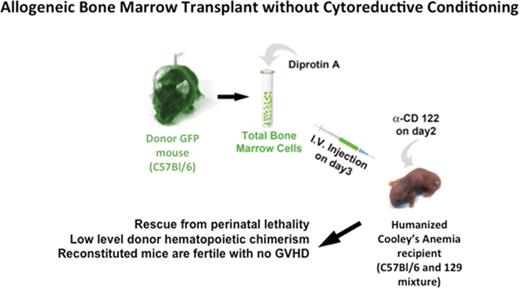
Key Points
After completion of the fetal-to-adult globin gene switch, humanized β-thalassemia major mice are transfusion dependent.
Perinatal humanized β-thalassemia major mice are rescued by bone marrow transplantation in the absence of cytoreductive conditioning.
Abstract
β-thalassemia is a group of inherited blood disorders that result in defects in β-globin chain production. Cooley anemia (CA), or β-thalassemia major, is the most severe form of the disease and occurs when an individual has mutations in both copies of the adult β-globin gene. Patients with CA fail to make adult hemoglobin, exhibit ineffective erythropoiesis, experience severe anemia, and are transfusion dependent for life. Currently, allogeneic bone marrow transplantation (BMT) is the only cure; however, few patients have suitable donors for this procedure, which has significant morbidity and mortality. In this study, a novel humanized murine model of CA is rescued from lethal anemia by allogeneic BMT in the absence of cytoreductive conditioning. A single intravenous postnatal injection of allogeneic bone marrow results in stable, mixed hematopoietic chimerism. Five months after transplantation, donor cells accounted for approximately 90% of circulating erythrocytes and up to 15% of hematopoietic stem and progenitor cells. Transplanted mice are transfusion independent, have marked improvement of hematological indices, exhibit no growth retardation or signs of graft-versus-host disease, and are fertile. This study describes a method for the consistent engraftment of allogeneic donor hematopoietic cells that rescues a humanized mouse model of CA from lethal anemia, all in the absence of toxic cytoreductive conditioning.
Introduction
β-thalassemia is a heterogeneous group of inherited blood disorders marked by defects in the production of the adult β-globin chains of hemoglobin. Cooley anemia (CA), or β-thalassemia major, is the most severe form of the disease and results in a complete absence of β-globin chains and thus the major adult form of hemoglobin (HbA).1-4 Without β-globin chains, functional α2β2 tetramers cannot be formed and the excess α-globin chains precipitate to form intracellular inclusions. This severe chain imbalance ultimately leads to the premature destruction of erythroid progenitors and peripheral red blood cell aplasia.5-7 The dearth of functional red blood cells (RBCs) stimulates a massive erythroid hyperplasia in the bone marrow; however, this ineffective erythropoiesis yields no HbA-containing RBCs. The limited, α-globin inclusion-laden RBCs that escape intramedullary marrow destruction undergo premature intravascular hemolysis or destruction by the splenic reticuloendothelial system.2,8,9
Fortunately in humans, newborns with CA are healthy because of the presence of high levels of gestational fetal hemoglobin (HbF) at birth, which is composed of two α-globin and two γ-globin chains (α2γ2).10,11 However, during the first year of life, a developmentally programmed hemoglobin switch is completed, which shifts hemoglobin production from HbF to the major and minor adult hemoglobin forms, HbA and HbA2, respectively. As postnatal RBC HbF levels drop, patients with CA experience severe microcytic, hypochromic anemia and become dependent on lifelong periodic blood transfusions.2 Unfortunately, chronic transfusions result in an increasing iron load that necessitates daily iron chelation therapy, an expensive and sometimes uncomfortable treatment.12-14 Ultimately, patients receiving transfusion and chelation therapies die of their disease at a young age, most commonly due to congestive heart failure, which is a consequence of iron toxicity.15
Currently, allogeneic bone marrow transplantation (BMT) is the only cure for CA.16-19 Donor hematopoietic stem and progenitor cells (HSPCs) engraft in the bone marrow niche and reconstitute the erythron with RBCs containing functional HbA. Successful BMT typically requires a willing, unaffected, HLA-matched related donor.20,21 Approximately 60% of CA patients do not have such a donor. If a nonrelative or only partial HLA-matched donor is used, there is a 55% chance of graft failure or rejection.22 Furthermore, the chemical myeloablation, total body irradiation, and immunosuppression used for BMT conditioning carry a high risk of complications. High-risk patients only have a 53% chance of event-free survival with this procedure; adverse events include death, graft rejection, graft-versus-host disease (GVHD), and infection.20,21,23,24
Nonmyeloablative conditioning regimens have been developed to minimize the morbidity and mortality associated with allogeneic BMT. Reduced intensity conditioning has been used for nonmalignant diseases in mouse models as well as for leukemia in humans.25-34 In particular, for older patients or patients who have comorbidities that prohibit the use of conventional conditioning regimens, a nonmyeloablative transplantation strategy could be a safer alternative.35-38 Nonlethal conditioning is especially attractive for patients with CA and sickle cell disease (SCD) who may have prolonged survival time with current medical treatment without adding the mortality risk associated with standard myeloablative preparation regimens. Nonmyeloablative transplantation for CA and SCD lowers transplant-related mortality risk while potentially establishing stable, mixed hematopoietic chimerism in the recipient.39-48 However, the variable outcomes from limited clinical experience warrant a more thorough investigation of the benefits and risks.
Early experiments by Billingham et al39 showed that tolerance can be induced in neonatal mice suggesting that ablative conditioning may be unnecessary for establishing mixed chimerism. For nonmalignant hematopoietic diseases such as CA, the question remains whether the low-level engraftment attained in the absence of ablative conditioning can be maintained long-term and produce enough RBCs to correct the clinical symptoms of anemia. We have developed humanized mouse models of CA that survive solely upon human HbF at birth.49-52 These mice die of lethal anemia before weaning age as they complete their fetal-to-adult hemoglobin switch to a nonfunctional adult human β-globin gene. In this report, we rescue a novel humanized CA mouse model from lethal postnatal anemia by a single intravenous injection of allogeneic bone marrow in the absence of cytoreductive conditioning.
