Key Points
The study developed a mouse model of bone disseminated myeloma disease as in humans.
The study established therapeutic potential of OPG variants to revert myeloma bone damage in vivo.
Abstract
The current treatment options for multiple myeloma (MM) osteolytic lesions are mainly combinations of chemotherapy and other small-molecule inhibitors, but toxic side effects still remain a major concern. Studies have shown that osteoclast activity is enhanced in MM patients through increased expression of receptor activator of nuclear factor κB ligand (RANKL), triggering RANK signaling on osteoclast precursors, which results in aggressive bone resorption. Furthermore, osteoprotegerin (OPG), a decoy receptor for RANKL, and the osteogenic potential of mesenchymal stem cells (MSCs) are significantly decreased in myeloma patients with multiple bone lesions. Thus, the use of OPG as a therapeutic molecule would greatly decrease osteolytic damage and reduce morbidity. However, in addition to inhibiting osteoclast activation, OPG binds to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), thereby rendering the tumor cells resistant to TRAIL-induced apoptosis and limiting the use of OPG for therapy. The present study developed a bone-disseminated myeloma disease model in mouse and successfully tested a cell therapy approach using MSCs, genetically engineered to express OPG variants that retain the capacity to bind RANKL, but do not bind TRAIL. Our results of skeletal remodeling following this regenerative stem cell therapy with OPG variants indicated a significant protection against myeloma-induced osteolytic bone damage in areas of major myeloma skeletal dissemination, suggesting the potential of this therapy for treating osteolytic damage in myeloma patients.
Introduction
Multiple myeloma (MM) is an incurable hematologic malignancy, primarily involving the skeleton, with >80% of patients presenting with bone pathology.1 Evidence suggests that skeletal events occur due to myeloma cell disruption of normal bone physiology.2 As myeloma cells proliferate within the bone microenvironment, their interaction with resident stromal cells leads to the production of osteolytic cytokines, including receptor activator of nuclear factor κB ligand (RANKL) and interleukin-6 that further stimulate RANKL expression in myeloma cells, thereby provoking a vicious cycle, leading to enhanced osteoclast activity.2-4 The dysregulation of osteoblastogenesis due to increased Dickkopf-related protein-1, as well as a significant reduction in circulating osteoprotegerin (OPG), partly due to myeloma cell internalization and degradation of this soluble decoy receptor, adds complexity to disease progression, causing an imbalance in the RANKL/RANK/OPG signaling cascade.2,5,6
Current therapy for myeloma consists of combination therapy that includes different combinations of drugs, including melphalan, prednisone, and proteasome inhibitors.7,8 Despite improvements in the antiresorptive bisphosphonate formulations, their use is associated with toxic side effects, including peripheral neuropathy, neutropenia, thrombocytopenia, inflammatory reaction, hypocalcemia, renal impairment, and/or osteonecrosis of the jaw, which affect quality of life.9,10 Recent studies demonstrated the potential of OPG in decreasing osteolytic lesions in a mouse myeloma model.11,12 Although reversing OPG homeostasis has the potential to prevent aggressive osteoclastogenesis and bone loss, therapeutic activity of OPG, used so far including that described above,11,12 is hampered due to its ability to bind and inhibit tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), an action that favors cancer cell growth. Recent studies have identified that TRAIL efficiently induces apoptosis in MM cells both in vitro and in vivo.13-15 Furthermore, stem cell–enriched CD138− myeloma cells have been shown to be sensitive to TRAIL, in combination with doxorubicin.16 Thus, uncoupling TRAIL binding of OPG would present a major breakthrough in the use of OPG to reverse osteolytic phenotype in myeloma. To overcome this limitation of OPG therapy, we recently identified domains on OPG that are important for TRAIL binding, developed and demonstrated functional ability of such OPG mutants, abolished in TRAIL binding, but retained in RANKL binding.17
The last decade has seen increasing therapeutic use of mesenchymal stem cells (MSCs).18,19 Along with their potential of self-renewal, MSCs can differentiate into adipocytes, chondrocytes, and osteoblasts.19-21 The fact that MSCs can modulate both innate and adaptive immune response makes them attractive agents for allogenic transplantation in regenerative medicine. Previous studies have shown a significant role of MSCs in immune-mediated pathologies such as graft-versus-host disease.19,20 It has also been shown that MSCs home to sites of injury and tumor, and this homing mechanism is being exploited as a means for therapeutic payload.22-25
Evaluation of preclinical therapeutic strategies for osteolytic lesions in myeloma bone disease is mostly limited to testing in localized tumor growth models following myeloma cell implantation. However, the disease in humans is characterized by osteolytic destruction in most major bones of the skeleton. In the current study, we first developed and characterized a preclinical mouse model with a human myeloma cell line, overexpressing heparanase, to simulate a rampant osteolysis, commonly observed in many myeloma patients. Next, using a systemic approach in this mouse model of bone-disseminated myeloma disease, we tested the potential of genetically engineered MSCs, transduced with a nonpathogenic recombinant viral vector encoding an OPG variant, retaining the capacity to bind RANKL, but not TRAIL. Results following this regenerative stem cell therapy with 2 different OPG variants demonstrated a significant protection against myeloma-induced osteolytic bone damage in major skeletal areas, suggesting the potential of this genetically engineered stem cell therapy for treating tumor-induced osteolytic damage in myeloma patients.
