Key Points
CLL patients value higher PFS but would accept significant reductions in PFS to avoid serious adverse events.
Adding even modest out-of-pocket costs changed treatment choices for hypothetical treatments, suggesting patients are sensitive to cost.
Abstract
Currently, in the United States, 130 000 people live with chronic lymphocytic leukemia (CLL), and almost 20 000 new cases of CLL are diagnosed each year. Little is known about the value patients place upon the attributes of available CLL treatments, which vary in efficacy, side effects, and mode of administration. We used a discrete-choice experiment (DCE) to investigate patients’ preferences for treatment attributes and the impact of out-of-pocket cost on patients’ choices. DCE surveys pose a series of choices between hypothetical treatment options, each defined by a set of attributes, and the responses provide quantitative estimates of the average relative preferences for treatment attribute. Each hypothetical treatment in this survey was defined by 5 attributes with predefined levels for efficacy, adverse events, and mode administration. A patient advocacy organization recruited 384 patients with a self-reported physician diagnosis of CLL to complete the online survey. Respondents placed the highest relative importance on longer progression-free survival (PFS). However, the risk of adverse events also was important, as significant additional PFS was needed to offset patients’ acceptance of worsening adverse events. A supplemental question with 2 treatments and varying costs was included to assess the impact of cost on choice. When costs were included, a large proportion of patients changed their choices between the 2 treatments. Given the available treatments and the high cost of some treatments, physicians may want to explore their patients’ preferences for different treatment features, including benefit–risk tradeoffs and out-of-pocket cost, when selecting the best treatment strategies for patients.
Introduction
In the United States, 130 000 people live with chronic lymphocytic leukemia (CLL).1 The American Cancer Society estimates that, in 2017, there will be 20 110 new cases of CLL in the United States, which represents approximately one-third of all leukemia diagnoses.2 Treatment options for patients have increased, with 4 new treatments approved since 2013 (obinutuzumab, ibrutinib, idelalisib, and venetoclax) and more under development.3,4 Because many patients relapse after treatment, patients may receive a number of different treatments.
Treatments available for CLL vary in efficacy, safety, routes of administration, and cost. With many available choices, patients’ preferences in treatment selection are becoming increasingly important. With the advent of value-based treatment decision-making like the American Society of Clinical Oncology value framework, which scores efficacy, safety and costs, the patients’ perspectives and preferences are often missing in the scoring algorithm.5 Understanding these perspectives and preferences is critical if we want to truly consider what patients value in the delivery of cancer care. Nonetheless, there is little systematic research in this area.
This study helps to fill the knowledge gap on patient preferences by conducting a relatively large-scale study of treatment preferences among patients with CLL in the United States using a discrete-choice experience (DCE) survey. The study includes a novel way to explore the impact of out-of-pocket cost on treatment choice. The study also investigates patient interest in more sensitive tests for minimal residual disease (MRD) or small numbers of cancer cells remaining after treatment.
Methods
Study design
We designed a patient preference survey that included a set of DCE questions that offer a choice between pairs of hypothetical treatments for CLL. DCE questions reflect the assumption that goods can be described by a set of attributes with varying levels and that individuals have preferences for certain combinations of attribute levels.6 We conducted 2 patient focus groups in February and June 2015 to learn about treatment attributes of interest to patients. Candidate attributes were developed based on the patient input, clinical input from experts in CLL, and data on existing CLL treatments. The final attributes and levels were selected at a meeting including representatives from the Leukemia & Lymphoma Society (LLS) and the Lymphoma Research Foundation.
Table 1 summarizes the final set of 5 attributes, which include progression-free survival (PFS), mode of administration and schedule, chance of diarrhea, chance of serious infection, and chance of organ damage (tumor lysis syndrome). The attribute levels were designed to cover most existing treatments as of the fall of 2015. The 2 modes of administration were assigned different durations: the IV mode was fixed at 6 months, and the pills were taken every day until the medicine stopped working (the length of PFS for that hypothetical treatment). Respondents were told they would experience the side effects and elevated risks (diarrhea, infection, and organ damage) as long as they were taking the medicine. Figure 1A presents a sample choice question from the survey.