Materials and methods
Mice
All animal procedures were approved by the University of Alabama at Birmingham’s (UAB's) Institutional Animal Care and Use Committee. Humanized CA mice were derived by targeted gene replacement in ES cells (supplemental Results). Donor bone marrow was obtained from green fluorescent protein (GFP) transgenic mice53 on C57BL/6 background (C57BL/6-Tg[UBC-GFP]30Scha/J, stock number 004353, The Jackson Laboratory, Bar Harbor, ME). Donor bone marrow was harvested from the tibias and femurs of euthanized mice.
Noncytoreductive allogeneic BMT
Recipient humanized CA and control mice were administered 8 μg of anti-CD122 antibody (eBioscience) per mouse on day 2 of life. Donor total bone marrow cells (BMCs) from GFP transgenic mice53 were incubated in 5 mg/mL Diprotin A (Peptides International, Louisville, KY) in Dulbecco’s phosphate-buffered saline (ThermoFisher Scientific) for 20 minutes at room temperature. A single dose of 1.5 × 107 BMCs in 20-μL Dulbecco’s phosphate-buffered saline was injected per mouse through the facial vein. Assuming 10% RBCs in total marrow and only 1 in 104 nucleated cells are hematopoietic stem cells (HSCs), this dose represents approximately 1350 HSCs injected.
Hematology and HPLC analysis of hemoglobin chains
Blood was collected periodically after transplant and analyzed on a HemaVet 1700 (Drew Scientific, Waterbury, CT) hematology analyzer for RBC counts and RBC distribution widths. Packed cell volume was measured in a JorVet J503 (Jorgenson Laboratories, Loveland, CO) microhematocrit centrifuge. Hemoglobin concentrations were determined after conversion to cyanmethemoglobin in Drabkin’s Reagent (Sigma-Aldrich, St. Louis, MO), removal of insoluble RBC membranes, measuring the absorbance at 540 nm on a spectrophotometer, and comparison with hemoglobin standards. Hemolysates were prepared and high-performance liquid chromatography (HPLC) performed as previously described.49
Flow cytometry analysis
The ratio of GFP-positive peripheral blood cells was periodically determined by flow cytometry of tail tip blood on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Peripheral blood white blood cells (WBCs) and BMCs were analyzed by flow cytometry on an LSR-II (Becton Dickinson). WBCs were separated from RBCs in 2% dextran followed by ammonium chloride lysis. The remaining WBCs were stained with the following fluorescently labeled antibodies: B220-Pacific Blue, Mac-1-APC, and Gr-1-PE-Cy7. For HSPC chimerism analysis, isolated BMCs were washed, pelleted, and stained with a blocking antibody to the FcγII/III receptor (2.4G2; BD Biosciences–Pharmingen, San Diego, CA) for 20 minutes at 4°C. After blocking, cells were stained for 25 minutes at 4°C with c-Kit-APC (2B8), Sca-1-Pacific Blue (E13-161.7), and Lineage-PE-Cy-7 cocktail. The Lineage cocktail included antibodies to CD3 (145-2C11), CD4 (GK1.5), CD5 (53-7.3), CD8 (53-6.7), Gr-1 (8C5), B220 (6B2), and Ter119.
GVHD determination
We evaluated acute GVHD by monitoring body weight and fur integrity. For determination of chronic GVHD, the lung, intestine, and skin were fixed in 70% alcoholic formalin 5 months after transplantation. The tissues were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Tissues were examined for the presence of lymphocytic infiltration as evidence of chronic GVHD.
Statistical analyses
Data values are reported as the mean ± standard deviation (supplemental Figure 3) or mean ± standard error of the mean (SEM) (Table 2). P values were calculated by the 2-tailed Student t test (Table 2; supplemental Figure 3) or a 1-way analysis of variance (Table 1).
Summarized chimerism data of CA mice and control mice in the absence of cytoreductive conditioning and with reduced-intensity myeloablation
| Genotype . | n . | Conditioning . | . | Final RBC chimerism . | HSPC chimerism . | Granulocyte chimerism . |
|---|---|---|---|---|---|---|
| α2α1/α2α1, γHPFHβ0/γHPFHβ0 | 12 | anti-CD122, Diprotin A | Mean | 87.9* | 5.2 | 4.8 |
| Range | 59.3-98.9 | 0.5-15.7 | 0.8-10.6 | |||
| SEM | 3.67 | 1.28 | 1.07 | |||
| α2α1/α2α1, γHPFHβ0/+ | 7 | Mean | 1.2* | ND | 1.7 | |
| Range | 0.0-4.2 | 0.0-6.0 | ||||
| SEM | 0.63 | 0.87 | ||||
| α2α1/α2α1, +/+ | 5 | Mean | 2.6* | ND | 1.5 | |
| Range | 0.0-6.1 | 0.0-6.4 | ||||
| SEM | 1.35 | 1.24 | ||||
| α2α1/α2α1, γHPFHβ0/γHPFHβ0 | 5 | 400 rad | Mean | 99.7 | 57.7 | 59.5 |
| Range | 98.8-99.9 | 15.1-97.1 | 18.8-83.7 | |||
| SEM | 0.22 | 16.84 | 12.91 |
| Genotype . | n . | Conditioning . | . | Final RBC chimerism . | HSPC chimerism . | Granulocyte chimerism . |
|---|---|---|---|---|---|---|
| α2α1/α2α1, γHPFHβ0/γHPFHβ0 | 12 | anti-CD122, Diprotin A | Mean | 87.9* | 5.2 | 4.8 |
| Range | 59.3-98.9 | 0.5-15.7 | 0.8-10.6 | |||
| SEM | 3.67 | 1.28 | 1.07 | |||
| α2α1/α2α1, γHPFHβ0/+ | 7 | Mean | 1.2* | ND | 1.7 | |
| Range | 0.0-4.2 | 0.0-6.0 | ||||
| SEM | 0.63 | 0.87 | ||||
| α2α1/α2α1, +/+ | 5 | Mean | 2.6* | ND | 1.5 | |
| Range | 0.0-6.1 | 0.0-6.4 | ||||
| SEM | 1.35 | 1.24 | ||||
| α2α1/α2α1, γHPFHβ0/γHPFHβ0 | 5 | 400 rad | Mean | 99.7 | 57.7 | 59.5 |
| Range | 98.8-99.9 | 15.1-97.1 | 18.8-83.7 | |||
| SEM | 0.22 | 16.84 | 12.91 |
Chimerism data were collected after 150 d upon euthanasia of recipients. Chimerism values represent percentage of GFP+ donor cells among RBCs, HSPCs (Lin–Sca1+cKit+), and granulocytes (Gr1+). Means, ranges, and SEMs are shown. RBC chimerism was significantly different between CA mice and control mice as determined by 1-way analysis of variance. By the same test, there was no statistically significant difference between granulocyte chimerism levels within the groups undergoing noncytoreductive conditioning.
