Key Points
Fibrocytes are progenitor cells that home to injured organs and contribute to fibrosis.
Levels of circulating fibrocytes are associated with pulmonary dysfunction in adults with SCD.
Abstract
Lung injury and fibrosis are common in patients with sickle cell disease (SCD). Fibrocytes, a population of circulating, bone marrow–derived cells, have been linked to development and progression of tissue fibrogenesis and have been implicated in the development of lung fibrosis in preclinical models of SCD. We tested the hypothesis that the levels and activation state of circulating fibrocytes during steady state are associated with abnormal pulmonary function in adults with SCD. In a prospective cohort of steady-state adults with SCD and healthy age- and race-matched control participants, we measured the concentration and activation state of circulating fibrocytes and assessed pulmonary phenotype with pulmonary function tests (PFTs), a respiratory questionnaire, 6-minute walk test, high-resolution chest computed tomography scan, and echocardiogram. Seventy-one adults with SCD and 26 healthy African American control participants were examined. Compared with control participants, patients with SCD demonstrated higher levels of circulating fibrocytes, a significant proportion of which expressed the activation marker α-smooth muscle actin. Within patients with SCD, elevated absolute concentrations of circulating fibrocytes were strongly and independently associated with impaired lung physiology, as measured by PFTs. We conclude that elevated circulating fibrocytes are associated with lung disease in adults with SCD during steady state, consistent with a role for these cells in pathogenesis of lung fibrosis in this disease. Circulating fibrocytes may represent a novel biomarker for progressive pulmonary fibrosis in patients with SCD.
Introduction
The lungs are among the most severely affected organs in patients with sickle cell disease (SCD).1 Acutely, episodic microvascular occlusion within the pulmonary vessels causes a form of lung injury known as acute chest syndrome (ACS).2 These episodes, characterized by fever, new infiltrates, and respiratory symptoms, occur at a rate of 8.8 per 100 patient-years in adults with SCD and affect 50% of patients during their lifetime.3 Chronically, evidence exists that fibrosis occurs in the lungs of adults with SCD: 74% have restriction on pulmonary function tests (PFTs),4 50% show increased interstitial markings on high-resolution computed tomography (HRCT),5 and 40% have pulmonary fibrosis on autopsy.6 When diagnosed in a patient with SCD, pulmonary fibrosis affects mortality. It is the primary cause of death in 20% of adults with SCD1 ; once pulmonary hypertension is present, it is associated with a median survival time of only 5 years.7 It is interesting to note that the rate of ACS episodes is not consistently associated with the development of pulmonary fibrosis, which suggests that acute, clinically apparent insults alone may not be sufficient to explain the pathogenesis of pulmonary fibrosis in SCD.4,8,9
Myofibroblasts, the key effector cells in fibrosis, were previously thought to be derived solely from the activation and proliferation of quiescent resident tissue fibroblasts at the site of injury.10 Contemporary evidence, however, indicates that myofibroblasts can also differentiate from epithelial or endothelial cells and also from a population of bone marrow–derived circulating progenitor cells, termed “fibrocytes.”11-14 A unique cell population characterized by co-expression of both leukocyte and fibroblast markers, fibrocytes are released from the bone marrow in response to injury; differentiate to myofibroblast-like cells (ie, express α-smooth muscle actin [α-SMA]); and home to the lungs, where they are associated with fibrosis.15,16
In the context of an animal model of SCD, fibrocytes are recruited to the lungs via the CXCL12/CXCR4 chemokine axis in response to episodes of hypoxia.17 An interruption of this recruitment reduced deposition of lung collagen and improved lung function.17 The concentration of circulating fibrocytes was also shown at steady state to be elevated in 2 separate cohorts of children and adults with SCD and increase further during episodes of clinical vaso-occlusion (VO).17,18 These data suggest that fibrocytes may be relevant to lung fibrogenesis when patients with SCD are at steady state, as well as during episodes of VO or ACS. To assess whether circulating fibrocyte levels are associated with pulmonary fibrosis in SCD, we tested the hypothesis that the levels and activation state of circulating fibrocytes during steady state are associated with abnormal pulmonary function in adults with SCD.