Attribute levels for the DCE
| Attribute . | Description in survey . | Levels . |
|---|---|---|
| How long until cancer advances | The goal of any medicine for CLL is to remove cancer cells from your body, especially from your blood and bone marrow. When the medicine is working, symptoms of CLL, such as swollen lymph nodes, tiredness, and weight loss, may go away or decrease and your blood counts can return to normal. Over time, the medicine can become less effective. Eventually, CLL symptoms may return and blood or bone marrow tests will show that the number of cancer cells is increasing. This means that the medicine is not working to control the cancer anymore, and the cancer is advancing (getting worse). When this happens, your doctor will talk to you about options, including starting a new CLL medicine. | 10 months 26 months 36 months 60 months |
| How you take the medicine | Medicines to treat CLL can be taken in different ways and follow different schedules. Some medicines are taken for a fixed amount of time, while other medicines need to be taken for as long as the medicine continues to work and the cancer does not advance. In this survey, we will ask you to think about 2 different ways you can take a hypothetical CLL medicine. IV (intravenous infusion) 1 time every month for 6 months ▪ An IV (intravenous infusion) administered at a chemotherapy clinic or hospital 1 day each month for 6 months. ▪ An IV tube is inserted into a blood vessel in your arm to administer the medicine. The IV infusion lasts from 1-1/2 to 4 hours. ▪ You need to spend about 6 hours at the hospital or clinic each time you need an IV infusion.Pills every day until the cancer advances. ▪ You take 1 to 4 pills by mouth either once or twice a day at the same time every day for as long as the medicine continues to work. | IV 1-3 times per month for 6 months Pill every day for X months* |
| Diarrhea† | Some medicines that treat CLL can cause diarrhea (loose stools). For this survey, assume that if a medicine causes diarrhea, the diarrhea would last for 2 to 3 days every month for as long as you are taking the medicine. Later in the survey, we will ask you to think about how severe the diarrhea may be with different hypothetical cancer medicines. There are 3 possibilities: ▪ None. You experience no diarrhea. ▪ Mild to moderate. You have fewer than 7 loose stools per day, and you may experience cramping. The diarrhea lasts for 2 to 3 days each month. ▪ Severe. You have 7 or more loose stools per day, and you have bowel control problems. You experience extreme fluid loss (dehydration) and may need IV fluids. You may need help taking care of yourself, including bathing, dressing, feeding yourself, using the toilet, and taking medications. The diarrhea lasts for 2 to 3 days each month. | None Mild to moderate for 2-3 days a month Severe for 2-3 days a month |
| Chance of serious infection† | For some people, medicines that treat CLL can also damage your body’s ability to make the healthy blood cells that protect your body from illness and infection. When this happens, your immune system becomes weaker and you are more likely to get a serious infection during treatment. People taking CLL treatments may develop different types of infections, including respiratory infections like pneumonia, infections with fever, skin infections with rashes or painful sores, hepatitis, or herpes simplex. When taking a medicine that makes you more likely to get a severe infection, you need to avoid situations where you could be exposed to infections. For example, you need to stay away from other people who are sick and avoid being in large groups of people. People who have a severe infection during treatment may need to go to the hospital, sometimes for as long as a couple of weeks. If not successfully treated, some infections can be life-threatening. Once the infection is gone, most people completely recover and no additional medical treatment is required for the infection. | None 5% 15% 30% |
| Chance of organ damage† | When tumor cells die, they break apart and chemicals from the cells are released into the blood. For some people who are treated for CLL, these chemicals can build up in the blood and cause damage to organs, including the kidneys, heart, and liver. People with organ damage can experience severe problems like vomiting, tiredness, muscle weakness, or seizures, and they need to be treated at the hospital for several days until their organs begin to function normally. Severe organ damage can be life-threatening if it is not treated, but this is very rare. Your doctor will monitor you for signs of organ damage. | None 1% 5% 8% |
| Attribute . | Description in survey . | Levels . |
|---|---|---|
| How long until cancer advances | The goal of any medicine for CLL is to remove cancer cells from your body, especially from your blood and bone marrow. When the medicine is working, symptoms of CLL, such as swollen lymph nodes, tiredness, and weight loss, may go away or decrease and your blood counts can return to normal. Over time, the medicine can become less effective. Eventually, CLL symptoms may return and blood or bone marrow tests will show that the number of cancer cells is increasing. This means that the medicine is not working to control the cancer anymore, and the cancer is advancing (getting worse). When this happens, your doctor will talk to you about options, including starting a new CLL medicine. | 10 months 26 months 36 months 60 months |