Methods
Cell lines and reagents
Human myeloma cell line, CAG, was established at the Myeloma Institute for Research and Therapy (Little Rock, AR), with stable modifications to overexpress heparanase (CAGHep).26 The CAGHep cell line was maintained in RPMI 1640 medium (Mediatech Inc, Hendron, VA) supplemented with 10% fetal bovine serum (FBS; Mediatech Inc), 1% glutamine, and 1% penicillin/streptomycin (Mediatech Inc). The murine myeloma cell line MPC-11 was obtained from ATCC and maintained in RPMI 1640, supplemented with 10% FBS, 2 mM l-glutamine, 0.05 M 2-mercaptoethanol, 1% penicillin-streptomycin as described earlier.27 The murine pre–osteoclast cell line RAW 264.7 was a kind gift from Xu Feng, The University of Alabama Birmingham (UAB), and was maintained in α-minimum essential medium supplemented with 10% FBS, 4 mM l-glutamine, and 1% penicillin-streptomycin. The human embryonic kidney cell line, HEK293, was purchased from ATCC and maintained in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% newborn calf serum and 1% penicillin-streptomycin. Recombinant OPG was purchased from R&D Systems, and RANKL was a kind gift from Xu Feng, UAB. All antibodies used in flow cytometry analysis (CD90, CD73, CD45, CD44, CD31, CD29, CD11b) were purchased from eBioscience (San Diego, CA). Reagents used for MSC differentiation: dexamethasone, β-glycerophosphate, l-ascorbic acid, insulin, and 3-isobutyl-1-methylxanthine, were purchased from Sigma Chemical Co (St. Louis, MO).
Animal use and care
Severe combined immunodeficient (SCID) and BALB/c mice of 6 to 8 weeks age were purchased from Charles River Laboratories and housed in the animal facility at UAB. Animal care and treatments were conducted in accordance with established guidelines and protocols approved by the UAB Institutional Animal Care and Use Committee.
Characterization of hMSCs
Human mesenchymal stem cells (hMSCs) were isolated from surgical bone marrow transplant remnants of healthy donors from UAB with Institutional Review Board approval. The cells were cultured in DMEM, 10% FBS, and 1% penicillin-streptomycin to confluency before immunophenotyping. Prior to using them for in vivo studies, MSCs were characterized for positive expression for CD90, CD73, CD44, and CD29, and negative for expression of CD11b and CD45. Verification of MSC pluripotency was confirmed by lineage differentiation into adipocytes and osteoblasts, as described earlier.28 Briefly, the sorted cells were cultured in either osteoblast differentiation medium (ODM) containing 10−7 M dexamethasone, 7 mM β-glycerophosphate, 5 µg/L l-ascorbic acid, or adipocyte differentiation medium (ADM) containing 25 mM glucose, 50 μg/mL gentamicin, 0.5 mM glutamine, 0.5 μg/mL fungizone, 10% fetal calf serum, 1 mM glutamine, and 1 μg/mL insulin for 2 weeks to confirm respective differentiation by Oil-Red-O staining for adipocyte differentiation and by Alizarin red, and von Kossa staining for osteoblast differentiation.
Production of recombinant adeno-associated virus expressing OPG
The OPG mutants (Y49R and F107A) were generated based on putative OPG residues identified from a structural model of the OPG/TRAIL complex.17 The recombinant OPG used comprises the ligand-binding domain of human OPG (1-201 amino acids), fused to the Fc domain of human immunoglobulin G in pcDNA3.1 vector, under the control of cytomegalovirus promoter. Variants of OPG containing mutations Y49R and F107A were created by site-directed mutagenesis as described earlier.17 To generate recombinant adeno-associated virus (rAAV) plasmids containing wild-type (WT) or mutant OPG, the open reading frame, encoding the transgene was excised from pcDNA3.1 and subcloned into the multicloning site (MCS) of rAAV plasmid. Packaging and production of rAAV-OPGWT/mut were performed by transient transfection in 293 cells with the helper plasmid pDP6 using an established protocol as described earlier.29 Purification of rAAV-OPGWT/mut was performed by iodixanol density gradient, and the particle titer of vectors was determined by quantitative slot-blot analysis.29
Development of a mouse myeloma model for skeletal dissemination of osteolytic malignancy
The goal of this study was to address the osteolytic component of myeloma using genetically modified OPG in a preclinical mouse model that exhibits osteolytic lesions in major bones of the skeletal system. While developing an appropriate in vivo model to test OPG therapy, we compared 2 different mouse models for their potential in manifesting disseminated skeletal lesions with a window for testing experimental therapies. The first model consisted of an immunocompetent BALB/c mouse with syngeneic MPC-11 cells following serial transplantation in vivo in the tibia, and the second model used an SCID mouse with a human myeloma cell line, CAG, which was genetically modified for high heparanase expression (CAGHep). The cells were systemically administered IV and analyzed for myeloma bone lesions. For each model, ∼5 × 105 cells were injected per mouse. Dissemination of the cells and engraftment in major skeletal sites was tested by noninvasive luciferase imaging and histology. Cohorts of mice were euthanized on days 7, 14, 28, and 45 after tumor cell transplantation to analyze skeletal dissemination. Each group consisted of at least 12 mice.