Methods
Study design
In a prospective cross-sectional study of adults with SCD (aged 18-60 years), we sought to determine the relationship between circulating fibrocyte concentration and pulmonary disease. All genotypes were eligible, and participants were recruited regardless of respiratory symptoms or pulmonary history. For complete inclusion and exclusion criteria, see the supplemental Methods. The institutional review board at the Medical College of Wisconsin approved the study. After written consent, blood samples for fibrocyte levels were obtained when patients with SCD were at steady state, at least 2 weeks from a hospital or emergency department admission. Within 60 days of fibrocyte measurement, PFTs with bronchodilator assessment, diffusing capacity, and lung volumes were performed and interpreted according to American Thoracic Society criteria.19-22 An oxygen assessment with pulse oximetry, a 2D echocardiogram, chest HRCT, a 6-minute walk test,23 and a visual analog pain score24 were also performed. Similar to fibrocyte measurements, participants had to be at steady state for 2 weeks prior to the pulmonary assessment. A chest radiologist blinded to clinical data assessed HRCT images for parenchymal abnormalities; a previously validated scoring system was used.25 In brief, CT findings were graded based on the severity and extent of the pulmonary interstitial disease:
• Grade 0: normal findings
• Grade 1: minimal disease (thickened interalveolar septa, ground-glass opacities)
• Grade 2: mild disease (more extensive thickening of the interalveolar septa and ground-glass opacities, reticular disease, and subpleural cysts)
• Grade 3: moderate disease (findings of grade 2 in addition to traction bronchiectasis)
• Grade 4: severe disease (findings of grade 3 involving the lung parenchyma diffusely)
Control participants were healthy African Americans without respiratory disease, symptoms, smoking history, or sickle cell trait. Control participants provided a blood sample and underwent PFTs with bronchodilator assessment, diffusing capacity of the lungs for carbon monoxide (DLCO), and lung volumes; they also underwent an oxygen assessment and a 6-minute walk test.
Fibrocyte measurements
Heparinized blood samples were shipped overnight at 4°C and were processed the following day. We performed fluorescence-activated cell-sorting analysis to quantify fibrocytes, defined as cells co-expressing the common leukocyte antigen, CD45, together with positive intracellular staining for collagen-1 (CD45+Col1+ cells). Subsets of fibrocytes were further quantified for expression of the myofibroblast activation marker α-SMA (CD45+Col1+α-SMA+ cells), as well as the CXCR4 chemokine receptor (CD45+Col1+CXCR4+ cells).11,12
Statistical analyses
We compared SCD participants with control participants using the χ2 test or Fisher’s exact test and Mann-Whitney U test for categorical and continuous variables, respectively. To determine the significance of predictors of pulmonary function, we chose to use a classification tree approach.26 This approach was selected because there are a large number of predictor variables to assess, and this approach allowed us to examine the interrelationship and importance of these variables in relationship to pulmonary function. It also allowed us to determine potential thresholds for the fibrocytes.26 Dependent variables were forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). PFT variables were analyzed as continuous and dichotomous variables. Ultimately, an examination of the data demonstrated that participants with lower PFTs differed from those with normal PFTs; therefore, we chose to perform the regression analyses with PFT variables as dichotomous. To categorize the PFT variables, abnormal FEV1, FVC, FEV1/FVC, total lung capacity (TLC), and DLCO were defined as less than 5% of the distribution of the values.21,27,28 For percent predicted TLC, a 12% adjustment was used to account for the effect of African American race.22 PFTs were interpreted per American Thoracic Society/European Respiratory Society guidelines.22 Predictor variables included in the tree model were fibrocytes, age, a history of smoking (at least 100 cigarettes in lifetime), asthma, hemoglobin levels, white blood cell count, SCD genotype, fetal hemoglobin levels, transfusion within 120 days, a history of ACS, and the number of hospitalizations. In the classification tree model, the relationship between dependent variables and each predictor variable was analyzed individually in order to determine which predictor variable and what threshold produced the highest significance. Using the information from the tree about potential interactions and requiring an importance of at least 20% in the tree, we chose the covariates for the logistic model. Variables with a P ≤ .25 on bivariate analyses were entered to the model selection procedure; variables with a P < .05 were retained in the model and considered significantly related to outcome. Missing data were excluded. For all analyses, a 2-sided P < .05 was considered significant. A post hoc power analysis revealed that, given 69 participants, there was at least 87% power to detect a difference in proportions of 41%. Statistical software used was SAS9.4 (SAS Institute, Cary, NC), SPSS21 (IBM Corporation, Armonk, NY), and Salford Systems CART (San Diego, CA).
Results
Participants
A total of 71 adults with SCD and 26 control participants were examined (Table 1). Median age was 32 years for adults with SCD and 28 years for control participants. The majority of SCD participants had HbSS disease (n = 45), and the remainder had HbSC (n = 17) and HbS-β+-thalassemia (n = 8). A diagnosis of asthma was present in 39% of participants with SCD, and 42% reported a lifetime smoking history of 5 packs or greater. Compared with control participants, those with SCD had lower median FEV1, FVC, and TLC values; a significant proportion of SCD participants had measures below the fifth percentile of the lower limit of normal. Of the SCD participants with complete PFTs (n = 69), 50 (72%) were classified as having normal measures, 4 (6%) were classified as having measures indicating obstruction, 12 (17%) had measures indicating restriction, and 3 (4%) had measures of mixed or unclassifiable classification. Eleven participants with SCD (16%) had evidence of fibrosis on chest HRCT.
