Key Points
A 19-year-old ataxia-telangiectasia patient with T-cell prolymphocytic leukemia harbored 2 JAK3-activating hotspot mutations.
The patient suffered toxicities with chemotherapy, but demonstrated a clinical response to novel use of a JAK3 inhibitor (tofacitinib).
Introduction
T-cell prolymphocytic leukemia (T-PLL) is an aggressive T-cell leukemia with a mature postthymic immunophenotype.1,2 T-PLL is rare (<2% of lymphoid leukemias), with a median age at diagnosis of 61 years3 ; however, it also accounts for nearly 3% of malignancies in patients with ataxia-telangiectasia (AT), where patients are diagnosed with T-PLL at 20 years and older.4 AT is a rare, autosomal-recessive disorder associated with progressive cerebellar ataxia, telangiectasias, genomic instability, immunodeficiency,4 and cancer predisposition; 10% to 25% of patients develop malignancy, particularly T-cell neoplasms.4-6 The responsible gene (AT mutation [ATM]), located on chromosome 11q22-23, encodes a 350-kDa serine-threonine kinase involved in DNA repair and damage response.5 Nonsense ATM mutations are common in AT, whereas missense mutations are common in sporadic T-PLL.5,7 Although mechanisms of malignancy in ATM are under exploration, biallelic ATM mutations are frequently detected in sporadic T-PLL patients8 and monoallelic carriers of the ATM mutation are at risk of cancers, especially breast cancer.9 With risk of excess toxicities, treating malignancies in AT poses challenges.4,10 There are no reports of T-PLL treatment in AT to our knowledge. We describe an adolescent AT patient with T-PLL harboring JAK3 mutations, and her response to standard and targeted therapy.
Case description
A 19-year-old woman with AT presented with a 3-week history of fatigue, anorexia, and 5-kg weight loss. At baseline, she was cognitively intact but wheelchair-dependent. Her family history included a maternal history of breast cancer. Physical examination revealed scoliosis, ocular telangiectasias, diffuse lymphadenopathy (0.5-2 cm), and hepatosplenomegaly. Complete blood count revealed mild normocytic anemia (hemoglobin, 10.2 g/dL), leukocytosis with lymphocytosis (white blood cell count [WBC], 18.3 × 109/L; lymphocytes, 5.5 × 109/L), and thrombocytopenia (platelets, 79 × 109/L). Imaging (ultrasound, computed tomography) revealed bilateral pleural effusions, hepatosplenomegaly, abdominal lymphadenopathy, and ascites. Left cervical lymph node excisional biopsy showed complete effacement of the lymphoid architecture by atypical monotonous medium-sized lymphocytes (Figure 1A). By immunostains, the atypical lymphocytes were CD8+ lymphocytes (Figure 1B) positive for CD3 and TCL1 (a subset) and negative for CD1a, CD4, CD56, CD57, and TdT. Epstein-Barr virus in situ hybridization was negative. Clonal T-cell receptor gene arrangement was documented. Review of peripheral blood smears (Figure 1C) and cytospin preparations of the pleural fluid (Figure 1D) revealed medium-sized lymphocytes with nongranular basophilic cytoplasm, occasional cytoplasmic blebs, irregular nuclei, and a visible nucleolus. Bone marrow biopsy was packed (>95% cellularity) with a diffuse interstitial infiltrate of atypical lymphocytes. Chromosomal analysis showed a normal female karyotype. Flow cytometry revealed atypical lymphocytes (CD1a−, CD2+, CD3+, CD4−, CD5+, CD7+, CD8+, CD16−, CD23−, CD25−, CD30−, CD34−, CD52+, CD56−, and CD57−). She received intrathecal cytarabine (70 mg) during a diagnostic lumbar puncture, which showed no evidence of leukemia. The final diagnosis was T-PLL associated with AT.
T-PLL involving lymph node, peripheral blood, pleural fluid, and bone marrow. Diffuse infiltrate of T-PLL in the cervical lymph node by morphology (A [original magnification ×400, hematoxylin and eosin stain]) and CD8 immunostain (B [original magnification ×400]). T-PLL in the peripheral blood (C [original magnification ×1000, Wright-Giemsa stain]) and pleural fluid (D [original magnification ×1000, Wright-Giemsa stain]). Persistent and diffuse involvement of bone marrow by T-PLL status post–4-week treatment with alemtuzumab by morphology (E [original magnification ×40, hematoxylin and eosin stain]) and CD8 immunostain (F [original magnification ×40]). Addition of JAK inhibitor tofacitinib to alemtuzumab markedly decreased the leukemic cells in a repeat bone marrow biopsy by morphology (G [original magnification ×40, hematoxylin and eosin stain]) and CD8 immunostain (H [original magnification ×40]).
