed on AML cells (CD34pos AML cells and KG1a cells) but not on normal CD34pos cells and could be considered a novel marker for this disease. Future studies using this glycoform of the protein as a target for the generation of mAbs may help to identify and target leukemic stem cells in the treatment of leukemia.86,87 CD34 marker expression in AML is reported to be associated with poor prognosis and apoptosis-resistance markers.88-90 Interestingly, CD34 fractions derived from AML samples with higher percentages of CD34 cells are more resistant to apoptosis than those derived from samples with a low CD34 percentage. In addition, apoptosis-related proteins are found to be differentially expressed among these populations,91 showing higher expression of antiapoptotic proteins such as Bcl-2 and Bcl-xL in CD34pos vs CD34neg subpopulations. It is appealing to consider that the special glycoform of CD34 identified in our work could be involved in mediating this anti-apoptotic behavior.
Despite CD34 being widely renowned as a marker on HSPCs, the data outlined here consider a likely function for CD34 in helping mediate the migration of HSPCs through the formation of microvilli structures and via direct interactions with vascular selectins.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors sincerely thank Samah Z. Gadhoum for her help and support throughout the project and Samir Hamdan for his time and fruitful discussions regarding the SPR analyses. The authors express their gratitude to Maryam Mih and Samar A. Rustum for their support in the management of the laboratory and also to Mohammad Imran Khan for his assistance with the confocal microscopy. Wei Xu at Imaging and Characterization core facility at King Abdullah University of Science and Technology (KAUST) was very helpful at providing analysis software and training on the confocal microscopes. The authors also thank Carolyn Unck from the KAUST Academic Writing Service for editing the manuscript. In addition, a special thanks to Aswini K. Panigrahi (MS assistance) and Alaguraj Dharmarajnadar (cell sorting on BD Influx) from the Bioscience Core Laboratory Facility at KAUST.
This research was supported by a KAUST Faculty Baseline Research Funding Program and by a Competitive Research Grant (CRG_R2_13_MERZ_KAUST_1) (J.S.M.).
Authorship
Contribution: D.B.A. designed, performed, and analyzed experiments and wrote the manuscript; F.A.A., A.S.A.-A., H.M.J.A., and A.F.A. designed, performed, and analyzed experiments; H.M.J.A., P.B., and R.S. designed, performed, and analyzed the TEM imaging experiments; C.J.C. performed and analyzed the MS experiment; and J.S.M. conceived, designed, and analyzed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.B.A. is Schepens Eye Research Institute, Massachusetts Eye & Ear, and Department of Ophthalmology, Harvard Medical School, Boston, MA.
Correspondence: Jasmeen S. Merzaban, Ibn Al Haytham Building, level 4, Office 4218, 4700 King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia; e-mail: jasmeen.merzaban@kaust.edu.sa.