ND, not determined.
P ≤ .00001.
Results
Establishment of the humanized CA mouse model
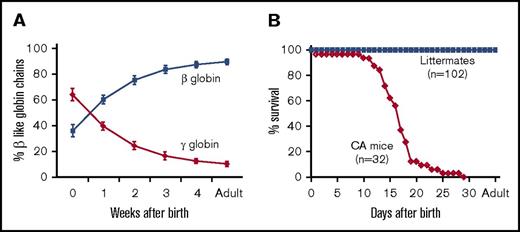
In an attempt to mimic the postnatal onset of anemia that is observed in patients with CA, the mouse adult β-globin genes were replaced by a human γHPFHβ0 globin gene cassette in mouse embryonic stem cells (supplemental Figure 1). The human γ-globin gene promoter contained the Greek-type hereditary persistence of fetal hemoglobin (HPFH) mutation54 to postpone the fetal-to-adult hemoglobin switch, and the human β-globin gene contained a splice donor site mutation to generate a nonfunctional β0 thalassemia allele.49-51 These γHPFHβ0 knock-in (KI) mice were further humanized by breeding to human α2α1 globin gene KI mice to produce animals that survive solely on human hemoglobin.49-52 Postnatal hemolysates from fully humanized compound heterozygous β-thalassemia trait mice (α2α1/α2α1, γHPFHβ0/γβA) demonstrate that the human γHPFHβ0 cassette delays the human fetal-to-adult globin gene switch in KI mice and mimics the postnatal hemoglobin-switching pattern observed in humans (Figure 1A). Homozygous CA mice (α2α1/α2α1, γHPFHβ0/γHPFHβ0) had a median life span of 17 days surviving solely on human HbF (Figure 1B). This 17‐day life span in the mouse corresponds to 1.5 to 2 years of age for a patient with untreated CA.
Hemoglobin switching and survival curve analyses of humanized CA mice. (A) Human fetal-to-adult globin gene switching was analyzed in humanized γHPFHβ0/γβA compound heterozygous mice after birth. Human γ-globin and β-globin chain levels in peripheral blood hemolysates were measured by HPLC and plotted as a percentage of total β-like chains across time. High fetal globin chain levels at birth are gradually replaced by adult chains during the first 5 weeks of life. (B) The fractional survival of fully humanized CA (α2α1/α2α1, γHPFHβ0/γHPFHβ0) and littermate control mice are plotted across time. The majority of CA mice die prior to weaning age with a median lifespan of 17 days, whereas all the control littermates survive into adulthood.
Hemoglobin switching and survival curve analyses of humanized CA mice. (A) Human fetal-to-adult globin gene switching was analyzed in humanized γHPFHβ0/γβA compound heterozygous mice after birth. Human γ-globin and β-globin chain levels in peripheral blood hemolysates were measured by HPLC and plotted as a percentage of total β-like chains across time. High fetal globin chain levels at birth are gradually replaced by adult chains during the first 5 weeks of life. (B) The fractional survival of fully humanized CA (α2α1/α2α1, γHPFHβ0/γHPFHβ0) and littermate control mice are plotted across time. The majority of CA mice die prior to weaning age with a median lifespan of 17 days, whereas all the control littermates survive into adulthood.
Allogeneic BMT of humanized CA mice without cytoreductive conditioning
As a positive control for these studies, nonmyeloablative BMT was performed on newborn CA mice by use of a moderate dose of radiation (supplemental Results). Although donor HSPC chimerism was quite variable at this dose (range, 15.1%‐97.1% GFP+ Lin−Sca1+ckit+ [LSK] cells), transplant-derived RBC chimerism rises to 99% by 9 weeks after transplantation (Table 1; supplemental Table 1)
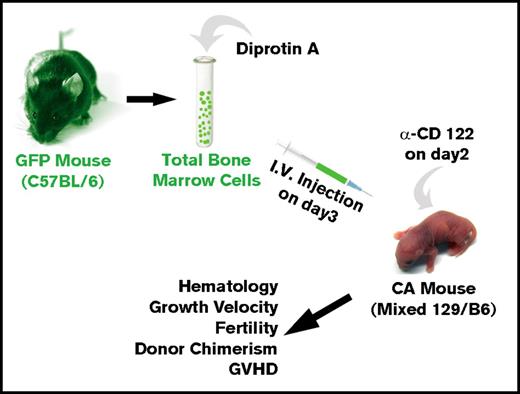
We then asked whether allogeneic BMT of neonatal CA mice in the absence of cytoreductive conditioning could result in high-enough engraftment levels to rescue CA mice from lethal anemia (Figure 2). Humanized CA mice have a mixed background of C57Bl/6 and 129 strains. Although the major histocompatibility locus of both of these strains is H-2b, there are differences in minor histocompatibility antigens between these 2 mouse strains. C57Bl/6 GFP transgenic mice were used for BMT donors. In addition to expressing GFP in their hematopoietic cells, the erythroid cells in the GFP mice express murine α-globin and β-globin, which are foreign to the CA recipient mice. Donor BMCs were treated with Diprotin A, a CD26 inhibitor, to increase their engraftment efficiency.55-57 The CA recipient mice received anti-CD122 antibody 1 day prior to BMT to suppress the immune system.58,59 Three-day-old CA mice were injected via the facial vein with 1.5 × 107 total donor BMCs (∼1350 HSCs). Transplanted mice were weaned at age 3 weeks and were bled periodically to evaluate their anemia and hematopoietic chimerism.
Method for allogeneic BMT of humanized CA mice in the absence of cytoreductive conditioning. Donor total BMCs from GFP transgenic mice were treated with Diprotin A and injected intravenously into 3-day-old humanized CA recipients that received a single injection of anti-CD122 antibody on day 2 after birth. Transplanted CA mice were analyzed across time for rescue from lethal anemia, hematology, growth velocity, fertility, hematopoietic chimerism, and GVHD.