Determination of the therapeutic effects of systemically administered, genetically engineered MSCs overexpressing OPG
hMSCs were cultured in DMEM, 10% FBS, and 1% penicillin-streptomycin to confluence prior to cell sorting. The cells were analyzed by flow cytometry, and the population with phenotype: CD90+, CD73+, CD44+, CD29+, CD11b−, and CD45−, was used as MSCs. Sort-purified MSCs were subjected to osteoblast and adipocyte differentiation assays as described.28 Media from hMSCs, transduced with vectors encoding OPG-WT or OPG-Mutants (Y49R or F107A), were collected and concentrated using a 30-kDa filter and analyzed by western blot using OPG and Fc antibodies. SCID mice were IV injected with 5 × 105 CAGHep cells. Each group consisted of 10 mice, and the experiments were performed at least twice for consistency. Engraftment and skeletal dissemination of the cells were followed by noninvasive imaging based on constitutive luciferase expression. Fourteen days after myeloma cell injection and upon establishment of tumor growth within the skeleton, 3 × 105 hMSCs that were transduced by rAAV6, encoding either OPG-WT or OPG-mutants (Y49R or F107A), were administered systemically. Mice were then monitored by noninvasive bioluminescence imaging weekly for assessment of tumor growth. Serum samples were collected once on day 14 and again during euthanization for enzyme-linked immunosorbent assay (ELISA) measurement of OPG. Cohorts of mice from each group were euthanized on day 28, a time point established as midpoint for osteolytic bone destruction, and harvested bones were analyzed by histology and microcomputer tomography (micro-CT) analysis.
Micro-CT analysis of bone tissue
Superficial CT scanning of the skeleton was performed using MicroCAT II (Imtek, Inc). For determination of the 3-dimensional (3D) architecture of the trabecular bones, mice were euthanized; tibia and spine were harvested and analyzed in an advanced micro-CT instrument (μCT 40; Scanco Medical AG). Two scans were performed on each of the bones: 1 for whole bone with 16-μm resolution and 1 for trabecular analysis with a 6-μm resolution. A 3D reconstruction of the images was created with a region of interest consisting of trabecular area under the growth plate. Scans of the trabecular bone were performed beginning 25 slices below the growth plate, and a total of 100 slices were used for analysis and 3D reconstruction. Data from this analysis were used for determination of connectivity density, trabecular number, and trabecular spacing.
Histology
Bone tissues were decalcified in 0.5 mol/L EDTA in Ca2+- and Mg2+-free Dulbecco's phosphate-buffered saline (Gibco) before embedding in paraffin. Histological sections from the tibia, femur, and spine were stained with hematoxylin and eosin to determine tumor growth in the bone and the extent of osteolysis in response to different treatments. Tartrate-resistant acid phosphatase (TRAP) staining was performed on bone sections to determine osteoclast activity as described earlier.30
ELISA
Blood was collected from mice prior to MSC injection, and 14 days after MSC injection. Serum samples from each time point were separated and frozen at −80°C. Determination of OPG levels was performed using a commercial kit as per the manufacturer’s instructions (eBioscience).
Statistical analysis
Results of the studies were analyzed using the Student t test. Values are provided as mean ± standard error. Level of significance for the experimental data was determined, and P < .05 was considered significant.
Results
Characterization of human MSCs
hMSCs were isolated from surgical bone marrow transplant remnants of healthy donors and were cultured to confluence for 10 days (4 days undisturbed followed by fresh media addition every 2 days). The MSC population was confirmed based on positive expression for CD44, CD29, and CD90, and negative for expression of CD11b and CD45. A 2-step purification was performed to obtain MSC population by flow cytometry. From the confluent cells, a distinct population of cells positive for expression of CD29 and CD44 was first sort-purified. From these purified cells, the population negative for CD45 was isolated. This population of ∼66% was used as hMSCs (Figure 1A).
Characterization of human MSCs. hMSCs were isolated from remnants of bone marrow transplant bags and cultured in DMEM with 10% FCS. Upon confluence, the cells were harvested and stained with antihuman CD11b to exclude the population that was positive for this marker. The remaining cells were sorted as positive for CD90, CD73, and CD44, and negative for CD45 (A) and cultured to confluence. The pluripotency of MSCs was determined by adipogenic and osteogenic differentiation assays using ADM and ODM, respectively. Fourteen days later, Oil-Red-O staining was performed for lipid droplets, and Alizarin red staining was performed for calcium deposition. Replicates of cells, grown in ODM, were also subjected to von Kossa staining on day 21 for mineralization. Original magnification ×100 (B).