Characteristics of adults with SCD and African American control participants
| Median (IQR), n (%), yes . | Control participants (n = 26) . | Adults with SCD (n = 71) . | P . |
|---|---|---|---|
| Demographics | |||
| Age, median years (range) | 28.3 (18.8-55.0) | 31.8 (19.7-63.2) | .07 |
| Female, n (%) | 15 (57.7) | 45 (63.3) | .61 |
| PFTs | |||
| FEV1, % predicted | 98.5 (90.0-106.0) | 81.0 (72.0-91.0) | <.0001 |
| No. of FEV1 (<5% LLN) | 1 (4.2) | 19 (27.5) | .019 |
| FVC, % predicted | 103.5 (94.0-111.0) | 85.0 (75.0-99.0) | <.0001 |
| No. of FVC (<5% LLN) | 0 | 16 (23.2) | .009 |
| FEV1/FVC, % predicted | 80.5 (76.0-84.0) | 78.0 (73.5-82.5) | .23 |
| No. of FEV1/FVC (<5% LLN) | 1 (3.9) | 6 (8.7) | .67 |
| TLC, % predicted | 107.0 (99.0-111.0) | 90.0 (81.0-103.0) | .004 |
| No. of TLC (<5% LLN) | 2 (8.7) | 32 (46.4) | .001 |
| DLCO, % predicted, hemoglobin adjusted | 79.9 (70.3-85.5) | 49.3 (41.4-57.7) | <.0001 |
| No. of DLCO (<80%) | 12 (50.0) | 68 (97.1) | <.0001 |
| PFT interpretation pattern, % | .12 | ||
| Normal | 23 (95.8) | 50 (72.5) | |
| Obstructive | 1 (4.2) | 4 (5.8) | |
| Restrictive | 0 | 12 (17.4) | |
| Mixed | 0 | 2 (2.9) | |
| Nonspecific | 0 | 1 (1.5) | |
| HRCT | |||
| No. with HRCT score ≥2 (%) | — | 11 (15.9) | — |
| Echocardiography | |||
| TR maximum velocity, cm/s | — | 228.0 (215.7-266.4) | — |
| Laboratory values | |||
| Hemoglobin, g/dL | 13.2 (12.4-13.9) | 9.5 (8.5-10.5) | <.0001 |
| WBC, ×109/L | 5.1 (4.1-6.9) | 8.6 (6.4-11.0) | <.0001 |
| Reticulocytes, % | 1.2 (1.-0-1.8) | 6.9 (3.3-9.8) | <.0001 |
| Resting SpO2, % | — | 98.0 (96.0-99.0) | — |
| 6-min walk test, m | — | 375.5 (330.0-417.5) | — |
| Fetal hemoglobin, % | — | 7.6 (2.2-15.7) | — |
| SCD characteristics | |||
| Pain length, cm | — | 2.4 (0.8-4.6) | — |
| History of ACS | — | 11 (15.5) | — |
| Hydroxyurea | — | 51 (71.8) | — |
| Transfusion in last 120 d, % | — | 14 (19.7) | — |
| Asthma characteristics | |||
| Asthma | — | 28 (39.4) | — |
| Eosinophils, % | 2.0 (1.0-2.5) | 2.0 (1.0-3.0) | >.9 |
| Median (IQR), n (%), yes . | Control participants (n = 26) . | Adults with SCD (n = 71) . | P . |
|---|---|---|---|
| Demographics | |||
| Age, median years (range) | 28.3 (18.8-55.0) | 31.8 (19.7-63.2) | .07 |
| Female, n (%) | 15 (57.7) | 45 (63.3) | .61 |
| PFTs | |||
| FEV1, % predicted | 98.5 (90.0-106.0) | 81.0 (72.0-91.0) | <.0001 |
| No. of FEV1 (<5% LLN) | 1 (4.2) | 19 (27.5) | .019 |
| FVC, % predicted | 103.5 (94.0-111.0) | 85.0 (75.0-99.0) | <.0001 |
| No. of FVC (<5% LLN) | 0 | 16 (23.2) | .009 |
| FEV1/FVC, % predicted | 80.5 (76.0-84.0) | 78.0 (73.5-82.5) | .23 |
| No. of FEV1/FVC (<5% LLN) | 1 (3.9) | 6 (8.7) | .67 |
| TLC, % predicted | 107.0 (99.0-111.0) | 90.0 (81.0-103.0) | .004 |
| No. of TLC (<5% LLN) | 2 (8.7) | 32 (46.4) | .001 |
| DLCO, % predicted, hemoglobin adjusted | 79.9 (70.3-85.5) | 49.3 (41.4-57.7) | <.0001 |
| No. of DLCO (<80%) | 12 (50.0) | 68 (97.1) | <.0001 |
| PFT interpretation pattern, % | .12 | ||
| Normal | 23 (95.8) | 50 (72.5) | |
| Obstructive | 1 (4.2) | 4 (5.8) | |
| Restrictive | 0 | 12 (17.4) | |
| Mixed | 0 | 2 (2.9) | |
| Nonspecific | 0 | 1 (1.5) | |
| HRCT | |||
| No. with HRCT score ≥2 (%) | — | 11 (15.9) | — |
| Echocardiography | |||
| TR maximum velocity, cm/s | — | 228.0 (215.7-266.4) | — |
| Laboratory values | |||
| Hemoglobin, g/dL | 13.2 (12.4-13.9) | 9.5 (8.5-10.5) | <.0001 |
| WBC, ×109/L | 5.1 (4.1-6.9) | 8.6 (6.4-11.0) | <.0001 |
| Reticulocytes, % | 1.2 (1.-0-1.8) | 6.9 (3.3-9.8) | <.0001 |
| Resting SpO2, % | — | 98.0 (96.0-99.0) | — |
| 6-min walk test, m | — | 375.5 (330.0-417.5) | — |
| Fetal hemoglobin, % | — | 7.6 (2.2-15.7) | — |
| SCD characteristics | |||
| Pain length, cm | — | 2.4 (0.8-4.6) | — |
| History of ACS | — | 11 (15.5) | — |
| Hydroxyurea | — | 51 (71.8) | — |
| Transfusion in last 120 d, % | — | 14 (19.7) | — |
| Asthma characteristics | |||
| Asthma | — | 28 (39.4) | — |
| Eosinophils, % | 2.0 (1.0-2.5) | 2.0 (1.0-3.0) | >.9 |
Bold indicates significance (P < .05).