Method for allogeneic BMT of humanized CA mice in the absence of cytoreductive conditioning. Donor total BMCs from GFP transgenic mice were treated with Diprotin A and injected intravenously into 3-day-old humanized CA recipients that received a single injection of anti-CD122 antibody on day 2 after birth. Transplanted CA mice were analyzed across time for rescue from lethal anemia, hematology, growth velocity, fertility, hematopoietic chimerism, and GVHD.
Transplanted CA mice are rescued from lethal anemia
CA recipients transplanted in the absence of cytoreductive conditioning were rescued from lethal anemia and survived 5 months after transplantation, at which time they were euthanized for analysis of their hematopoietic system and histopathological features (Table 1; supplemental Table 1). It is interesting to note that control transplantations performed in the absence of either Diprotin A or anti-CD122 treatment failed to rescue CA mice (Table 1; supplemental Table 1). Because patients with CA experience growth retardation, hypogonadism, and infertility due to a combination of their anemia, siderosis, and/or toxicity from chemotherapeutic agents during BMT,60,61 we examined the growth velocity and fertility of the recipient CA mice after transplantation. Humanized CA mice have stunted growth due to anemia during their limited life span (supplemental Figures 2 and 3). In the first week after transplantation, the body weights of CA mice were significantly lower than those of the littermates (P < .01). From the second week after transplantation through adulthood, there was no significant difference between the transplanted CA mice and the littermate control mice (supplemental Figure 3). This growth pattern is consistent with CA mice that are rescued by chronic transfusion therapy (data not shown).
Conventional treatment and conditioning regimens for BMT in patients with CA result in iron overload, hypogonadism, delayed sexual maturation, and sterility.60,62 Therefore, we investigated whether CA mice transplanted without cytoreductive conditioning were healthy enough to sire offspring. We tested the fertility of transplanted CA male mice by breeding to β-thalassemia trait females in the post-transplant period. One CA male sired 5 litters, producing 23 pups that were a mixture of homozygous β-thalassemia major and β-thalassemia trait genotypes (supplemental Table 2). These data demonstrate that CA mice that were rescued from lethal anemia by BMT in the absence of cytoreductive conditioning had normal growth velocity and were fertile.
Transplantation results in stable, mixed-lineage hematopoietic chimerism
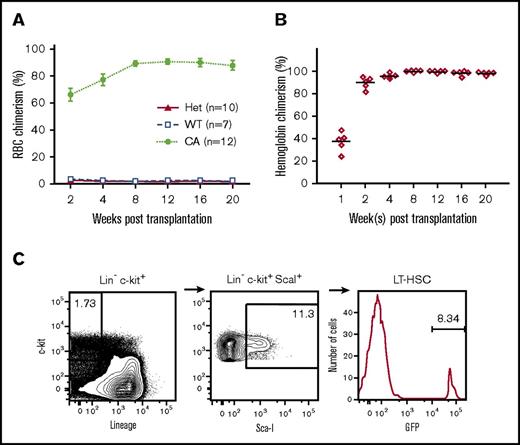
Erythroid reconstitution by transplanted donor cells was monitored across time by analysis of peripheral RBC chimerism by flow cytometry and hemoglobin chimerism in hemolysates by HPLC (Figure 3A-B, respectively). The GFP-positive donor RBCs in the peripheral blood of CA mice increased to more than 90% by 8 weeks after transplantation (Figure 3A). Peripheral blood hemolysates demonstrated that the donor-derived murine globin chains increased from 37.2 ± 3.8% of total chains 1 week after transplantation to 98.8 ± 0.4% at 2 months after transplantation and remained stable thereafter (Figure 3B). It is interesting to note that wild-type and heterozygous control recipients had markedly lower donor RBC chimerism that matched their low granulocyte (Gr-1+) levels (Figure 3; Table 1; supplemental Table 1). The short-lived peripheral blood granulocyte levels are a surrogate measure for the HSPC chimerism present in the bone marrow.
Stable hematopoietic chimerism established in transplanted CA mice in the absence of cytoreductive conditioning. (A) The percentage of donor GFP+ RBCs in peripheral blood was measured across time. Stable donor RBC chimerism levels at approximately 90% were maintained from 8 to 20 weeks after transplantation in CA mice compared with levels of less than 5% in wild-type (WT = α2α1/α2α1, +/+) and heterozygous (Het = α2α1/α2α1, γHPFHβ0/+) littermate control mice. (B) Donor hemoglobin levels measured across time in CA mice after transplantation. Donor mouse β-globin and recipient human γ-globin chain levels were measured in peripheral blood hemolysates by HPLC. Stable donor-derived globin chain levels greater than 95% of total globin chains were achieved by 8 weeks after transplantation. (C) HSPCs were detected in the bone marrow of CA mice 20 weeks after transplantation. Recipient CA mice were euthanized 20 weeks after transplantation and their BMCs analyzed by flow cytometry after staining with fluorescently labeled antibodies that recognize c-kit+, ScaI+, and a cocktail of lineage (Lin) antigens. Donor HSPCs were determined by the percentage of GFP+ cells in the Lin–c-kit+ScaI+ population. Relatively low levels of donor HSPCs (range, 0.5%‐15.7%) resulted in high-donor RBC chimerism and rescue from lethal anemia.
Stable hematopoietic chimerism established in transplanted CA mice in the absence of cytoreductive conditioning. (A) The percentage of donor GFP+ RBCs in peripheral blood was measured across time. Stable donor RBC chimerism levels at approximately 90% were maintained from 8 to 20 weeks after transplantation in CA mice compared with levels of less than 5% in wild-type (WT = α2α1/α2α1, +/+) and heterozygous (Het = α2α1/α2α1, γHPFHβ0/+) littermate control mice. (B) Donor hemoglobin levels measured across time in CA mice after transplantation. Donor mouse β-globin and recipient human γ-globin chain levels were measured in peripheral blood hemolysates by HPLC. Stable donor-derived globin chain levels greater than 95% of total globin chains were achieved by 8 weeks after transplantation. (C) HSPCs were detected in the bone marrow of CA mice 20 weeks after transplantation. Recipient CA mice were euthanized 20 weeks after transplantation and their BMCs analyzed by flow cytometry after staining with fluorescently labeled antibodies that recognize c-kit+, ScaI+, and a cocktail of lineage (Lin) antigens. Donor HSPCs were determined by the percentage of GFP+ cells in the Lin–c-kit+ScaI+ population. Relatively low levels of donor HSPCs (range, 0.5%‐15.7%) resulted in high-donor RBC chimerism and rescue from lethal anemia.
