Key Points
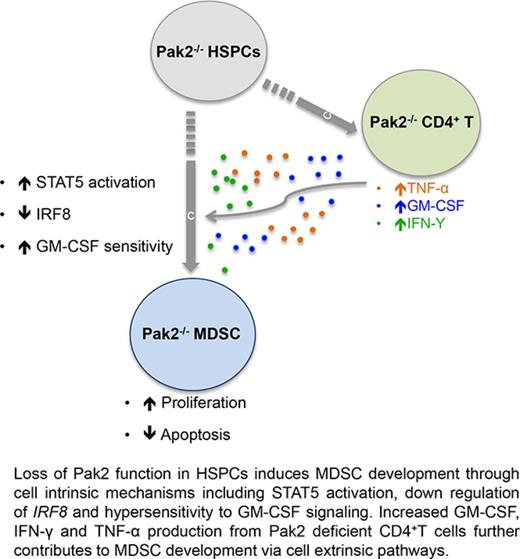
Pak2 negatively regulates CD11bhighGr1high MDSC development in mice via both cell-intrinsic and extrinsic mechanisms.
Pak2 disruption activates STAT5 while downregulating the expression of IRF8, a well-described myeloid transcription factor.
Abstract
Myeloid-derived suppressor cells (MDSCs) are CD11b+Gr1+ cells that induce T-cell hyporesponsiveness, thus impairing antitumor immunity. We have previously reported that disruption of Pak2, a member of the p21-activated kinases (Paks), in hematopoietic stem/progenitor cells (HSPCs) induces myeloid lineage skewing and expansion of CD11bhighGr1high cells in mice. In this study, we confirmed that Pak2-KO CD11bhighGr1high cells suppressed T-cell proliferation, consistent with an MDSC phenotype. Loss of Pak2 function in HSPCs led to (1) increased hematopoietic progenitor cell sensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling, (2) increased MDSC proliferation, (3) decreased MDSC sensitivity to both intrinsic and Fas-Fas ligand–mediated apoptosis, and (4) promotion of MDSCs by Pak2-deficient CD4+ T cells that produced more interferon γ, tumor necrosis factor α, and GM-CSF. Pak2 disruption activated STAT5 while downregulating the expression of IRF8, a well-described myeloid transcription factor. Together, our data reveal a previously unrecognized role of Pak2 in regulating MDSC development via both cell-intrinsic and extrinsic mechanisms. Our findings have potential translational implications, as the efficacy of targeting Paks in cancer therapeutics may be undermined by tumor escape from immune control and/or acceleration of tumorigenesis through MDSC expansion.
Introduction
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells that are present during physiologic and pathologic conditions. This cellular phenotype can increase in different pathologic settings such as cancer, infection, or inflammation, displaying suppressive function.1-4 MDSCs contribute to tumor evasion by suppressing cell-mediated immunity. Phenotypically, MDSCs and normal polymorphonuclear neutrophils (PMNs) are defined by expression of CD11b and Gr1. Based on the differential expression of Ly6C and/or Ly6G, murine MDSCs are subcategorized into granulocytic (CD11b+Ly6G+Ly6Clow) or monocytic MDSCs (CD11b+Ly6Glow/−Ly6Chi). Although these subsets can have various functions and distribution depending on their environment, the capacity to induce T-cell hyporesponsiveness can be seen in both subsets.5 While much research has focused on the tumor-promoting qualities of MDSCs, studies on the regulation of MDSC development during hematopoiesis remain limited.6,7
p21-activated kinases (Paks) are serine/threonine kinases that regulate diverse cellular activities, including cytoskeletal remodeling, cell motility, proliferation, apoptosis, and mitosis.8-11 Despite active research on pharmacological inhibition of group I Paks (Pak1, Pak2, and Pak3) in treating solid tumors,10-14 few studies have examined the role of Paks in modulating normal hematopoiesis.15-17 Utilizing a conditional Pak2-KO murine model, we previously demonstrated that Pak2 disruption in hematopoietic stem/progenitor cells (HSPCs) induces myeloid lineage skewing and CD11bhighGr1high cell expansion.18 In contrast to the positive effects on myelopoiesis, particularly granulopoiesis, Pak2 disruption in HSPCs impaired lymphopoiesis, profoundly diminishing mature T- and B-cell populations in mice with Pak2-KO bone marrow (BM).18 In the current study, we aimed to further characterize these Pak2-deficient CD11bhighGr1high cells and understand how Pak2 regulates their development. Herein, we demonstrate that Pak2 disruption in HSPCs enhances hematopoietic progenitor cell (HPC) sensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling and induces CD11bhighGr1high MDSC development via both cell-intrinsic and extrinsic mechanisms.