| How you take the medicine | Medicines to treat CLL can be taken in different ways and follow different schedules. Some medicines are taken for a fixed amount of time, while other medicines need to be taken for as long as the medicine continues to work and the cancer does not advance. In this survey, we will ask you to think about 2 different ways you can take a hypothetical CLL medicine. IV (intravenous infusion) 1 time every month for 6 months ▪ An IV (intravenous infusion) administered at a chemotherapy clinic or hospital 1 day each month for 6 months. ▪ An IV tube is inserted into a blood vessel in your arm to administer the medicine. The IV infusion lasts from 1-1/2 to 4 hours. ▪ You need to spend about 6 hours at the hospital or clinic each time you need an IV infusion.Pills every day until the cancer advances. ▪ You take 1 to 4 pills by mouth either once or twice a day at the same time every day for as long as the medicine continues to work. | IV 1-3 times per month for 6 months Pill every day for X months* |
| Diarrhea† | Some medicines that treat CLL can cause diarrhea (loose stools). For this survey, assume that if a medicine causes diarrhea, the diarrhea would last for 2 to 3 days every month for as long as you are taking the medicine. Later in the survey, we will ask you to think about how severe the diarrhea may be with different hypothetical cancer medicines. There are 3 possibilities: ▪ None. You experience no diarrhea. ▪ Mild to moderate. You have fewer than 7 loose stools per day, and you may experience cramping. The diarrhea lasts for 2 to 3 days each month. ▪ Severe. You have 7 or more loose stools per day, and you have bowel control problems. You experience extreme fluid loss (dehydration) and may need IV fluids. You may need help taking care of yourself, including bathing, dressing, feeding yourself, using the toilet, and taking medications. The diarrhea lasts for 2 to 3 days each month. | None Mild to moderate for 2-3 days a month Severe for 2-3 days a month |
| Chance of serious infection† | For some people, medicines that treat CLL can also damage your body’s ability to make the healthy blood cells that protect your body from illness and infection. When this happens, your immune system becomes weaker and you are more likely to get a serious infection during treatment. People taking CLL treatments may develop different types of infections, including respiratory infections like pneumonia, infections with fever, skin infections with rashes or painful sores, hepatitis, or herpes simplex. When taking a medicine that makes you more likely to get a severe infection, you need to avoid situations where you could be exposed to infections. For example, you need to stay away from other people who are sick and avoid being in large groups of people. People who have a severe infection during treatment may need to go to the hospital, sometimes for as long as a couple of weeks. If not successfully treated, some infections can be life-threatening. Once the infection is gone, most people completely recover and no additional medical treatment is required for the infection. | None 5% 15% 30% |
| Chance of organ damage† | When tumor cells die, they break apart and chemicals from the cells are released into the blood. For some people who are treated for CLL, these chemicals can build up in the blood and cause damage to organs, including the kidneys, heart, and liver. People with organ damage can experience severe problems like vomiting, tiredness, muscle weakness, or seizures, and they need to be treated at the hospital for several days until their organs begin to function normally. Severe organ damage can be life-threatening if it is not treated, but this is very rare. Your doctor will monitor you for signs of organ damage. | None 1% 5% 8% |
X months is the same as length of time until cancer advances, meaning you take the pill every day until the cancer advances.
In the DCE questions, respondents were reminded that the chance of serious infection and organ damage and diarrhea lasted for the duration of treatment (6 months or PFS, depending on whether mode of administration was an IV or a pill). See Figure 1A for a sample DCE question.
DCE question and questions with cost attribute. (A) An example of the medicine-choice question that respondents answered in the online survey. (B-C) Different costs of medicine A and B. Respondents were randomly assigned to one version of the cost question.
DCE question and questions with cost attribute. (A) An example of the medicine-choice question that respondents answered in the online survey. (B-C) Different costs of medicine A and B. Respondents were randomly assigned to one version of the cost question.
The online survey instrument started with screening questions and informed consent. After providing consent, the respondents were asked background questions about their disease and treatment histories. Each DCE attribute was described individually using the text in Table 1, followed by questions about the attribute. The risk-based attributes (chance of serious infection and chance of organ damage) were presented both numerically and graphically. Risk levels were presented graphically using risk grids, each including 100 human figures, in which each figure represents 1 person who takes the medicine and figures in color represent patients for whom the event occurred.7 Respondents were asked a comprehension question about the risk grid and presented with the correct answer to reinforce the information. The survey text was pretested in semistructured, face-to-face interviews with 15 patients prior to survey administration.