Characterization of human MSCs. hMSCs were isolated from remnants of bone marrow transplant bags and cultured in DMEM with 10% FCS. Upon confluence, the cells were harvested and stained with antihuman CD11b to exclude the population that was positive for this marker. The remaining cells were sorted as positive for CD90, CD73, and CD44, and negative for CD45 (A) and cultured to confluence. The pluripotency of MSCs was determined by adipogenic and osteogenic differentiation assays using ADM and ODM, respectively. Fourteen days later, Oil-Red-O staining was performed for lipid droplets, and Alizarin red staining was performed for calcium deposition. Replicates of cells, grown in ODM, were also subjected to von Kossa staining on day 21 for mineralization. Original magnification ×100 (B).
To determine the pluripotency of hMSC, the sorted cells were cultured in either ODM or ADM for 2 weeks to confirm respective lineage differentiation. After 14 days, hMSC, cultured in ADM, revealed increased Oil-Red-O staining for lipid droplets, when compared with control cells (Figure 1B). Similarly, hMSC cultured in ODM revealed increased Alizarin red staining compared with control cells, indicating positive calcium deposition as an indicator of osteogenesis. A replicate of ODM-conditioned hMSC was also confirmed to be positive for von Kossa staining 21 days later, indicating mineralization (Figure 1B). These results confirmed that the sorted hMSCs were pluripotent.
Disseminated mouse myeloma disease model
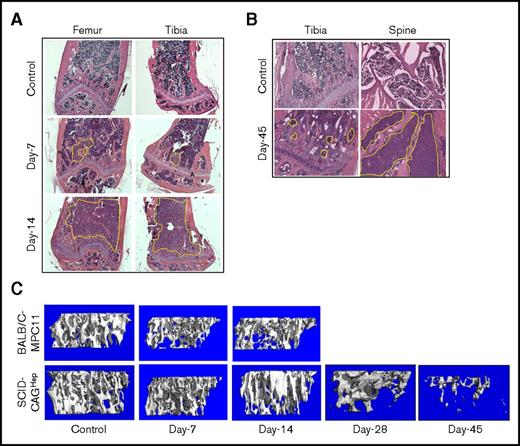
To establish a murine myeloma model with disseminated osteolytic disease, cohorts of BALB/c and SCID mice were IV injected with MPC-11 and CAGHep cells, respectively. After tumor cell injections, groups of mice were euthanized at day 7 and day 14 to analyze bone architecture and systemic dissemination of the tumor. Mice were also monitored for physical signs of pathological morbidity. Mice injected with MPC-11 cells displayed spinal compression around day 12 followed by hind limb paralysis around day 14. Mice were euthanized, and bones were analyzed by micro-CT and histology. Results revealed bone destruction on day 7 that progressively worsened with time. Histological sections revealed the presence of tumors in the bone microenvironment in femur and tibia on day 7 with extensive tumor infiltration by day 14 (Figure 2A). Spinal sections also revealed the presence of tumor growth on day 14 demonstrating skeletal dissemination of the conditioned MPC-11 cell line (data not shown). Mice injected with CAGHep cells indicated tumor growth in both tibia and femur with evidence of skeletal growth on day 7, albeit reduced. Compared with the MPC-11-BALB/c model, SCID mice, challenged with CAGHep cells, survived longer with spinal compression and hind limb paralysis being evident only ∼45 days after myeloma cell injection when a significant volume of tumor cells were found in the tibia and spine (Figure 2B). Mice were euthanized, and bone tissues were harvested for micro-CT analysis, which confirmed substantial bone destruction as early as day 7 in the BALB/c model, but relatively to a lesser degree in the SCID-CAGHep model. Micro-CT analysis of the tibia revealed an increase in trabecular bone destruction in both BALB/c and SCID models on day 14 and day 45, respectively (Figure 2C). Although the MPC-11-BALB/c model recapitulated myeloma phenotype, the aggressive growth of the MPC-11 cell line required euthanasia by week 3. However, disseminated growth of CAGHep cells in the SCID model persisted for >7 weeks, without debilitating consequences, allowing adequate therapeutic window for evaluating the efficacy of therapy with hMSC-OPG. Based on this result, the SCID model with CAGHep cells was used to evaluate the potential of genetically engineered MSC-OPG therapy for myeloma bone damage.
Establishment of a mouse myeloma model with skeletal dissemination of tumor and osteolysis. Two different mouse models were tested for myeloma osteolytic dissemination. Groups of BALB/c mice were challenged with 5 × 105 MPC-11 cells, and SCID mice were challenged with 5 × 105 CAGHep cells via tail vein. Based on constitutive expression of firefly luciferase in both cell lines, mice were monitored by noninvasive imaging to monitor tumor growth within the tibia, femur, and spine. Cohorts of mice from each group were euthanized at the indicated time points, and bones were subjected to histology (A-B; original magnification ×100, hematoxylin and eosin stain) and micro-CT (C). Representative data indicate aggressive tumor growth and skeletal damage in the BALB/c-MPC-11 model by 2 weeks. However, the growth of myeloma cells in the SCID-CAGHep model was significantly delayed, allowing a window of >6 weeks before aggressive bone destruction was noted.