IQR, interquartile range; LLN, lower limit of normal; SpO2, blood oxygen saturation; TR, tricuspid regurgitation; WBC, white blood cell count.
Fibrocyte measurements
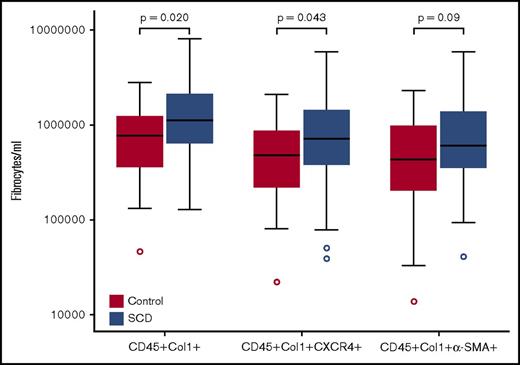
Similar to our previous studies in patients with SCD,17,18 the concentration of total circulating fibrocytes, fibrocytes expressing the chemokine receptor CXCR4, and the fibrocyte subset expressing the activation marker α-SMA were all higher in SCD participants compared with those of control participants (Figure 1). Because fibrocyte values were not normally distributed, we examined different cutoff values to maximize the sensitivity and specificity to predict abnormal pulmonary function in patients with SCD (Table 2; supplemental Table 1). Using cutoff values for fibrocytes (CD45+Col1+ cells), CXCR4+ fibrocytes, and α-SMA+–activated fibrocytes, we observed significant relationships between higher levels of circulating fibrocytes and reduced FEV1, FVC, and TLC. However, the strongest association was with FEV1 and FVC; only α-SMA+–activated fibrocytes were associated with TLC. Fibrocyte concentrations were not associated with resting pulse oximetry (P = .25), 6-minute walk test (P = .57), or moderate to severe fibrosis on HRCT (P = .52).
Box plots of fibrocytes (CD45+Col1+), CXCR4+fibrocytes, and α-SMA+fibrocytes in adults with SCD and African American control participants. Boundaries of box represent 75th and 25th percentiles; median is line within the box; whiskers are 1.5 quartiles; outliers are shown in circles.
Box plots of fibrocytes (CD45+Col1+), CXCR4+fibrocytes, and α-SMA+fibrocytes in adults with SCD and African American control participants. Boundaries of box represent 75th and 25th percentiles; median is line within the box; whiskers are 1.5 quartiles; outliers are shown in circles.
Associations between circulating fibrocytes and pulmonary function in adults with SCD
| . | . | χ2 test P value and sensitivity, specificity . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fibrocyte . | Threshold . | Abnormal FEV1 . | Abnormal FVC . | Abnormal TLC . | ||||||
| P . | P . | P . | ||||||||
| CD45+Col1+ | 1 500 000 | .009 | TPR | 0.63 | .006 | TPR | 0.69 | .150 | TPR | 0.53 |
| 95% CI | (0.38-0.84) | 95% CI | (0.41-0.89) | 95% CI | (0.29-0.77) | |||||
| SPC | 0.72 | SPC | 0.72 | SPC | 0.67 | |||||
| 95% CI | (0.58-0.84) | 95% CI | (0.58-0.83) | 95% CI | (0.55-0.80) | |||||
| CD45+Col1+CXCR4+ | 1 100 000 | .007 | TPR | 0.63 | .003 | TPR | 0.69 | .360 | TPR | 0.47 |
| 95% CI | (0.38-0.84) | 95% CI | (0.41-0.89) | 95% CI | (0.23-0.71) | |||||
| SPC | 0.72 | SPC | 0.72 | SPC | 0.65 | |||||
| 95% CI | (0.56-0.84) | 95% CI | (0.58-0.83) | 95% CI | (0.52-0.78) | |||||
| CD45+Col1+α−SMA+ | 420 000 | .010 | TPR | 0.90 | .036 | TPR | 0.88 | .020 | TPR | 0.88 |
| 95% CI | (0.67-0.99) | 95% CI | (0.62-0.99) | 95% CI | (0.72-0.99) | |||||