Because HSPC engraftment is critical for sustained hematopoietic chimerism, we investigated the donor HSPC chimerism at 5 months after transplantation. BMCs were collected from CA recipient mice and analyzed for the presence of donor GFP+ LSK cells. GFP+ LSK cells were detected in all analyzed mice with an average level of 5.2% ± 2.5% (range, 0.54%‐15.7%) (Figure 3C; Table 1; supplemental Table 1). In a similar manner, donor-derived myeloid and lymphoid lineages were also present at levels corresponding to the low level of donor HSPC chimerism (Figure 4; supplemental Table 1). These data demonstrate that in the absence of cytoreductive conditioning, stable, mixed-lineage hematopoietic chimerism was established in CA mice. It is remarkable that even the lowest level of 0.54% HSPC chimerism resulted in transfusion-independent survival, although the histopathological and hematological features in animals with less than 5% donor HSPC engraftment were similar to those in β-thalassemia intermedia mice.50,63 It is important to note that CA mice with donor HSPC chimerism levels of 8% and higher had corrected phenotypes comparable to those of wild-type control mice (Table 2).
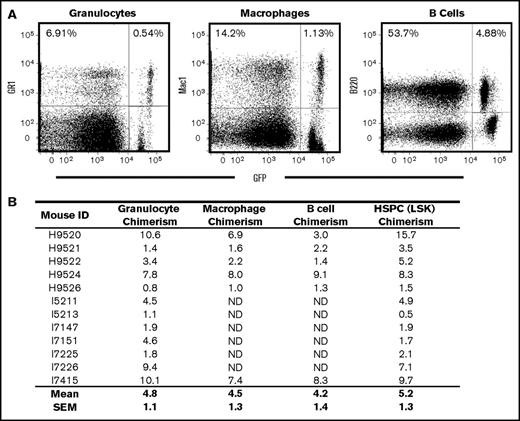
Myeloid and lymphoid chimerism in humanized CA mice after BMT in the absence of cytoreductive conditioning. (A) Bone marrow cells from CA mice 20 weeks after transplantation were stained with fluorescently labeled antibodies that recognize myeloid (Gr-1 and Mac1) and lymphoid (B220) antigens and analyzed by flow cytometry. Donor-derived myeloid and lymphoid cells were determined by GFP fluorescence. Chimerism levels are determined by the ratio of GFP-positive cells to total positive cells for each lineage antibody tested. For this representative mouse, donor-derived Gr-1, Mac1, and B220-positive cells were 7.8% (0.54/6.91), 8.0% (1.13/14.2), and 9.1% (4.88/53.7), respectively. (B) Donor chimerism was consistent between multiple lineages and HSPCs. Overall, the mean (± SEM) granulocyte (Gr-1), macrophage (Mac1), B-cell (B220), and HSPC (LSK) donor chimerism levels for 6 of the transplanted CA mice tested were 5.2% (±1.6%), 4.5% (±1.3%), 4.2% (±1.4%), and 7.4% (±2.1%), respectively.
Myeloid and lymphoid chimerism in humanized CA mice after BMT in the absence of cytoreductive conditioning. (A) Bone marrow cells from CA mice 20 weeks after transplantation were stained with fluorescently labeled antibodies that recognize myeloid (Gr-1 and Mac1) and lymphoid (B220) antigens and analyzed by flow cytometry. Donor-derived myeloid and lymphoid cells were determined by GFP fluorescence. Chimerism levels are determined by the ratio of GFP-positive cells to total positive cells for each lineage antibody tested. For this representative mouse, donor-derived Gr-1, Mac1, and B220-positive cells were 7.8% (0.54/6.91), 8.0% (1.13/14.2), and 9.1% (4.88/53.7), respectively. (B) Donor chimerism was consistent between multiple lineages and HSPCs. Overall, the mean (± SEM) granulocyte (Gr-1), macrophage (Mac1), B-cell (B220), and HSPC (LSK) donor chimerism levels for 6 of the transplanted CA mice tested were 5.2% (±1.6%), 4.5% (±1.3%), 4.2% (±1.4%), and 7.4% (±2.1%), respectively.
Hematology data of control and CA mice with and without transplantation
| Genotype . | n . | RBC, 106/μL . | Hb, g/dL . | PCV, % . | RDW, % . |
|---|---|---|---|---|---|
| Wild-type control | 8 | 8.8 ± 0.2 | 13.8 ± 0.4 | 43.8 ± 0.8 | 16.1 ± 0.2 |
| CA mice nontransplanted | 10 | 2.6 ± 0.4 | 2.5 ± 0.4 | 13.6 ± 1.9 | 39.9 ± 1.2 |
| CA mice transplanted | 5 | 6.4 ± 0.7* | 11.5 ± 1.4* | 32.4 ± 3.0* | 18.9 ± 1.2* |
| Genotype . | n . | RBC, 106/μL . | Hb, g/dL . | PCV, % . | RDW, % . |
|---|---|---|---|---|---|
| Wild-type control | 8 | 8.8 ± 0.2 | 13.8 ± 0.4 | 43.8 ± 0.8 | 16.1 ± 0.2 |
| CA mice nontransplanted | 10 | 2.6 ± 0.4 | 2.5 ± 0.4 | 13.6 ± 1.9 | 39.9 ± 1.2 |
| CA mice transplanted | 5 | 6.4 ± 0.7* | 11.5 ± 1.4* | 32.4 ± 3.0* | 18.9 ± 1.2* |
Hematology data were collected 8 weeks after birth, except the nontransplanted CA mice, which were analyzed before death (median age, 17 d). Values represent mean ± SEM. Statistical significances were determined for the transplanted CA mice compared with the nontransplanted CA mice. P values were calculated by the 2-tailed Student t test.