Establishment of a mouse myeloma model with skeletal dissemination of tumor and osteolysis. Two different mouse models were tested for myeloma osteolytic dissemination. Groups of BALB/c mice were challenged with 5 × 105 MPC-11 cells, and SCID mice were challenged with 5 × 105 CAGHep cells via tail vein. Based on constitutive expression of firefly luciferase in both cell lines, mice were monitored by noninvasive imaging to monitor tumor growth within the tibia, femur, and spine. Cohorts of mice from each group were euthanized at the indicated time points, and bones were subjected to histology (A-B; original magnification ×100, hematoxylin and eosin stain) and micro-CT (C). Representative data indicate aggressive tumor growth and skeletal damage in the BALB/c-MPC-11 model by 2 weeks. However, the growth of myeloma cells in the SCID-CAGHep model was significantly delayed, allowing a window of >6 weeks before aggressive bone destruction was noted.
In vivo analysis of OPG mutants in SCID-CAGHep myeloma model
To determine the in vivo effect of OPG variants in a disseminated myeloma disease by a systemic approach for skeletal remodeling, the SCID-CAGHep myeloma model was used in the study. We first confirmed bone homing of hMSC upon the formation of myeloma bone lesions, utilizing hMSC infected with rAAV-EGFP (supplemental Figure 1A). Micro-CT analysis confirmed CAGHep myeloma bone lesion within the tibia, and immunofluorescence and IHC analyses of bone sections confirmed hMSC homing to the lesions upon tail vein route injection of hMSC-EGFP (supplemental Figure 1B). This result indicated the feasibility of harnessing hMSC and the cell-mediated OPG augmentation in the MM bone lesions.
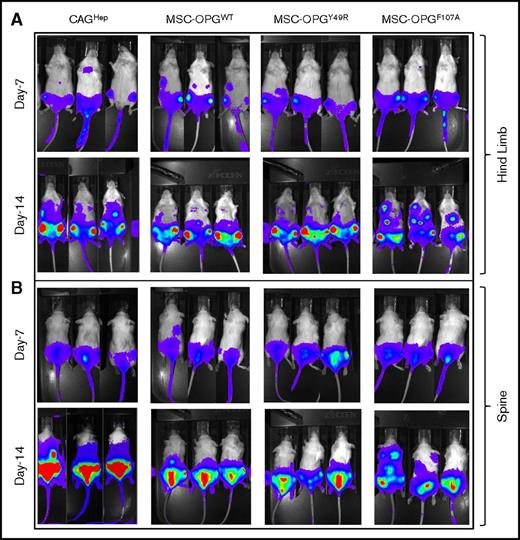
After confirmation of hMSCs by fluorescence-activated cell sorting (FACS) analysis and differentiation assays for multipotency, rAAV encoding WT or mutant OPG (Y49R or F107A) was used to transduce hMSC, prior to injection into tumor-bearing mice. Cohorts of SCID mice were first IV injected with 5 × 105 CAGHep cells followed by noninvasive imaging. Fourteen days after myeloma cell injection, and upon establishment of tumor growth within the skeleton, as confirmed by noninvasive image analysis, 3 × 105 hMSCs that were transduced by rAAV, expressing either OPG-WT or OPG-mutantsY49R/F107A, were administered systemically. Mice were monitored weekly by noninvasive bioluminescence imaging for tumor growth. Results demonstrated localization of CAGHep cells within tibia and femur as early as day 7 (Figure 3A), and by day 14, CAGHep cells disseminated to the vertebral column (Figure 3B). Fourteen days after hMSC-OPG administration, noninvasive imaging showed an overall delay in tumor growth in cohorts of mice treated with either OPGwt or OPGmut when compared with the untreated group, and this was more pronounced in the spine (Figure 3B). The control experiment with hMSC, transduced with rAAV-EGFP, showed no significant changes in myeloma bone lesions, compared with the group of mice challenged with CAGHep cells alone (supplemental Figure 1C), indicating that hMSC alone is not sufficient to revert bone damage in our experimental model.
Noninvasive bioluminescence imaging as a measure of therapeutic potential of OPG variants as compared with WT OPG. Groups of SCID mice were challenged with 5 × 105 CAGHep cells via tail vein followed by noninvasive imaging for establishment of tumor cells around day 14. Cohorts of mice were then left untreated or administered with hMSCs modified to express WT OPG (OPGwt), or OPG variants: OPGY49R/OPGF107A. Tumor growth following therapy was monitored by noninvasive imaging on day 7 (day 21 after tumor challenge), and day 14 (day 28 after tumor challenge) after initiation of therapy.
Noninvasive bioluminescence imaging as a measure of therapeutic potential of OPG variants as compared with WT OPG. Groups of SCID mice were challenged with 5 × 105 CAGHep cells via tail vein followed by noninvasive imaging for establishment of tumor cells around day 14. Cohorts of mice were then left untreated or administered with hMSCs modified to express WT OPG (OPGwt), or OPG variants: OPGY49R/OPGF107A. Tumor growth following therapy was monitored by noninvasive imaging on day 7 (day 21 after tumor challenge), and day 14 (day 28 after tumor challenge) after initiation of therapy.