Each respondent answered 8 DCE questions, choosing between pairs of experimentally designed hypothetical CLL treatments, each a combination of the 5 attributes shown in Table 1 with varying levels. Following good research practices in conjoint analysis and DCE,8 we created a set of 48 DCE questions, known as the experimental design, using the SAS implementation of a common D-optimal design.9,10 The 48 DCE questions were divided into 6 blocks of 8 questions. Respondents were randomly assigned to one of these blocks, and the order of the DCE questions within a block was randomized to control for question order effects.
There are significant cost differences in treatments for CLL, which can translate to variation in out-of-pocket costs depending on a patient’s insurance coverage. To explore the impact of cost on preferences, we added a question after the DCE questions in which respondents were again asked to choose between medicine A and medicine B, only this time with out-of-pocket cost included. We created 2 versions of the cost question, and respondents were randomly assigned to 1 of the 2 versions. The noncost attributes for medicine A and medicine B were fixed at the attribute levels presented in Figure 1A. In the first version, medicine A was $200 per month and medicine B was $600 per month. In the second version, medicine A was $25 per month and medicine B was $100 per month. The 2 versions are presented in Figure 1B-C.
Detection of MRD can help inform patient and physician decisions about frequency of monitoring and potential length of remission. After the cost questions, we assessed respondent interest in more sensitive blood and bone marrow tests of MRD using a 5-point Likert scale (see Figure 5 for the questions and response categories). Before the questions on interest in blood or bone marrow tests of MRD, we provided a brief description of MRD and MRD testing (see the supplemental Appendix for the text used to describe the tests). For the bone marrow test, we explained that physicians are interested in whether there are cancer cells in both the blood and the bone marrow, because bone marrow disease is a major concern in patients with CLL. We described the process of getting a bone marrow aspiration and noted that the test can be painful.
Sample
A convenience sample was recruited through the LLS during March and April 2016. The LLS sent e-mails to potential respondents in their database of patients. The e-mails contained an invitation to the survey and a unique link to the survey. Respondents were offered a $20 incentive to complete the survey. Adults aged ≥18 years with a self-reported physician diagnosis of CLL and who were able to provide informed consent were eligible to participate in the online survey.
The study was approved by an institutional review board at RTI International.
Analysis
DCE analysis.
The DCE questions generate panel data, which were estimated using a main effects random-parameters logit (RPL) model with NLOGIT Software version 5.0 (Econometric Software, Inc., Plainview, NY).11 RPL accounts for differences in preferences across respondents that can bias results from conventional conditional logit models.12 Parameter estimates from the model can be interpreted as relative preference weights that indicate the average relative preference for one attribute level compared with other attribute levels. The mean preference weights were used to calculate the relative importance of each attribute and to calculate the minimum acceptable benefit in terms of the months of PFS respondents required in order to accept a worsening of the other attributes.
In order to assess whether patients’ treatment status influenced their response, we performed subgroup analysis, dividing the sample into 3 categories: first-line patients, relapsed/refractory patients, and treatment-naive patients. The first-line category included respondents who were receiving or had completed a first-line oral or IV treatment at the time of the survey. The relapse category included respondents who reported having been treated more than once with oral or IV treatment and who reported that their condition worsened after experiencing a complete or partial remission. The treatment-naive category included respondents who had not received an oral or IV treatment of CLL.
Cost sensitivity analysis.
The percentage of respondents who selected medicine A and medicine B in the 2 versions of the cost question was compared with an estimate of the percentage of respondents who would have selected each medicine based on a prediction using the coefficients from the RPL model, where cost was not included. Posterior preferences for each individual in the sample were computed, conditional on the pattern of observed choices.12-14
Interest in testing for MRD.
The percentage of respondents selecting each point on the 5-point scale was tabulated. The 2 questions regarding interest in a blood or bone marrow MRD test were analyzed using an ordered logistic regression model.15,16 The dependent variable was a categorical variable ranging from 1 to 5 indicating the respondent’s level of interest in the blood test. A 5 indicated high interest, and a 1 indicated no interest. The independent variables were age, gender, education, geographic residence, employment, race, type of health insurance, treatment line, years since diagnosis, and whether a respondent has had a bone marrow test in the past (for the bone marrow question).
Results
Response rate and sample characteristics
Of 4420 people in the LLS patient database who were sent an invitation e-mail by LLS, 610 accessed the survey, 432 met the eligibility criteria, and 384 provided consent and completed the survey. Table 2 presents select demographic and treatment experience summary statistics. The age, race, and gender of the sample are similar to what is known about the population of patients with CLL.17 The average age of the sample was 65 years, approximately half were male, and the majority were white.