Hb, hemoglobin; PCV, packed cell volume; RDW, red cell distribution width.
P ≤ .0001.
Donor-derived erythroid cells have survival advantage over CA erythroid cells
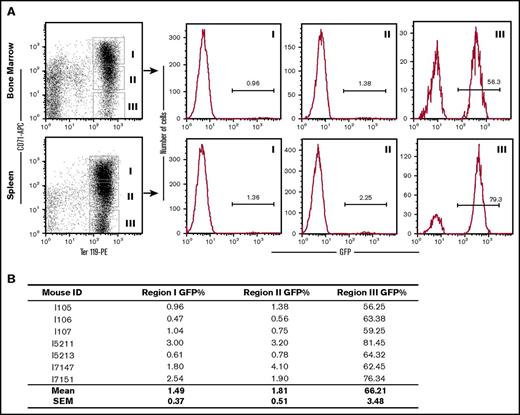
We sought to understand how the low level of donor HSPC chimerism produced near-normal levels of donor erythrocytes. Erythroid differentiation was quantitatively evaluated by flow cytometry in a cohort of CA mice at 2 to 3 months after transplantation. Early and late erythroblasts from the bone marrow and spleen were grouped into 3 populations enriched for basophilic/polychromatophilic erythroblasts (CD71highTer119+), polychromatophilic/orthochromatic erythroblasts (CD71lowTer119+), and orthochromatic erythroblasts/RBCs (CD71−Ter119+) and analyzed for the fraction of wild-type, donor-derived cells based on their GFP expression. In the early erythroblast stages (CD71highTer119+ and CD71lowTer119+), donor-derived GFP+ erythroid progenitors were 1% to 2% of the cell populations in both the bone marrow and spleen (Figure 5). However, in the late erythroblast/RBC stage (CD71−Ter119+), donor-derived GFP+ erythroid cells represented the majority of the population (Figure 5). These results indicate that the endogenous CA erythroid progenitor cells are short lived. Furthermore, erythroid cells derived from the donor HSPCs have a tremendous survival advantage compared with the endogenous erythroid cells and reconstitute the vast majority of the erythron.
Donor-derived erythroid cells have a selective advantage over endogenous CA erythroid cells. (A) GFP+ donor erythroid cells were quantified by flow cytometry in the bone marrow and spleen of several CA recipients 2 to 3 months after transplantation. Cells were stained with fluorescently labeled antibodies that recognize the transferrin receptor (CD71) and the erythroid-specific antigen Ter119. Ter119+ erythroid cells were grouped into CD71 high (Region I), low (Region II), and negative (Region III) populations. The donor-derived erythroid cells in each region were determined by the percentage of GFP+ cells in the histograms to the right. Although donor-derived erythroid cells were only a small fraction of the early erythroblasts in Regions I and II, they represent the majority of late erythroblast and enucleated RBCs in Region III. (B) Low-donor chimerism in early erythroblasts (Regions I and II) and simultaneous high-donor chimerism in late erythroid cells (Region III) was consistent in all of the transplanted animals that were analyzed.
Donor-derived erythroid cells have a selective advantage over endogenous CA erythroid cells. (A) GFP+ donor erythroid cells were quantified by flow cytometry in the bone marrow and spleen of several CA recipients 2 to 3 months after transplantation. Cells were stained with fluorescently labeled antibodies that recognize the transferrin receptor (CD71) and the erythroid-specific antigen Ter119. Ter119+ erythroid cells were grouped into CD71 high (Region I), low (Region II), and negative (Region III) populations. The donor-derived erythroid cells in each region were determined by the percentage of GFP+ cells in the histograms to the right. Although donor-derived erythroid cells were only a small fraction of the early erythroblasts in Regions I and II, they represent the majority of late erythroblast and enucleated RBCs in Region III. (B) Low-donor chimerism in early erythroblasts (Regions I and II) and simultaneous high-donor chimerism in late erythroid cells (Region III) was consistent in all of the transplanted animals that were analyzed.
Transplanted CA mice have markedly improved hematological and histopathological status
All of the transplanted humanized CA mice were rescued from lethal anemia and had a marked improvement in RBC indices. The RBC indices of transplanted CA mice were measured 8 weeks after transplantation and were compared with normal wild-type control and nontransplanted CA mice, which die at approximately 10 to 20 days after birth (Figure 1; Table 2). Transplanted CA mice had RBC count, hemoglobin, packed-cell volume, and red cell distribution width values that were significantly improved compared with those of nontransplanted CA mice (P ≤ .0001) (Table 2).
The RBC morphological status in peripheral blood smears from transplanted CA mice confirmed the improved hematological measurements. The vast majority of RBCs in transplanted CA mice were enucleated and normocytic in appearance (Figure 5A). Nontransplanted CA mice have significant numbers of nucleated erythroblasts and hypochromic, microcytic, and targeted RBCs (Figure 6A; supplemental Figure 4).
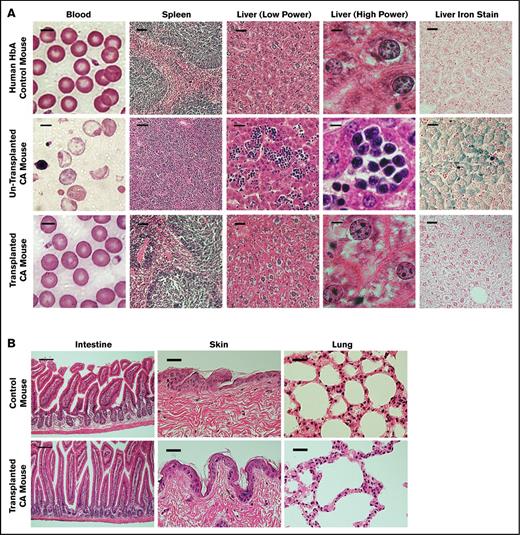
Control and CA mouse histopathological features before and after transplantation. (A) Peripheral blood smears and histopathological features of spleen and liver sections of humanized HbA control, nontransplanted CA, and transplanted CA mice. The morphological status of RBCs in the blood of nontransplanted CA mice shows marked anisopoikilocytosis and hypochromia with numerous nucleated RBCs. In contrast, the blood of transplanted CA mice is normocytic and normochromic, similar to the nonanemic HbA control mice. Nontransplanted CA mice have splenomegaly with a massive expansion of erythroid progenitors, whereas the spleens of transplanted CA mice are normalized, similar to HbA control mice. There is marked extramedullary hematopoiesis and excess iron in the livers of nontransplanted CA mice. After transplantation the livers of CA mice are normalized, similar to control mice. Scale bar is 5 μm for the blood, 50 μm for the spleen, 25 μm for the liver (low power), 5 μm for the liver (high power), and 25 μm for the liver iron stain. (B) Histological sections of the intestine, skin, and lung of wild-type control and transplanted CA mice were examined for evidence of GVHD. Five months after transplantation, the skin, intestine, and lung were fixed and stained with hematoxylin and eosin. No GVHD was observed in CA mice after BMT in the absence of cytoreductive conditioning. Scale bar is 100 μm for the intestine and 50 μm for the lung and skin.