| SPC | 0.46 | SPC | 0.43 | SPC | 0.44 | |||||
| 95% CI | (0.32-0 .61) | 95% CI | (0.30-0.58) | 95% CI | (0.31-0.58) | |||||
| . | . | χ2 test P value and sensitivity, specificity . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fibrocyte . | Threshold . | Abnormal FEV1 . | Abnormal FVC . | Abnormal TLC . | ||||||
| P . | P . | P . | ||||||||
| CD45+Col1+ | 1 500 000 | .009 | TPR | 0.63 | .006 | TPR | 0.69 | .150 | TPR | 0.53 |
| 95% CI | (0.38-0.84) | 95% CI | (0.41-0.89) | 95% CI | (0.29-0.77) | |||||
| SPC | 0.72 | SPC | 0.72 | SPC | 0.67 | |||||
| 95% CI | (0.58-0.84) | 95% CI | (0.58-0.83) | 95% CI | (0.55-0.80) | |||||
| CD45+Col1+CXCR4+ | 1 100 000 | .007 | TPR | 0.63 | .003 | TPR | 0.69 | .360 | TPR | 0.47 |
| 95% CI | (0.38-0.84) | 95% CI | (0.41-0.89) | 95% CI | (0.23-0.71) | |||||
| SPC | 0.72 | SPC | 0.72 | SPC | 0.65 | |||||
| 95% CI | (0.56-0.84) | 95% CI | (0.58-0.83) | 95% CI | (0.52-0.78) | |||||
| CD45+Col1+α−SMA+ | 420 000 | .010 | TPR | 0.90 | .036 | TPR | 0.88 | .020 | TPR | 0.88 |
| 95% CI | (0.67-0.99) | 95% CI | (0.62-0.99) | 95% CI | (0.72-0.99) | |||||
| SPC | 0.46 | SPC | 0.43 | SPC | 0.44 | |||||
| 95% CI | (0.32-0 .61) | 95% CI | (0.30-0.58) | 95% CI | (0.31-0.58) | |||||
Bold indicates significance (P < .05).
CI, confidence interval; SPC, specificity (true-negative rate); TPR, sensitivity (true-positive rate).
We next determined whether fibrocyte concentrations correlated with PFT variables. We examined PFTs as a continuous and dichotomous (normal/abnormal) variable. When analyzed as a continuous variable, fibrocytes and CXCR4+ fibrocytes were significantly associated with FEV1 (P = .032), whereas α-SMA+ fibrocytes were associated with FEV1 and FVC (P = .015 and P = .032, respectively). Otherwise, there were no significant associations between fibrocytes, CXCR4+ fibrocytes, or α-SMA+ fibrocytes and FEV1, FVC, TLC, or DLCO. For the remainder of the analyses, we examined PFTs as normal/abnormal. First, we assessed for interactions between potential variables with a classification tree analysis (supplemental Figure 1A-B). In this analysis, fibrocytes had the strongest association with abnormal FEV1 and FVC. From variables selected in the classification tree analysis (see “Methods” for criteria), multivariable logistic regression analyses were performed with FEV1 and FVC as the dependent variables. We focused on these variables because FVC is the PFT variable most strongly associated with outcome in interstitial lung disease,29 and FEV1 decline is associated with mortality in SCD.30 The logistic regression analyses demonstrated that circulating fibrocyte levels were strongly and independently associated with abnormal FEV1 and FVC (Table 3). In addition to fibrocytes, transfusion within 120 days was found to be independently associated with impaired FEV1 but not FVC (Table 3).