Respondent summary statistics (N = 384)
| Name . | Statistic . |
|---|---|
| Age, mean (SD), range, y | 64.6 (9.7) 30, 89 |
| Female | 53 |
| White | 94 |
| Black or African American | 4 |
| Private insurance* | 70 |
| Public insurance (Medicare, Medicaid, or Veterans Health Insurance) | 69 |
| Employed full-time | 24 |
| Employed part-time | 9 |
| Retired from employment | 48 |
| Time since first diagnosed with CLL, mean (SD), range, y | 9.4 (6.4) 1, 58 |
| Treatment naive | 20 |
| First-line treatment | 23 |
| Relapsed/refractory treatment | 39 |
| Other treatment† | 17 |
| Currently receiving treatment of CLL | 38 |
| Ever received oral therapy (pills) for CLL | 39 |
| Ever received IV therapy for CLL | 72 |
| Received financial aid (n = 301)‡ | 53 |
| Ability to pay out-of-pocket costs (n = 142)§ | |
| Very difficult | 13 |
| Difficult | 27 |
| Neither difficult nor easy | 42 |
| Easy | 11 |
| Very easy | 6 |
| Participated in a clinical trial | 26 |
| Lives in a rural area | 17 |
| Lives in a suburban area | 58 |
| Lives in an urban area | 23 |
| Previously had a bone marrow aspiration test | 67 |
| Name . | Statistic . |
|---|---|
| Age, mean (SD), range, y | 64.6 (9.7) 30, 89 |
| Female | 53 |
| White | 94 |
| Black or African American | 4 |
| Private insurance* | 70 |
| Public insurance (Medicare, Medicaid, or Veterans Health Insurance) | 69 |
| Employed full-time | 24 |
| Employed part-time | 9 |
| Retired from employment | 48 |
| Time since first diagnosed with CLL, mean (SD), range, y | 9.4 (6.4) 1, 58 |
| Treatment naive | 20 |
| First-line treatment | 23 |
| Relapsed/refractory treatment | 39 |
| Other treatment† | 17 |
| Currently receiving treatment of CLL | 38 |
| Ever received oral therapy (pills) for CLL | 39 |
| Ever received IV therapy for CLL | 72 |
| Received financial aid (n = 301)‡ | 53 |
| Ability to pay out-of-pocket costs (n = 142)§ | |
| Very difficult | 13 |
| Difficult | 27 |
| Neither difficult nor easy | 42 |
| Easy | 11 |
| Very easy | 6 |
| Participated in a clinical trial | 26 |
| Lives in a rural area | 17 |
| Lives in a suburban area | 58 |
| Lives in an urban area | 23 |
| Previously had a bone marrow aspiration test | 67 |
Data are reported as percentage of respondents unless otherwise indicated.
SD, standard deviation.
There is overlap between the private and public insurance categories, as many respondents have both private and public insurance.
Other includes respondents who provided answers that did not fit the categories for treatment naive, first line, or relapsed/refractory.
Those who stated they had received at least 1 treatment of CLL were asked, “Some patients find it difficult to pay for their CLL treatment. Do you currently or have you ever received financial assistance from a patient support program through your doctor, hospital, drug maker or from a patient organization such as the Leukemia and Lymphoma Society or the Lymphoma Research Foundation?”
Among respondents who have received at least 1 treatment and paid some amount for treatment, “How difficult did you find it to pay your out-of-pocket costs for your current or most recent CLL treatment?”
Sixty-nine percent of respondents had a form of public insurance (Medicare, Medicaid, and/or Veterans Health Insurance), and approximately half of the respondents were retired. Fifty-three percent of respondents had received financial aid from patient support programs through their doctor, hospital, or drug maker or from a patient organization for their out-of-pocket treatment costs. Forty percent of patients who had to pay something out of pocket for their medicines reported difficulty in paying out-of-pocket costs. Many respondents (26%) stated they were either participating in a clinical trial for CLL or had participated in one in the past.