Control and CA mouse histopathological features before and after transplantation. (A) Peripheral blood smears and histopathological features of spleen and liver sections of humanized HbA control, nontransplanted CA, and transplanted CA mice. The morphological status of RBCs in the blood of nontransplanted CA mice shows marked anisopoikilocytosis and hypochromia with numerous nucleated RBCs. In contrast, the blood of transplanted CA mice is normocytic and normochromic, similar to the nonanemic HbA control mice. Nontransplanted CA mice have splenomegaly with a massive expansion of erythroid progenitors, whereas the spleens of transplanted CA mice are normalized, similar to HbA control mice. There is marked extramedullary hematopoiesis and excess iron in the livers of nontransplanted CA mice. After transplantation the livers of CA mice are normalized, similar to control mice. Scale bar is 5 μm for the blood, 50 μm for the spleen, 25 μm for the liver (low power), 5 μm for the liver (high power), and 25 μm for the liver iron stain. (B) Histological sections of the intestine, skin, and lung of wild-type control and transplanted CA mice were examined for evidence of GVHD. Five months after transplantation, the skin, intestine, and lung were fixed and stained with hematoxylin and eosin. No GVHD was observed in CA mice after BMT in the absence of cytoreductive conditioning. Scale bar is 100 μm for the intestine and 50 μm for the lung and skin.
Histopathological status of the spleen and liver of transplanted CA mice, which were examined 5 months after transplantation, showed dramatic correction of the anemia-induced pathological changes observed in nontransplanted CA mice.49 The massive erythroid splenomegaly in CA mice was normalized to the red-and-white pulp architecture of normal control mice after BMT (Figure 6A; supplemental Figure 4). The extensive extramedullary hematopoiesis in the livers of nontransplanted CA mice was greatly reduced after transplantation. In a similar manner, iron overload due to ineffective erythropoiesis and increased intestinal iron absorption was greatly reduced in the livers of transplanted CA mouse (Figure 6A; supplemental Figure 4). The alleviation in splenic and hepatic pathological features was proportional to the level of donor HSPC engraftment and subsequent reduction of anemia. These data demonstrate that BMT of CA mice in the absence of cytoreductive conditioning renders the CA mice transfusion-independent by alleviating their anemia and anemia-related pathological patterns.
Absence of GVHD in transplanted CA mice
GVHD is a common complication after allogeneic BMT. Because the major target sites for acute GVHD are the intestine and skin, we closely observed transplanted CA mice for loss of body weight and fur integrity after transplantation. No hair loss or ulceration was observed on the skin of CA recipients. By the second week following transplantation, CA recipient mice showed no significant difference in body weight compared to control littermates (supplemental Figure 3). The intestine, lung, and skin were examined 5 months after transplantation for evidence of chronic GVHD. There was no histological evidence of chronic GVHD in the transplanted CA mice compared with nontransplanted control mice (Figure 6B; supplemental Figure 5). There was no lymphocyte infiltration in the lung. Intestinal villi were morphologically normal without any crypt destruction or lymphocyte infiltration. No lymphocyte infiltration or hyperkeratosis was found in the skin sections analyzed. These data demonstrate that there was no evidence of acute or chronic GVHD after transplantation of CA mice in the absence of cytoreductive conditioning.
Discussion
In this report, we demonstrate that allogeneic BMT in the absence of cytoreductive conditioning can rescue a novel preclinical animal model of CA. BMT among immunocompetent adult recipients requires cytotoxic conditioning to open up HSC niches for incoming donor cells and prevent graft rejection by the host immune system. Previous studies have suggested that in the absence of cytoreductive conditioning, little capacity exists for significant long-term engraftment of donor stem cells, presumably because of the lack of available HSC niche space in the bone marrow.28,64,65 In unconditioned BMT, rare, empty HSC niches can be engrafted by donor BMCs leading to low-level chimerism but only by syngeneic cells.28,66 Without conditioning, the small differences used to distinguish congenic donor cells in the host after transplantation are enough to result in graft rejection.67-69 Although GFP-expressing BMCs can readily reconstitute the hematopoietic system of recipients after myeloablative or nonmyeloablative pretreatment,70-72 congenic GFP-expressing cells are lost in unconditioned mice.67
The cells from donor and recipient mice used in this study differ in 3 important ways. Wild-type donor cells are from inbred adult C57Bl/6 strain, contain exclusively mouse α-globin and β-globin chains in erythroid cells, express GFP in all hematopoietic cells, and have solely C57Bl/6 minor histocompatibility antigens. Recipient CA mice are a mixed background, have differences in minor histocompatibility antigens, contain only human α-globin and γ-globin chains, and do not express GFP in any cells. It is remarkable that allogeneic BMT of CA mice in the absence of cytoreductive conditioning resulted in long-term, stable hematopoietic chimerism.
We propose that a combination of factors led to the successful rescue of the humanized CA mice by allogeneic BMT without conditioning. First, rather than transplant adult recipients, newborn CA mice were transplanted at age 3 days while there is still a functional immaturity of the immune system.73 Classical experiments by Billingham et al39 demonstrated that immune tolerance could be induced by injection of live cells from donor strain A into recipient strain CBA during fetal life and in 10% of injected newborns without any additional treatment.