OPG mutants protect against myeloma-induced osteoclastogenesis
Four weeks after MSC-OPG therapy, a time established as midpoint for tumor burden in untreated mice, bones were harvested from cohorts of mice for micro-CT analysis of trabecular architecture. Results demonstrated that mice treated with hMSCs overexpressing either OPGWT or OPGmut (Y49R or F107A) displayed a significant decrease in osteolytic bone damage induced by CAGHep cells, when compared with untreated mice (naive; CAGHep cells only). The therapeutic potential of both OPGmut (Y49R and F107A), abolished in TRAIL binding, was comparable to OPGWT (Figure 4A tibia), confirming for the first time that mutant OPG, devoid of TRAIL binding, provides efficient therapeutic benefit for bone remodeling against myeloma-induced bone loss. 3D reconstruction of micro-CT images showed significant destruction to the trabecular bone under the growth plate in the tibia of mice challenged with CAGHep cells (Figure 4A). Similarly, significant bone destruction was observed in the spine, particularly the L4 bone of the lumbar region in the spinal column of untreated control mice as 3D reconstruction images showed excessive bone destruction of the L4 bone (Figure 4B). However, images of bone 3D reconstruction in cohorts of mice treated with OPG (WT or mutant) therapy showed intact tibia and lumbar spinal bone with bone density and trabecular architecture, comparable to age-matched, control mice (Figure 4A-B). Data obtained from micro-CT were analyzed for connectivity density, trabecular number, and trabecular spacing in both tibia and spine following treatment. Results indicated a significant increase in connectivity density and trabecular number in both tibia and spine following treatment with OPGWT/mut, and a significant decrease in trabecular spacing in both following treatment (Figure 4A-B).
Micro-CT and quantitative analysis of tibia and spine following hMSC-OPG therapy. Four weeks after tumor challenge, a time established as midpoint for the degree of osteolytic damage, cohorts of mice from untreated and indicated treatment groups were euthanized, and tibia (A) and spine (B) were subjected to micro-CT analysis. Data obtained from micro-CT were used in 3D reconstruction images and quantitative analysis of respective bones for connectivity density, trabecular number, and trabecular spacing (*P < .05; **P < .01, compared with untreated mice following tumor challenge).
Micro-CT and quantitative analysis of tibia and spine following hMSC-OPG therapy. Four weeks after tumor challenge, a time established as midpoint for the degree of osteolytic damage, cohorts of mice from untreated and indicated treatment groups were euthanized, and tibia (A) and spine (B) were subjected to micro-CT analysis. Data obtained from micro-CT were used in 3D reconstruction images and quantitative analysis of respective bones for connectivity density, trabecular number, and trabecular spacing (*P < .05; **P < .01, compared with untreated mice following tumor challenge).
Decreased osteoclast activation following MSC-OPG therapy
To establish that the observed decrease in bone loss in mice treated with OPGmut was due to inhibition of osteoclast activity through inhibition of RANKL, harvested bones were decalcified and sectioned for analysis by TRAP staining to assess osteoclast activity. Results indicated a striking increase in osteoclast activity in tumor-challenged mice when compared with the age-matched controls (Figure 5). However, when the sections were compared between hMSC-OPG(WT/mut)–treated cohorts and untreated mice, osteoclast activity was significantly decreased in both tibia and spine in the treatment group, confirming therapeutic effects of OPGmut in decreasing osteoclast activation (Figure 5).
Osteoclast staining in tibia and spine following hMSC-OPG therapy. Bone tissues were harvested from mice in control and indicated treatment groups 28 days following tumor challenge. The bones were decalcified, and 5-µM sections were made. The slides were stained with TRAP stain on both tibia and spine for osteoclast activity within the bone microenvironment. Original magnification ×100.
Osteoclast staining in tibia and spine following hMSC-OPG therapy. Bone tissues were harvested from mice in control and indicated treatment groups 28 days following tumor challenge. The bones were decalcified, and 5-µM sections were made. The slides were stained with TRAP stain on both tibia and spine for osteoclast activity within the bone microenvironment. Original magnification ×100.