T-PLL involving lymph node, peripheral blood, pleural fluid, and bone marrow. Diffuse infiltrate of T-PLL in the cervical lymph node by morphology (A [original magnification ×400, hematoxylin and eosin stain]) and CD8 immunostain (B [original magnification ×400]). T-PLL in the peripheral blood (C [original magnification ×1000, Wright-Giemsa stain]) and pleural fluid (D [original magnification ×1000, Wright-Giemsa stain]). Persistent and diffuse involvement of bone marrow by T-PLL status post–4-week treatment with alemtuzumab by morphology (E [original magnification ×40, hematoxylin and eosin stain]) and CD8 immunostain (F [original magnification ×40]). Addition of JAK inhibitor tofacitinib to alemtuzumab markedly decreased the leukemic cells in a repeat bone marrow biopsy by morphology (G [original magnification ×40, hematoxylin and eosin stain]) and CD8 immunostain (H [original magnification ×40]).
Methods
She required mechanical ventilation and chest tube placement prior to treatment with IV alemtuzumab (day 1, 3 mg; day 3, 10 mg; day 5 then 3 times weekly, 30 mg]. St. Jude Children’s Research Hospital AT supportive care guidelines were followed.10 After 14 days of alemtuzumab (day A14), she was without supplemental oxygen or chest tubes. Her peripheral blood lymphocytes were trending down (Figure 2A). However, bone marrow biopsy day A33 revealed a packed marrow (>95% cellularity) with diffuse infiltration by CD8+ lymphocytes (Figure 1E-F) and pleural fluid showed leukemic cells (92% abnormal CD8+ lymphocytes by flow cytometry). She continued alemtuzumab (30 mg 3 times weekly). Pentostatin (4 mg/m2) was added. Despite pre- and posthydration along with continuous IV fluids, she developed acute renal failure requiring hemodialysis (peak creatinine, 2.7 mg/dL; cystatin-C, 3.71 mg/L; glomerular filtration rate, 21 mL per minute). No further pentostatin was given. She required mechanical ventilation and bilateral chest tube placement. Due to her underlying disease and clinical status, it was felt she would not tolerate alkylators or comparable treatment. After review of the literature, a JAK3 inhibitor (tofacitinib) was added (5 mg daily due to renal compromise and concomitant fluconazole, a strong CYP3A4 inhibitor) to alemtuzumab. Deep sequencing of the lymph node (results returned after urgent initiation of therapy) revealed 2 activating mutations of JAK3 (A573V, M511I), nonsense mutation of ATM R457, and missense mutation of vascular endothelial growth factor receptor-3 (FLT4) G71R; the latter has not been previously reported in T-PLL, and its functions have not been characterized.
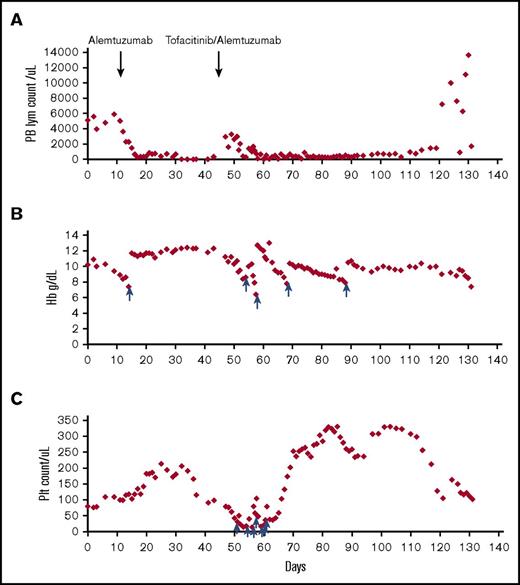
Trends of peripheral counts through treatment. Absolute lymphocyte count (A), hemoglobin (B), and platelet count (C) in the peripheral blood before and after treatment are shown in the y-axis; x-axis represents timeline in days. The time points at which alemtuzumab and tofacitinib were added are indicated by arrows. The time points at which the patient received red blood cell transfusions for panel B and platelet transfusions for panel C are indicated by blue arrows. Hb, hemoglobin; PB, peripheral blood; Plt, platelet.
Trends of peripheral counts through treatment. Absolute lymphocyte count (A), hemoglobin (B), and platelet count (C) in the peripheral blood before and after treatment are shown in the y-axis; x-axis represents timeline in days. The time points at which alemtuzumab and tofacitinib were added are indicated by arrows. The time points at which the patient received red blood cell transfusions for panel B and platelet transfusions for panel C are indicated by blue arrows. Hb, hemoglobin; PB, peripheral blood; Plt, platelet.
Two days after starting tofacitinib (day T2), she was extubated and chest tubes were removed (day T3, day T9). Pleural fluid (day T8) showed reduced CD8+ lymphocytes (0.7% of abnormal CD8+ lymphocytes by flow cytometry). Pleural effusions and ascites reaccumulated on day T23 and tofacitinib was increased to 5 mg twice daily, with normalized creatinine and fluconazole discontinued. She weaned off supplemental oxygen; day T52 bone marrow showed reduced cellularity with 10% of CD8+ lymphocytes (Figure 1G-H). Figure 2 presents a summary of peripheral counts through treatment.
Results and discussion
The patient was in rehabilitation (on room air) on day T62 when she developed progressive abdominal pain and subcutaneous nodules. Peripheral blasts became evident (18%) on day T70, she resumed supplemental oxygen, and palliative chest tubes were placed to avoid intubation. On day T72, she completed 16 weeks of alemtuzumab, and her WBC rose to 57 000 (66% blasts). She was discharged home with hospice on day T75 with a WBC of 171 000 (90% blasts). She died peacefully on day T77.