Bivariate and multivariable models to demonstrate the association between high fibrocytes and abnormal FEV1 and FVC
| Variable . | Odds ratio (95% CI) . | χ2 . | P . |
|---|---|---|---|
| FEV1 | |||
| Univariate | |||
| Fibrocytes | 4.408 (1.441-13.484) | 6.763 | .009 |
| Age | 0.960 (0.908-1.016) | 2.015 | .156 |
| Transfusion in last 120 d | 4.278 (1.209-15.134) | 5.083 | .024 |
| Hemoglobin | 0.715 (0.496-1.030) | 3.242 | .072 |
| WBC | 1.142 (0.980-1.332) | 2.880 | .090 |
| No. of hospitalizations | 1.042 (0.949-1.144) | 0.750 | .386 |
| Smoking history | 0.970 (0.332-2.833) | 0.032 | .955 |
| Fetal hemoglobin | 0.990 (0.924-1.060) | 0.082 | .775 |
| Sex | 1.412 (0.478-4.168) | 0.390 | .532 |
| Asthma | 1.747 (0.597-5.114) | 1.037 | .309 |
| Multivariable | |||
| Fibrocytes | 4.462 (1.385-14.376) | 6.276 | .012 |
| Transfusion in last 120 d | 4.352 (1.125-16.837) | 4.537 | .033 |
| FVC | |||
| Univariate | |||
| Fibrocytes | 5.573 (1.655-18.771) | 7.688 | .006 |
| Age | 0.942 (0.882-1.006) | 3.212 | .073 |
| Hemoglobin | 0.713 (0.484-1.051) | 2.927 | .087 |
| WBC | 1.128 (0.967-1.317) | 2.344 | .126 |
| No. of hospitalizations | 0.977 (0.873-1.093) | 0.169 | .684 |
| Fetal hemoglobin | 1.016 (0.947-1.091) | 0.204 | .652 |
| Smoking | 0.530 (0.161-1.742) | 1.093 | .296 |
| Transfusion in last 120 d | 2.557 (0.699-9.357) | 2.011 | .156 |
| Asthma | 1.392 (0.447-4.335) | 0.325 | .568 |
| Multivariable | |||
| Fibrocytes | 5.573 (1.655-18.771) | 7.688 | .006 |
| Variable . | Odds ratio (95% CI) . | χ2 . | P . |
|---|---|---|---|
| FEV1 | |||
| Univariate | |||
| Fibrocytes | 4.408 (1.441-13.484) | 6.763 | .009 |
| Age | 0.960 (0.908-1.016) | 2.015 | .156 |
| Transfusion in last 120 d | 4.278 (1.209-15.134) | 5.083 | .024 |
| Hemoglobin | 0.715 (0.496-1.030) | 3.242 | .072 |
| WBC | 1.142 (0.980-1.332) | 2.880 | .090 |
| No. of hospitalizations | 1.042 (0.949-1.144) | 0.750 | .386 |
| Smoking history | 0.970 (0.332-2.833) | 0.032 | .955 |
| Fetal hemoglobin | 0.990 (0.924-1.060) | 0.082 | .775 |
| Sex | 1.412 (0.478-4.168) | 0.390 | .532 |
| Asthma | 1.747 (0.597-5.114) | 1.037 | .309 |
| Multivariable | |||
| Fibrocytes | 4.462 (1.385-14.376) | 6.276 | .012 |
| Transfusion in last 120 d | 4.352 (1.125-16.837) | 4.537 | .033 |
| FVC | |||
| Univariate | |||
| Fibrocytes | 5.573 (1.655-18.771) | 7.688 | .006 |
| Age | 0.942 (0.882-1.006) | 3.212 | .073 |
| Hemoglobin | 0.713 (0.484-1.051) | 2.927 | .087 |
| WBC | 1.128 (0.967-1.317) | 2.344 | .126 |
| No. of hospitalizations | 0.977 (0.873-1.093) | 0.169 | .684 |
| Fetal hemoglobin | 1.016 (0.947-1.091) | 0.204 | .652 |
| Smoking | 0.530 (0.161-1.742) | 1.093 | .296 |
| Transfusion in last 120 d | 2.557 (0.699-9.357) | 2.011 | .156 |
| Asthma | 1.392 (0.447-4.335) | 0.325 | .568 |
| Multivariable | |||
| Fibrocytes | 5.573 (1.655-18.771) | 7.688 | .006 |
High fibrocytes are defined as >1 500 000 cells/mL; abnormal FEV1 and FVC, <5% LLN. The variables in the univariate section for each outcome were chosen by using the information from the tree and requiring an importance of at least 20% in the tree (see supplemental Figure 1A-B).
Bold indicates significance (P < .05).
Abbreviations are explained in Tables 1 and 2.
Finally, we compared SCD participants with high circulating fibrocytes vs SCD participants with low circulating fibrocytes to determine whether these 2 groups differed in characteristics other than pulmonary function (Table 4). We found no associations between higher fibrocyte levels and demographic variables, pulmonary fibrosis on HRCT or other measures of pulmonary disease, or SCD characteristics.