DCE
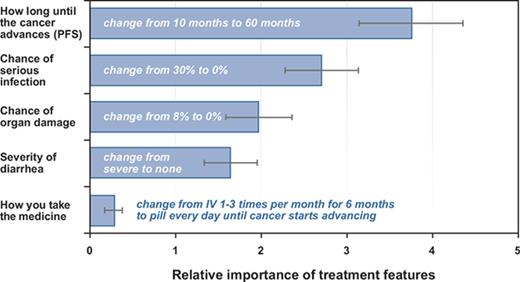
Respondents placed the most importance on a change in PFS from 10 months to 60 months, followed by a change in the chance of infection from a 30% risk to no risk, a change in the chance of organ damage from an 8% risk to no risk, a change in diarrhea from severe to none, and a change in mode of administration from IV to pill. Figure 2 presents the normalized preference weights for each attribute level. The preference weights indicate the relative strength of preference for each attribute, where larger positive numbers indicate greater preference and smaller, negative numbers indicate less preference. All the levels within each attribute are statistically different from each other at the 5% level. The results followed the expected ordering, with stronger preferences for better levels of the attributes. The vertical distance between the most-preferred and least-preferred level within each attribute represents the degree of importance of that attribute within the ranges presented for the survey.
Estimated preference weights. The vertical axis is the normalized mean preference weight for each attribute level using the results from the RPL model. The vertical bars around each mean parameter estimate represent the 95% confidence intervals about the point estimate.
Estimated preference weights. The vertical axis is the normalized mean preference weight for each attribute level using the results from the RPL model. The vertical bars around each mean parameter estimate represent the 95% confidence intervals about the point estimate.
In the subgroup analysis of differences among first-line patients, relapsed/refractory patients, and treatment-naive patients, there were no statistically significant differences between the preferences of any subgroup and the full sample, or across subgroups.
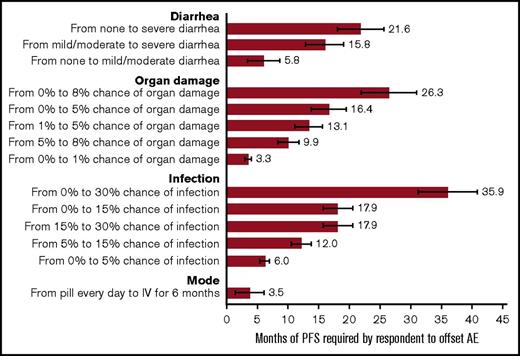
Figure 3 provides the results from the minimum acceptable benefit calculation. The graph shows the additional months of PFS necessary for the average respondent to accept a worsening in the other attributes. Moving from the lowest risk of serious infection to the highest generated the largest minimum acceptable benefit. On average, 36 additional months of PFS would compensate respondents for an increase in the risk of serious infection from 0% to 30%.
Additional months of PFS required by respondents to offset a change in an adverse event or change mode of administration. The bars display the minimum acceptable benefit (MAB) calculation (the number of months of PFS needed to offset a change in the attribute level). The horizontal bars at the end of the MAB bar represent the 95% confidence intervals about the point estimate. AE, adverse event.
Additional months of PFS required by respondents to offset a change in an adverse event or change mode of administration. The bars display the minimum acceptable benefit (MAB) calculation (the number of months of PFS needed to offset a change in the attribute level). The horizontal bars at the end of the MAB bar represent the 95% confidence intervals about the point estimate. AE, adverse event.
Cost sensitivity assessment
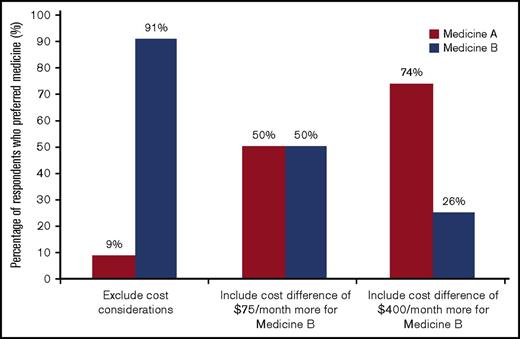
Figure 4 presents the results from the cost sensitivity assessment. Using results from the DCE in absence of cost, it was forecasted that 91% of respondents would choose the medicine with the longest PFS, medicine B. However, when respondents were presented with a choice between the 2 medicines and their out-of-pocket cost, 75% of respondents who saw Figure 1B ($400 difference in out-of-pocket cost per month) chose the lower-cost medicine, and 50% of the respondents who saw Figure 1C ($75 difference in out-of-pocket cost per month) chose the lower-cost medicine. The difference between respondents’ forecasted choice based on the DCE results and their actual choice was most pronounced for respondents who were assigned to the version with the largest difference in cost between the 2 medicines (Figure 1B).