Methods
Mice, transplantation, Mx1Cre induction, and tumor inoculation
Mice were housed in specific pathogen-free conditions and cared for according to the guidelines of the University of Arizona Institutional Animal Care and Use Committee. To generate the conditional Pak2-KO mice, Pak2flox/flox mice were bred to Mx1Cre mice as previously described.18 CD45.2+Pak2flox//floxMx1Cre+ or Pak2flox//floxMx1Cre− BM low-density mononuclear cells (LDMNCs) were injected into lethally irradiated CD45.1+ BoyJ mice. Each recipient mouse received 2 × 106 LDMNCs. Two months following transplantation, Cre expression in reconstituted BM cells was induced by intraperitoneal injections of poly I poly C (polyIC, Sigma).18 Mice that received Pak2flox//floxMx1Cre+ or Pak2flox//floxMx1Cre− BM and subsequent polyIC treatment are referred to as mice reconstituted with Pak2-KO and wild-type (WT) BM, respectively. In some experiments, mice were inoculated with 0.1 × 106 Lewis lung carcinoma (LLC) cells or phosphate-buffered saline (PBS) subcutaneously. The quantity and suppressive function of splenic CD11bhighGr1high cells were evaluated by flow cytometry and T-cell suppression assays 1 week following inoculation, when the tumors were palpable.
Peripheral blood isolation and complete blood cell count
Peripheral blood was collected by tail tipping. White blood cells (WBCs) were isolated by incubating blood in red blood cell lysis buffer (BD Biosciences). Complete blood counts were determined using a Hemavet 950 (Drew Scientific).
Flow cytometry and intracellular staining
Splenocytes or WBCs were incubated with fluorochrome-conjugated anti-mouse antibodies as previously described.18 The list of antibodies used can be found in supplemental Methods. For splenocyte spontaneous apoptosis staining, freshly isolated splenocytes were stained with anti-CD11b and anti-Gr1 antibodies along with propidium iodide and Annexin V fluorescein isothiocyanate using the FITC Annexin V Apoptosis Detection kit (BD Pharmingen). For intracellular Ki-67 and T regulatory cell (Treg) staining, cells were stained with anti-CD45.2, anti-CD11b, and anti-Gr1 or anti-CD4 antibodies and then fixed, permeabilized, and stained with anti-Ki-67 or anti-FoxP3, respectively. The absolute numbers of phenotypically defined cell populations in the spleen and blood were obtained by multiplying the percentage of the indicated cell population by the absolute number of splenocytes and WBC count, respectively.
Isolation of BM cells and c-kit–positive cells
BM LDMNCs were isolated by density gradient centrifugation.19 c-kit+ cells were isolated by magnetic cell sorting using a CD117 isolation kit (Miltenyi Biotec) with >90% selection purity (data not shown). Unless otherwise mentioned, cells were cultured in RPMI (Hyclone) with 10% fetal bovine serum (FBS; Hyclone), 1% penicillin/streptomycin (Life Technologies), and 1% l-glutamine (Life Technologies).
Colony assays
Methylcellulose-based colony assays were performed using BM LDMNCs as previously described18 ; details can be found in supplemental Methods. In some experiments, colonies were collected and resuspended in PBS. Cells obtained from the colonies are referred to as GM-CSF colony progenies.
BM-derived MDSC generation and MDSC isolation from the spleen
To generate MDSCs from the BM, BM cells were cultured with 10 ng/mL murine GM-CSF, interleukin-6 (IL-6; Peprotech), and human granulocyte colony-stimulating factor (G-CSF; Amgen) in Dulbecco’s modified Eagle medium with 10% FBS for 3 days.20,21 Gr1highLy6G+ cells were isolated from splenocytes using an MDSC isolation kit from Miltenyi Biotec. Over 98% of the isolated cells were positive for CD11b (supplemental Figure 1A). CD45.2+ cells accounted for >95% and >85% cells in the spleens of mice with WT and Pak2-KO BM, respectively (supplemental Figure 1B). CD45.2+ donor cells were therefore not selected from the splenic Gr1highLy6G+ cells to minimize the ex vivo manipulation of cells.
MDSC suppression assay
T cells were isolated from splenocytes using a Pan T-cell Isolation Kit II (Miltenyi Biotec), stained with CellTrace Violet (Life Technologies) and stimulated with CD3/CD28 beads (Life Technologies). MDSCs were coincubated with T cells at the indicated ratios in RPMI 1640 with 10% FBS and 55 μM β-mercaptoethanol (Sigma-Aldrich) for 3 days, stained for CD4 and CD8, and analyzed by flow cytometry as previously described.20 Modfit analysis was used to determine the proliferation index (PI). Proliferation was determined as follows: proliferation (%) = (PI of sample − PI of unstimulated T cells)/(PI of stimulated T cells − PI of unstimulated T cells). Suppression (%) was calculated as = 100 − proliferation (%).