Treatment with modified hMSCs elevates OPG levels in vivo
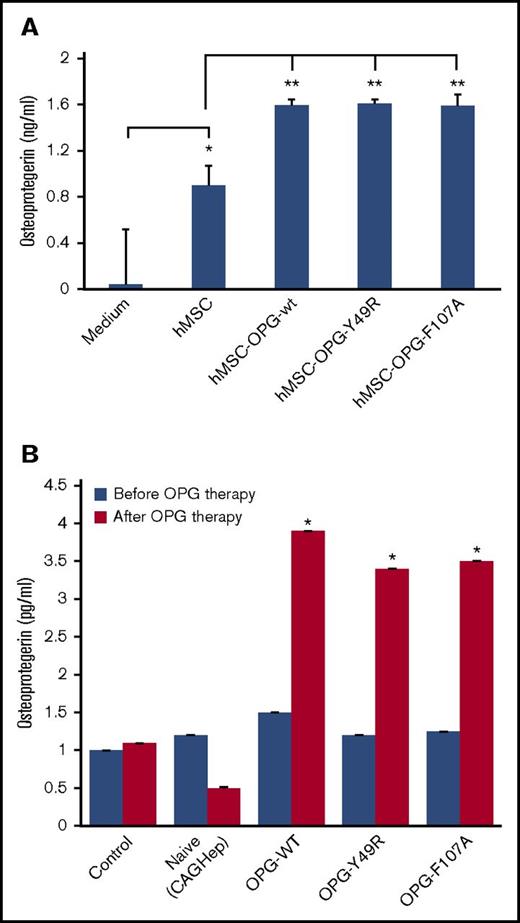
To quantify the amount of OPG produced by hMSC per cell, prior to in vivo administration, supernatants from hMSC, transduced by rAAV-OPGwt and rAAV-OPGmut (Y49R and F107A), were analyzed by ELISA. Results of the assay indicated a significant increase in OPG levels from all 3 groups of vector-transduced hMSCs, compared with supernatant from untransduced hMSCs. A significantly higher OPG level in supernatants from untransduced, control hMSC, compared with culture medium, confirmed previous reports of modest, endogenous OPG expression in MSCs31 (Figure 6A). To correlate the therapy effects with systemic OPG levels, serum samples were collected both prior to beginning of hMSC therapy and on day 14 after hMSC administration and analyzed by ELISA. Results of this quantitative analysis indicated a significant increase in OPG levels in mice treated with MSCs, transduced with rAAV-OPGWT/mut, compared with the group that received MSCs without vector transduction (Figure 6B; P < .05). Although not statistically significant, it was interesting to note an observable decrease in serum OPG levels in the group of mice that were challenged with CAGHep cells, but without follow-up therapy with hMSCs.
ELISA for OPG levels. Forty-eight hours after transduction of hMSCs with AAV-OPGwt or AAV-OPGY49R or AAV-OPGF107A culture, supernatants were analyzed by ELISA for OPG levels using a commercial kit (A). OPG levels were also quantitated in mouse serum following therapy. Blood samples were obtained from untreated and hMSC-OPG–treated mice both prior to initiating hMSC therapy and 14 days after the treatment and similarly analyzed by ELISA for OPG levels (B). Data presented are mean ± standard error from triplicate experiments (*P < .05; **P < .01).
ELISA for OPG levels. Forty-eight hours after transduction of hMSCs with AAV-OPGwt or AAV-OPGY49R or AAV-OPGF107A culture, supernatants were analyzed by ELISA for OPG levels using a commercial kit (A). OPG levels were also quantitated in mouse serum following therapy. Blood samples were obtained from untreated and hMSC-OPG–treated mice both prior to initiating hMSC therapy and 14 days after the treatment and similarly analyzed by ELISA for OPG levels (B). Data presented are mean ± standard error from triplicate experiments (*P < .05; **P < .01).
Discussion
A major challenge for testing novel therapies aimed at curing disseminated myeloma is the involvement of major bones of the skeleton. In myeloma, tumor-induced bone lesions can be discrete, widespread, osteopenic, or multiple lytic types affecting spine, skull, and long bones.32 With poor prognosis of patients with higher osteoclast lesions, current frontline therapy for disseminated myeloma bone disease is aminobisphosphonates, such as zoledronate and pamidronate.33,34 Despite the initial response, prolonged use of aminobisphosphonates often results in osteonecrosis of the jaw and atypical stress fractures.35-41 Although the classical pathways activating osteoclast functions are well known in myeloma bone lesions, testing of novel approaches is hampered by a lack of appropriate preclinical models exhibiting adequate window for disease progression with characteristics of disseminated disease in major bones. Toward advancing and testing a genetically engineered stem cell therapy by a systemic approach, targeting osteolytic pathology in myeloma, we first developed a mouse model using the human myeloma cell lines, overexpressing heparanase.24 The SCID/CAGHep model has been used previously for its resemblance of myeloma pathology. Early studies showed that subcutaneous injection of CAGHep cells resulted in bone-specific growth of myeloma cells, enhanced by syndecan-1.26,42 Studies determined that syndecan-1 not only is an important molecule involved in myeloma cell growth and survival but also exerted a role in tumor angiogenesis because tumors with low syndecan-1 expression developed poor vasculature due to decreased VEGF.43 Other studies have shown that overexpression of heparanase in CAG cells promoted local and systemic bone destruction through secretion of RANKL and enhanced the expression and activity of hepatocyte growth factor.26,42 Based on this, we reasoned that CAG cells overexpressing heparanase would serve to establish an ideal preclinical model for testing the effects of a novel cell therapy in a setting of skeletal disease dissemination following systemic administration.