Impact of cost on medicine choice. The bars indicate the percentage of the sample that selected medicine A or B. The first bar represents the forecast of the percentage selecting medicine A or B based on predictions from the model results for the DCE when cost was excluded. The next 2 bars show the percentage of the sample that selected medicine A or B when cost was included (see Figure 1 for medicine definitions).
Impact of cost on medicine choice. The bars indicate the percentage of the sample that selected medicine A or B. The first bar represents the forecast of the percentage selecting medicine A or B based on predictions from the model results for the DCE when cost was excluded. The next 2 bars show the percentage of the sample that selected medicine A or B when cost was included (see Figure 1 for medicine definitions).
Interest in testing for MRD
Approximately 82% and 58% of respondents were interested or very interested in the blood test or bone marrow test for MRD, respectively (Figure 5). The results of the ordered logistic regression for the blood test show that interest in a blood test for MRD decreased with age, was lower for women, and was higher for respondents with public insurance. Interest in the bone marrow test for MRD was also lower for women but higher for respondents living in rural areas, those who were employed part-time, those who were black, and those who had previously had a bone marrow test (results in supplemental Tables 1 and 2; supplemental Appendix).
Respondents’ interest in MRD tests. Response to the question, “Suppose that you have finished a 6-month course of medicine for CLL. The standard blood test does not find any cancer cells in your blood. Your doctor offers you one of the new, more sensitive blood tests. How interested would you be in getting this new [blood test/bone marrow aspiration test]?”
Respondents’ interest in MRD tests. Response to the question, “Suppose that you have finished a 6-month course of medicine for CLL. The standard blood test does not find any cancer cells in your blood. Your doctor offers you one of the new, more sensitive blood tests. How interested would you be in getting this new [blood test/bone marrow aspiration test]?”
Discussion
This study investigated patient preferences for different, hypothetical CLL treatments; the relationship between treatment preferences and patient out-of-pocket cost; and patient interest in more sensitive tests for MRD. The study included a relatively large sample of patients with CLL, including first-line patients, relapsed/refractory patients, and treatment-naive patients. The respondents valued efficacy, but they also valued safety. Any increase in adverse events or the risk of adverse events would require a potentially large improvement in efficacy to offset the disutility to respondents associated with the adverse events. For simplicity, the chance of organ damage (tumor lysis syndrome) is presented as constant for the duration of treatment. This overstates the actual risk, which is highest at the start of treatment.18,19 Cost had a big impact on treatment choice.
Evidence suggests that patients prefer oral over IV mode of administration in oncology.20 In the context of the treatment attributes included in this survey, respondents were generally unwilling to trade off more convenient dosing for lower efficacy and risk attributes.
We did not find any statistically significant differences among the preferences of first-line, relapsed/refractory, and never-treated respondents. This suggests that patient preferences may be stable over the stages of the disease; however, it is possible that sample size was not large enough to estimate the difference in preferences across the groups.
The impact of out-of-pocket costs for care to patients has become an important issue in cancer care, and studies have found that higher out-of-pocket costs may be associated with noncompliance.21-23 In our study, more than half the respondents had received financial aid from patient support programs, and almost half of patients who had to pay something out of pocket for their treatments reported difficulty in paying out-of-pocket costs.
Including costs in a DCE when the cost levels are considered too high for some fraction of respondents to pay presents a challenge to studies examining the impact of cost on treatment choice. If too many respondents select a hypothetical treatment based only on cost in the DCE questions, nothing is learned about relative preferences for the other attributes. When respondents ignore the cost attribute in the DCE because they cannot afford either option, it reduces the precision of all the estimates. We used a novel approach to collecting information on the impact of cost on preferences, adding a fixed follow-up question that contained the same attributes as the DCE and added cost. The addition of cost to the treatment profiles resulted in a large swing in the predicted choices toward the treatments with lower out-of-pocket costs. These results give a strong indication of cost sensitivity in patients with CLL.
Detection of MRD can help inform patient and physician decisions about the frequency of monitoring and potential length of remission. The value of MRD as a prognostic factor in CLL has been demonstrated by several studies, including randomized trials,24-26 but very little is known about patients’ interest in knowing their MRD status. In this study, a significant number of patients indicated interest in a test of their MRD status, especially if the sample was taken from the peripheral blood rather than by bone marrow aspiration. If further studies establish the usefulness of MRD testing for treatment decisions, our study suggests that patients may be interested.