The pretreatment of donor BMCs with Diprotin A and recipient CA mice with anti-CD122 antibody were used to increase hematopoietic chimerism and perinatal tolerance. Diprotin A inhibits dipeptidyl peptidase IV (DPPIV or CD26), a cell-surface glycoprotein found on both HSCs and T cells.55-57,74,75 DPPIV cleaves the amino terminal dipeptide from the chemokine stromal cell–derived factor 1, also known as CXCL12, thereby blocking HSC homing to and engraftment in the bone marrow niche. Inhibition of DPPIV increases allogeneic HSC engraftment severalfold during unconditioned in utero transplantation57 or in adults after myeloablation.56 Using Diprotin A in our study raised the hematopoietic chimerism level severalfold (mean, 5.2%) above the 1.3% level reported earlier in unconditioned syngeneic transplants.66 On T cells, DPPIV activity has been shown to have costimulatory activity.74,75 Inhibition of this activity could decrease activation and interleukin-2 (IL-2) production of T cells in the donor bone marrow, which helps to establish tolerance and limit GVHD. In a similar manner, antibody against CD122, the IL-2 receptor β chain that is shared by both IL-15 and IL-2 receptors, enhances allogeneic BMT success by blocking IL-15 and IL-2 stimulation of natural killer (NK) cells and T cells, respectively.58,59,76,77 By treating CA recipients 1 day prior to allogeneic BMT with anti-CD122 antibody, potential graft rejection by recipient NK cells and GVHD by stimulation of donor T cells were likely avoided.
The generation of stable hematopoietic chimerism after transplantation in the absence of cytoreductive conditioning has been used to treat lymphoid, myeloid, and erythroid disorders in the mouse. Reconstitution of the lymphoid system in adult severe combined immunodeficiency mice was achieved using purified donor HSCs, but this required an immune-incompetent host using congenic donor cells, and only low-level engraftment (0.1%-1.0%) of the HSC compartment was observed.78 Mucopolysaccharidosis type VII disease was ameliorated by multiple high-dose BMTs in unconditioned newborn mice. Recipients of mucopolysaccharidosis type VII had low-level lymphoid and myeloid lineage donor cells present throughout life.33 Similar experiments for RBC disorders were more variable. Jaundiced mice (ja/ja) that had RBC β-spectrin deficiency were not rescued from postnatal lethality by a single low-dose injection of donor BMCs into newborns79 ; however, repeated high doses of BMCs resulted in transient peripheral RBC chimerism that lasted for months.26 Of 9 control SCD mice that received T-cell–depleted bone marrow and costimulation blockade but no busulfan conditioning, 5 went on to have some RBC chimerism.31 Fetal liver cells transplanted into unconditioned newborn β-thalassemia mice resulted in trace peripheral blood chimerism (<1%) with significant donor hemoglobin chimerism (>30%) in the majority of animals.27 In contrast to these previous studies, our data demonstrate the consistent, stable establishment of hematopoietic chimerism (5.2% ± 1.3% of HSPCs and 98.8% ± 0.4% of hemoglobin) in all of the animals after a single, low-dose injection of allogeneic bone marrow into newborn CA mice in the absence of cytoreductive conditioning.
Less toxic nonmyeloablative protocols for allogeneic BMT have been developed to alleviate some of the safety concerns associated with myeloablative conditioning. Nonmyeloablative BMT in patients with CA and SCD has demonstrated the establishment of beneficial hematopoietic chimerism.35,36,40,41 However, the majority of these patients, including those with stable hematopoietic chimerism for more than 1 year, experienced graft rejection on removal of immunosuppression, and their disease returned.36,41 Based on our study, earlier intervention may result in better establishment of mixed chimerism in the absence of myeloablation. Furthermore, because of the great survival advantage of wild-type donor RBCs compared with the short-lived ineffective erythroid cells present in CA, even low-level HSPC chimerism can generate the majority of the erythron. Thus, this BMT strategy in the absence of cytoreductive conditioning could be useful for additional clinical disorders whenever diseased endogenous cells have a relatively short life span compared with normal cells. Other hemoglobinopathies such as SCD would also benefit from this approach, although there will always be significant levels of circulating sickled erythrocytes. It is important to note that these data suggest relatively low levels of β-globin gene correction by gene editing in autologous HSPCs of patients with CA could be therapeutic or curative. Although conditioning would be necessary for gene-edited autologous BMT, the immunological benefits of using the patient’s bone marrow may outweigh the risks associated with myeloablation.
In summary, a simple noncytotoxic preparative regimen for allogeneic BMT in the absence of cytoreductive conditioning was tested in a novel preclinical humanized mouse model of CA. Donor BMCs were treated with Diprotin A to increase HSC homing and engraftment. Recipient CA mice received a bolus of anti-CD122 antibody the day before transplantation to reduce T-cell and NK-cell activation. Humanized CA recipients were tolerant of their grafts and established stable hematopoietic chimerism. Transplanted CA pups were rescued from lethal anemia, had markedly improved hematological and pathological status, and were transfusion-independent for the duration of the experiment.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Joseph Ruisi, UNICO National (Italian American Service Organization), and the Thalassemia–Cooley's Anemia Group at UAB for support.
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH) (R01 HL072351, R01 HL073440, and R56 HL072351 [T.M.R.]; and F31 HL120614 [J.R.L.]); and a UAB grant (1 G20RR022807-1) (J.R.L.). Flow cytometry experiments were supported by the UAB Comprehensive Flow Cytometry Core supported by NIH, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P30 AR048311 and National Institute of Allergy and Infectious Diseases grant P30 AI027667. This work was supported by a Research Fellowship grant from the Cooley's Anemia Foundation (S.L.). This work was sponsored in part by the Alabama Institute of Medicine Stem Cell Research Grant Program.
The content of this publication does not necessarily represent the official views of the State of Alabama or the Department of Public Health of the State of Alabama.
Authorship
Contribution: Y.H., J.R.L., and T.M.R. designed the research; Y.H., J.R.L., S.L., S.F., and M.B. performed experiments; Y.H., J.R.L., S.L., S.F., M.B., and T.M.R. analyzed the results; Y.H. and J.R.L. created the figures; and Y.H., J.R.L., and T.M.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas M. Ryan, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, 1918 University Blvd, MCLM 566A, Birmingham, AL 35294; e-mail: tryan@uab.edu.
References
Author notes
Y.H. and J.R.L. contributed equally to this study.