Upon establishing this model, we assessed the effects of genetically engineered cell therapy for controlling osteolytic bone damage in myeloma. Human MSCs were used to deliver OPG variants, abolished in TRAIL binding. A previous study from our laboratory determined therapeutic effects of murine bone marrow–derived MSCs in a localized osteolytic skeletal lesion.30 Although MSCs in the bone microenvironment produce OPG to prevent excess osteoclast activation, upon osteolytic tumor growth, the homeostasis shifts toward less OPG production.44 Furthermore, because of a decreased osteogenic potential of MSCs in myeloma,45-47 the osteoprogenitor cell source becomes limited to remodel bone following aggressive osteoclast damage. During this shift in balance that negatively affects OPG level,44 supplementation of MSCs overexpressing OPG offers a dual benefit to decreasing osteoclast activation and providing additional source of osteoprogenitors. In pathophysiology of myeloma bone disease, the coordinated function of osteoclasts and osteoblasts is shifted toward increased osteoclastic activity.44,48 RANKL, a member of the TNF superfamily, is produced by bone marrow stromal cells, osteoblasts, and activated T lymphocytes. Interaction of myeloma cells in the bone microenvironment stimulates bone marrow stromal cells and osteoblasts to increase production of RANKL.49,50 Elevated levels of RANKL in myeloma patients are also associated with cancer cell–mediated internalization and degradation of OPG, consequently increasing the RANKL/OPG ratio with poor prognosis.51,52 Thus, restoring RANKL/OPG homeostasis is crucial to manage bone pathology in myeloma. Despite the potential of OPG reversing RANKL activation of osteoclasts,11,12 the use of OPG is limited because of its ability to bind to TRAIL, thereby serving as a survival factor for tumor cells from TRAIL-induced apoptosis. Thus, it becomes crucial to uncouple RANKL binding and TRAIL binding properties of OPG for therapeutic utility. In this regard, we recently identified critical domains on OPG that bind to TRAIL and successfully developed OPG variants, abolished in TRAIL binding, yet retained in OPG binding.15 In a similar effort, a recent study also demonstrated that a DR5-specific variant (TRAIL D269H/E195R) displayed a significantly decreased affinity to OPG, indicating uncoupling OPG and TRAIL interaction could effectively improve clinical utility of both OPG and TRAIL for restoring bone damage and inducing myeloma cell apoptosis, respectively.53
Mice treated with the hMSC-rAAV-OPG (WT, or mutants Y49R or F107A) were protected against myeloma-induced osteolysis, irrespective of the treatment groups. More importantly, the level of protection was observed in both the tibia and the spine by systemic administration of MSC expressing OPG. The most surprising outcome was the amount of bone protection observed in cohorts of mice treated with OPGwt or OPGmut (Y49R or F107A) despite the presence of aggressively growing tumors. In cohorts of mice that were administered hMSC-OPG therapy after tumor challenge, 3D reconstruction from bone micro-CT analysis clearly showed intact spinal lumbar bones as evidence for bone protection. Cohorts of mice treated with hMSC-OPG mutants showed results similar to the hMSC-OPGWT group and untreated age-matched control group in trabecular bone architecture and trabecular connectivity density. Although quantitative analysis showed a significant difference in connectivity density, there was no significant difference in trabecular number and trabecular spacing when comparing control group to hMSC-OPG–treated groups, suggesting a reversal of bone phenotype to normal. Results indicated that even though there was an increased production of RANKL due to the presence of CAGHep cells, serum OPG levels in the 3 treated groups indicated a significant elevation, suggesting that the observed increase was sufficient to control RANKL-induced bone destruction. Overall, results of this study suggest that OPG therapy using genetically modified OPG, abolished in TRAIL binding, can be an alternative therapeutic approach to treating myeloma bone disease and may be used in combination with conventional therapy.
The use of MSC in cancer therapy has been a subject of debate.54-58 Although MSCs possess advantages of immune modulation, studies have also reported tumorigenic potential of culture-expanded MSCs upon in vivo administration.59 Hence, future studies using humanized mice models should define an optimal amount of MSC without deleterious consequences, prior to clinical translation.
Although the potential of OPG inhibiting osteoclast activity is known, its ability to bind TRAIL poses a major problem as a survival factor for tumor cells. Results of our study provided the first evidence that a systemic, cell-based therapy approach using modified OPG variants with abolished TRAIL binding, but not the RANKL binding, can be successfully used to revert damages in major bones in a disseminated MM model. Our study initially employed the BALB/c-MPC-11 model to test efficacy of OPG-engineered MSC in disseminated myeloma bone lesions. Although systemic dissemination of disease was evident, the osteolytic progression was too rapid. Hence, we resorted to the SCID-CAGHep model, and positive results obtained in this model should be further validated in a humanized mouse model bearing human myeloma growth prior to clinical translation.
Further analysis, combining TRAIL therapy to synergize bone remodeling to induction of tumor cell apoptosis, should move this unique combination closer to human clinical trials for not only myeloma but also other osteolytic metastases, including carcinomas of the breast, kidney, lung, and thyroid.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank UAB Pathology Core, Small Animal Phenotyping Core Research Laboratories, Vinayak Khattar and Kenneth Hough for help with fluorescence-activated cell sorting analysis.
This work was supported by National Institutes of Health, National Cancer Institute grants R01CA133737 (S.P.), R01CA184770 (S.P.), and R01CA138340 (R.D.S.), and National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR060948 (S.P.).
Authorship
Contribution: J.T.H. and J.H.L. performed experiments, analyzed data, and wrote the manuscript; H.W. and C.Y.H. performed experiments; V.C.R., R.D.S., and D.C. contributed reagents; and S.P. designed the work, provided resources, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Selvarangan Ponnazhagan, Department of Pathology, The University of Alabama at Birmingham, 1825 University Blvd, SHEL 814, Birmingham, AL 35294; e-mail: pons@uab.edu.