Characteristics of adults with SCD with low and high circulating fibrocytes
| Median (IQR), n (%), yes . | Low fibrocytes (n = 44) . | High fibrocytes (n = 27) . | P . |
|---|---|---|---|
| Demographics | |||
| Age, median (range) | 33.8 (21.2-59.8) | 31.0 (19.7-63.2) | .20 |
| Female, n (%) | 26 (59.1) | 19 (70.4) | .33 |
| Smoking history, n (%) | 20 (46.5) | 10 (37.0) | .44 |
| HRCT | |||
| No. ≥2 on fibrosis score (%) | 8 (18.2) | 3 (11.5) | .52 |
| Echocardiography | |||
| TR maximum velocity, cm/s | 227.0 (211.9-271.5) | 231.8 (222.2-249.7) | .82 |
| Laboratory values | |||
| Hemoglobin, g/dL | 9.8 (8.7-10.7) | 9.2 (8.1-10.1) | .15 |
| WBC, ×109/L | 8.6 (6.4-10.1) | 9.9 (6.6-11.9) | .08 |
| Reticulocytes, % | 6.3 (3.3-8.3) | 8.6 (4.6-15.0) | .08 |
| Resting SpO2, % | 98.0 (96.5-99.0) | 97.0 (95.0-99.0) | .25 |
| 6-min walk test, m | 376.0 (317.0-418.0) | 374.0 (350.0-417.0) | .57 |
| PFT interpretation pattern, % | .026 | ||
| Normal | 36 (83.7) | 14 (53.9) | |
| Obstructive | 2 (4.7) | 2 (7.7) | |
| Restrictive | 5 (12.1) | 7 (31.8) | |
| Mixed | 0 | 2 (7.7) | |
| Nonspecific | 0 | 1 (3.9) | |
| SCD characteristics | |||
| HbSS, % | 26 (59.1) | 19 (70.4) | .34 |
| Pain length, cm | 2.3 (0.5-4.6) | 2.7 (0.8-4.8) | .79 |
| ACS | 8 (18.2) | 3 (11.1) | .52 |
| Hydroxyurea | 31 (70.5) | 20 (74.1) | .74 |
| Fetal hemoglobin, % | 6.8 (2.1-15.0) | 11.5 (3.2-16.1) | .55 |
| Transfusion in last 120 d, % | 7 (15.9) | 7 (25.9) | .30 |
| Asthma characteristics | |||
| Asthma | 17 (38.6) | 11 (40.7) | .86 |
| Eosinophils, % | 1.0 (1.0-3.0) | 2.0 (2.0-3.0) | .10 |
| Median (IQR), n (%), yes . | Low fibrocytes (n = 44) . | High fibrocytes (n = 27) . | P . |
|---|---|---|---|
| Demographics | |||
| Age, median (range) | 33.8 (21.2-59.8) | 31.0 (19.7-63.2) | .20 |
| Female, n (%) | 26 (59.1) | 19 (70.4) | .33 |
| Smoking history, n (%) | 20 (46.5) | 10 (37.0) | .44 |
| HRCT | |||
| No. ≥2 on fibrosis score (%) | 8 (18.2) | 3 (11.5) | .52 |
| Echocardiography | |||
| TR maximum velocity, cm/s | 227.0 (211.9-271.5) | 231.8 (222.2-249.7) | .82 |
| Laboratory values | |||
| Hemoglobin, g/dL | 9.8 (8.7-10.7) | 9.2 (8.1-10.1) | .15 |
| WBC, ×109/L | 8.6 (6.4-10.1) | 9.9 (6.6-11.9) | .08 |
| Reticulocytes, % | 6.3 (3.3-8.3) | 8.6 (4.6-15.0) | .08 |
| Resting SpO2, % | 98.0 (96.5-99.0) | 97.0 (95.0-99.0) | .25 |
| 6-min walk test, m | 376.0 (317.0-418.0) | 374.0 (350.0-417.0) | .57 |
| PFT interpretation pattern, % | .026 | ||
| Normal | 36 (83.7) | 14 (53.9) | |
| Obstructive | 2 (4.7) | 2 (7.7) | |
| Restrictive | 5 (12.1) | 7 (31.8) | |
| Mixed | 0 | 2 (7.7) | |
| Nonspecific | 0 | 1 (3.9) | |
| SCD characteristics | |||
| HbSS, % | 26 (59.1) | 19 (70.4) | .34 |
| Pain length, cm | 2.3 (0.5-4.6) | 2.7 (0.8-4.8) | .79 |
| ACS | 8 (18.2) | 3 (11.1) | .52 |
| Hydroxyurea | 31 (70.5) | 20 (74.1) | .74 |
| Fetal hemoglobin, % | 6.8 (2.1-15.0) | 11.5 (3.2-16.1) | .55 |
| Transfusion in last 120 d, % | 7 (15.9) | 7 (25.9) | .30 |
| Asthma characteristics | |||
| Asthma | 17 (38.6) | 11 (40.7) | .86 |
| Eosinophils, % | 1.0 (1.0-3.0) | 2.0 (2.0-3.0) | .10 |
Bold indicates significance (P < .05).
Abbreviations are explained in Tables 1 and 2.
Discussion
Lung fibrosis is an important cause of morbidity and mortality in adults with SCD.1,7 VO, the inciting event in the pathophysiology of fibrosis, produces ischemia and tissue injury. Although the prevailing thought has been that fibroblasts that reside at the site of injury are largely responsible for fibrogenesis, more recently, a population of bone marrow–derived mesenchymal stem cells called fibrocytes has been discovered.31 Fibrocytes enter circulation, home to sites of injury, and are associated with progression of fibrosis.15 They have been implicated in the pathogenesis of several interstitial lung diseases.32-34 Previous data, largely on animal models, have suggested that fibrocytes are also involved in the pathogenesis of lung fibrosis in SCD.17 Our previous data on patients show that circulating fibrocytes are increased and activated in patients with SCD compared with control participants; circulating fibrocytes also are increased further during VO crises.17,18 For the first time, our study extends these findings, which demonstrate that fibrocytes are not only increased and activated in adults with SCD compared with control participants but also are associated with impaired PFTs.