Despite responses to chemo/immunotherapy (alemtuzamab11,12 with/without pentostatin,13 hematopoietic cell transplantation,14 fludarabine/mitoxantrone/cyclophosphamide,15 or methylpredinisolone16 and other alkylators),17 the median progression-free survival (8-12 months) and overall survival (20-24 months) in T-PLL remain dismal.18 Longer survival is seen with hematopoietic cell transplantation; however, 33% to 47% of patients relapse within 36 months and treatment-related mortality is almost as high.3 AT patients face surplus treatment-related toxicities4 due to underlying hypersensitivity to DNA-damaging agents. However, AT patients who achieve a response to cancer treatment see increased survival.4 Thus, it is crucial to identify approaches to T-PLL in patients with AT that are tolerable and avoid end-organ complications in order to optimize quality and quantity of life.
Targeted agents provide precision in therapy that is well suited to patients at high risk of end-organ complications, but use in these patients is not widely documented. AT patients with malignancy are ideally suited to this approach due to their additional risks. Mutational analysis in sporadic T-PLL (n = 51) revealed mutations in ATM (73%), JAK1 (6%), and JAK3 (21%), among which JAK3 mutation is associated with poor prognosis.19 Similarly, whole-exome sequencing of sporadic T-PLL (n = 50) detected JAK1 (8%) and JAK3 mutations (30%).20 An additional TPLL series detected JAK3 missense mutations (43%), with M511I and A573V identified as primary and secondary hotspot-activating mutations21 ; M511I led to the most efficient oncokinase with the highest transforming activities.22 Both mutations were identified concurrently in our patient. However, findings regarding the lack of correlation between specific mutations (JAK3) and successful ex vivo inhibition (tofacitinib) are consistent with the occasional poor correlation between appropriately targeted therapy and clinical outcome.23 However, tofacitinib additionally salvaged refractory T-PLL in 1 elderly patient,24 and 9 T-cell large granular lymphocytic leukemia patients,25 including 8 with associated rheumatoid arthritis; tofacitinib is currently US Food and Drug Administration approved for refractory rheumatoid arthritis. Separating the independent effects of JAK3 inhibition in this case is challenging; prospective studies are necessary to evaluate JAK3 inhibition in T-PLL, especially with combination therapies.
This case identifies a promising response to JAK3 inhibition with tofacitinib in T-PLL with underlying AT, providing the opportunity for rehabilitation and meaningful interaction before allowing natural death to progress from disease. It is conceivable that had a targeted agent without surplus toxicity been tried earlier in her course, quality and quantity of life could have been optimized.
Acknowledgments
This work was supported by the St. Baldrick’s Scholar Career Development Award (J.W.).
Authorship
Contribution: J.W. and G.L. wrote the manuscript; E.W. reviewed and revised the manuscript; and G.L., J.W., and E.W. all participated in acquisition of data and interpretation of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie Wolfson, 1600 7th Ave S, Lowder 500, Birmingham, AL 35233; e-mail: jwolfson@peds.uab.edu.

![Figure 1. T-PLL involving lymph node, peripheral blood, pleural fluid, and bone marrow. Diffuse infiltrate of T-PLL in the cervical lymph node by morphology (A [original magnification ×400, hematoxylin and eosin stain]) and CD8 immunostain (B [original magnification ×400]). T-PLL in the peripheral blood (C [original magnification ×1000, Wright-Giemsa stain]) and pleural fluid (D [original magnification ×1000, Wright-Giemsa stain]). Persistent and diffuse involvement of bone marrow by T-PLL status post–4-week treatment with alemtuzumab by morphology (E [original magnification ×40, hematoxylin and eosin stain]) and CD8 immunostain (F [original magnification ×40]). Addition of JAK inhibitor tofacitinib to alemtuzumab markedly decreased the leukemic cells in a repeat bone marrow biopsy by morphology (G [original magnification ×40, hematoxylin and eosin stain]) and CD8 immunostain (H [original magnification ×40]).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/27/10.1182_bloodadvances.2017010470/3/m_advances010470f1.jpeg?Expires=1769136865&Signature=WZnN7A3FDQTnVohkrbuTOjpPY8EwqBDVanO1EPGd6Az2EiM7D-4qfQiZjjHFCiLYZ~INXTUjiYyZGQ8mmTXamZX0hS0SKue2wDTQCY0wY~-nr2O5Om8bGGfsnYk~hTrUL9EZBIVnSlMOcts1fKEX~KThKhX7qtq3Gcax9JVaVmtT3VAbB~upF6ix98ZqWYh2AOQeyWTCNB4ANqfj3hssXihnVyQRMNVIxVIoacC6C7Z8vZgTxUsV4cd6PMdy6ub95hKWepewxLyCLs5-H1mY5vxTT1PeRg~W7aoic2q-E8CQOXqZGZOBR6dEM3I9I1WlztyUF3Wxl2iW-ziYdSx8MA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)