The only other DCE study to investigate treatment preferences for CLL using a DCE was that conducted by Landfeldt et al.27 The study included physicians, the general population, and a small sample of relapsed/refractory patients in Germany and Sweden. Among 6 attributes (overall survival, PFS, fatigue, nausea, risk of serious infections, and treatment administration), overall survival was the most important attribute for all 3 groups, and treatment administration was more important to patients than it was to physicians. We selected PFS as our measure of efficacy because patients often receive a series of treatments followed by remission and relapse and there is more consistent clinical data on PFS across existing treatments. Although in the larger picture, patients may care more about overall survival, patients with CLL often take a series of treatments, and it can be hard to tie one particular treatment to a change in overall survival. During the focus groups, patients talked more about remission than overall survival, and in the pretests, no patients asked about overall survival, so the use of PFS as an efficacy end point appeared to be acceptable to patients.
The study results should be viewed in light of several limitations. The respondents were recruited through a patient advocacy organization, and this population may represent a subgroup of the overall CLL patient. The DCE method relies on hypothetical scenarios, and only a limited number of treatment attributes can be included. Although the attributes and choices reflected in the survey are similar to choices that patients face, the answers do not carry the weight of real choices. In addition, the results are conditional on the set of attributes included and the range of levels. If different attributes or ranges had been considered, the relative preferences for any individual attribute might shift.
The study demonstrates the importance of efficacy to respondents and a willingness to trade off efficacy to avoid serious risks. Cost considerations drove a large shift in preferences, and patients expressed interest in MRD testing. Combined with the responses to other questions indicating difficulty paying for current treatments, cost is an important factor in patient decisions. Patients with CLL have a number of options for treating their disease. These results could help patients play a more active role in discussions about treatments and help physicians work with their patients to find treatments that best fit patients’ preferences and circumstances.
The full-text version of this article contains a data supplement.
Acknowledgments
Kimberly Moon (RTI Health Solutions) was responsible for overall project management for this study. Alicia Patten (LLS) provided assistance with patient recruitment. Editorial assistance during preparation of this manuscript was provided by Joyce Hicks (RTI Health Solutions) and funded by Genentech, Inc.
This study was supported by research funding from Genentech, Inc., to RTI Health Solutions.
Authorship
Contribution: C.M., A.M., E.W., M.G., J.W., J.L., and C.R. contributed to the conception and design of the work; C.M., J.S., and M.B. conducted the analysis; and all authors were involved in the acquisition of data, interpretation of results, drafting the work, and revising it critically for important intellectual content.
Conflict-of-interest disclosure: C.M., M.B., and J.S. are full-time employees of RTI Health Solutions and were paid contractors of Genentech, Inc., in the development of the survey, the analysis and interpretation of the data, and preparation of the study report. A.M., J.L., and C.R. are employees of Genentech, Inc. The remaining authors declare no competing financial interests.
Correspondence: Carol Mansfield, RTI Health Solutions, 200 Park Offices Dr, PO Box 12194, Research Triangle Park, NC 27709-2194; e-mail: carolm@rti.org.





![Figure 5. Respondents’ interest in MRD tests. Response to the question, “Suppose that you have finished a 6-month course of medicine for CLL. The standard blood test does not find any cancer cells in your blood. Your doctor offers you one of the new, more sensitive blood tests. How interested would you be in getting this new [blood test/bone marrow aspiration test]?”](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/24/10.1182_bloodadvances.2017007294/8/m_advances007294f5.jpeg?Expires=1769121044&Signature=LUMtxLvaFEQgjPDWDg4wBG-6TC~CAeP01LuSh30uL75QH4TXCkV8HMV-cpGyuJccP9oglMbSy76usWj2DJ3KzobJCPrEsX9kYvNVx1hrxq-nWKvor8SbK0DcTv78RVNm9Vh1VErks8SILUjkL8~VVZYgFgKRLVyBjQHp23~ZhhtogSdrx7Gl3OoJ9qS2rEZRD1n5~rl-jQxCLJ2ySt4Dj6iJceRIm7JNyYiofcN8mOn81lWLTitGZPZgcxUz7PBXRsB4STJmn6JfhOzx9ebhqNxbL8zjN4d0aIdkYEhRXfAfom38OOunm7-G3DR7bXETLpSdTTLN3ZAGV7IbetcYwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)