Our findings demonstrate that even during steady state, levels of circulating fibrocytes are significantly elevated in adults with SCD compared with control participants, similar to findings in the SCD mouse model and prior cohorts of children and adults with SCD.17,18 These data underscore the point that steady-state SCD is not biologically quiescent and subclinical VO contributes to chronic hemolysis, inflammation, and tissue injury even during steady state.8,9 These findings support a model of SCD with persistent and variable VO and hemolytic intensity, which result in varying clinical symptoms; it is a process that is associated with ongoing inflammation and tissue injury even during asymptomatic subclinical periods. In this model, organ dysfunction is not simply the consequence of painful episodes or ACS events but also is the sum of subclinical and clinically apparent VO. Hypoxia has been shown to stimulate fibrocytes to express CXCR4 in a hypoxia-inducible factor-1-α–mediated mechanism,16 promoting their migration along a chemokine gradient in response to CXCL12 generated by tissue injury in the lung.15 This model of ongoing tissue damage and fibrogenesis may explain the inconsistent relationships between the rate of ACS episodes and abnormal pulmonary function, because ACS only represents the most severe ischemic episodes.
A key finding of this study was that higher levels of circulating fibrocytes were present during steady state and were associated with decreased FEV1 and FVC consistent with fibrotic lung disease. It is noteworthy that the percentage of SCD participants with abnormal PFTs was less than that seen in the largest study of adults with SCD.4 Although we cannot determine for certain, selection bias may have affected the results in the previous study because nearly 4000 patients were enrolled in the Cooperative Study of Sickle Cell Disease, but only 310 were analyzed for pulmonary function.4 Potentially, participants who exhibited a need for PFTs were more likely to undergo testing. In our study, we enrolled participants regardless of a clinical need for a pulmonary evaluation, which could explain our lower prevalence of abnormal pulmonary function.4 To examine potential factors that could affect pulmonary function, we performed a regression analysis. In this analysis, the level of fibrocytes was strongly associated with reduced FEV1 and FVC. The presence of these associations on a 1-time measure of circulating fibrocytes and pulmonary function is strong evidence of a pathogenic relationship. Previous studies have shown that levels of circulating fibrocytes increase during VO, which suggests that fibrocytes increase and decrease depending on clinical status, similar to other biomarkers in patients with SCD.17 We hypothesize that the contribution of fibrocytes to the development of pulmonary fibrosis occurs across time, with periods of higher and lower levels of circulating fibrocytes depending on the clinical status of the patient. In this scenario, a 1-time fibrocyte measurement may not be optimal to determine future risk of pulmonary fibrosis. Although we found a highly significant relationship between circulating fibrocytes and decreased pulmonary function, this hypothesis predicts an even stronger relationship in a longitudinal study with repeated measures in different disease states, as has been reported in other fibrotic lung diseases.32 Future studies are aimed at exploring this hypothesis and its predictions.
Our study did not detect a significant relationship between most fibrocyte populations and TLC, in contrast to FVC. In a similar manner, we did not detect associations between fibrocyte concentration and other measures of lung disease (DLCO, HRCT, 6-minute walk test). It is possible that the cross-sectional design of our study limited the power of our study to detect these correlations. A planned longitudinal study design, with repeated measures across time, may detect these additional relationships. A second possible explanation is that some of the measurements of lung disease studied have not been validated in quantifying the degree of parenchymal lung disease in SCD. For example, although the prevalence of reticulation and ground glass on HRCT in SCD has been defined, their relationship with physiologic measures of lung function in SCD awaits further study confirmation and validation.35-37 In addition, DLCO measurements are likely to be affected by the extent of anemia and microvascular disease, which are presumably not directly related to lung fibrogenesis. In a similar manner, walking distance may be affected by anemia, as well as cardiovascular and musculoskeletal fitness, independent of lung disease. Finally, it is possible that lung fibrosis may not be identifiable by noninvasive measurements in younger patients, yet higher levels of circulating fibrocytes could still predict long-term risk.
In summary, we identified a significant relationship between elevated circulating fibrocytes and impaired pulmonary physiologic measures. Consistent with the murine models of SCD, these data suggest that circulating fibrocytes are associated with pulmonary dysfunction, which, we propose, is attributed to lung fibrosis.17 Because fibrocytes are measurable in the circulation, they may represent biomarkers of future lung fibrosis.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank research nurse coordinator Debora Nischik for her dedication to the study.
This work was supported by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (RO1HL098526) (B.M., J.J.F., and R.M.S.).
Authorship
Contribution: B.M., R.M.S., and J.J.F. conceived and designed the studies; M.D.B., N.J.W., K.S.S., L.Z., and P.M.S. acquired and analyzed data; B.M., L.Z., P.M.S., and J.J.F. interpreted the data; J.J.F. wrote the manuscript; and B.M., M.D.B., N.J.W., L.Z., P.M.S., and J.J.F. edited the manuscript.
Conflict-of-interest disclosure: J.J.F. has received past or current research support from NKT Therapeutics Inc, Prolong Pharmaceuticals, Ironwood Pharmaceuticals, and Astellas. R.M.S. is employed by Novartis. The remaining authors declare no competing financial interests.
Correspondence: Joshua J. Field, Medical Sciences Institute and Blood Research Institute, BloodCenter of Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: joshua.field@bcw.edu.