Giemsa staining
Gr1highLy6G+ cells were isolated from splenocytes using an MDSC isolation kit, stained for CD45.2, and then subjected to FACS sorting. Sorted cells were pelleted on to a glass slide using a cytospin centrifuge. Cells were fixed in methanol and stained with Giemsa using a Giemsa staining kit (Sigma). Samples were analyzed with an Olympus BX41 light microscope using a 60× objective lens. Photographs were takes with an Olympus DP21 digital camera.
CD4+ splenic T-cell isolation and cytokine measurement
CD4+ T cells were isolated from splenocytes using a CD4+ T-cell negative selection kit (Miltenyi Biotec) and stimulated with CD3/CD28 beads for 3 days. Supernatant was collected, and the amounts of GM-CSF, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) were determined using enzyme-linked immunosorbent assay kits (eBioscience). Since the majority of cells in the spleen were donor derived (supplemental Figure 1B), we did not separate CD45.2+ T cells from host-derived T cells.
Quantitative real-time PCR
Quantitative real-time polymerase chain reaction (PCR) was performed using messenger RNA (mRNA) isolated from splenic Gr1highLy6G+ cells or GM-CSF colony progenies as previously described.18 Details are in supplemental Methods.
Statistics
Statistical analyses were performed with GraphPad Prism 5.0 or Microsoft Excel. Data are reported as mean ± standard error and were analyzed using unpaired 2-tailed Student t tests or analysis of variance with appropriate post-hoc comparisons. Differences yielding P < .05 were defined as statistically significant.
Results
Genetic disruption of Pak2 in HSPCs induces MDSC expansion in the spleen
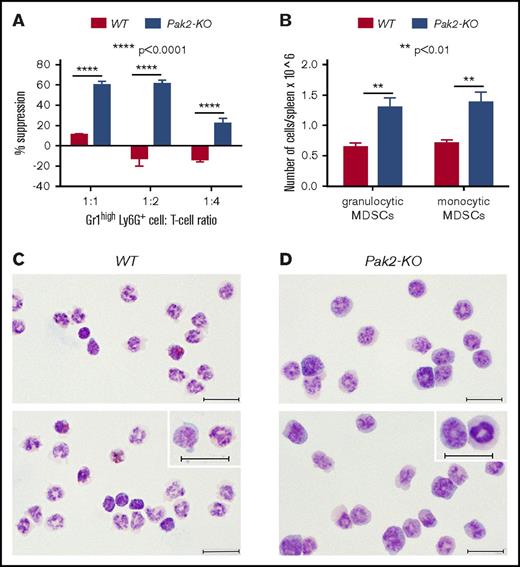
We have previously reported and again demonstrate in the current study a significantly higher number of CD45.2+CD11bhighGr1high cells in the spleens of mice reconstituted with Pak2-KO BM than in those reconstituted with WT cells (supplemental Figure 1C).18 We next tested their suppressive function. Gr1highLy6G+ cells isolated from the spleens of mice with Pak2-KO BM exhibited significantly greater T-cell suppressive function in vitro, consistent with an MDSC phenotype. In contrast, WT Gr1highLy6G+ cells displayed minimal suppressive or even stimulatory effects on T-cell proliferation (Figure 1A; representative Modfit analysis shown in supplemental Figure 1D). We further characterized these cells phenotypically and found a near twofold increase in the numbers of both granulocytic and monocytic MDSCs (Figure 1B; representative flow shown in supplemental Figure 1E). Comparison of the morphologic features of splenic CD45.2+Gr1highLy6G+ cells showed that majority of the WT cells were mature neutrophils with condensed chromatin, whereas the Pak2-KO cells showed a greater number of immature forms with open chromatin and high nuclear cytoplasmic ratio (Figure 1C). Together, these data clearly demonstrate that Pak2 disruption in HSPCs results in splenic MDSC expansion.
Genetic disruption of Pak2 in HSPCs induces MDSC expansion in the spleen. CD45.1+ naive BoyJ mice were noncompetitively transplanted with CD45.2+Pak2flox/floxMx1Cre+ (Pak2-KO) or CD45.2+Pak2flox/floxMx1Cre− (WT) BM cells and treated with polyIC. (A) Gr1highLy6G+ cells that were isolated from spleens suppressed T-cell proliferation. (B) The numbers of phenotypic granulocytic (CD11b+Ly6G+Ly6Clow) and monocytic (CD11b+Ly6Glow/−Ly6Chi) MDSCs are shown per spleen. (C-D) WT (C) and Pak2-KO (D) splenic CD45.2+Gr1highLy6G+ cells were stained with Giemsa. Inserts show morphology of cells at a high magnification. All scale bars represent 20 mm. Representative data from at least 3 experiments with 3 to 9 mice per genotype are shown.
Genetic disruption of Pak2 in HSPCs induces MDSC expansion in the spleen. CD45.1+ naive BoyJ mice were noncompetitively transplanted with CD45.2+Pak2flox/floxMx1Cre+ (Pak2-KO) or CD45.2+Pak2flox/floxMx1Cre− (WT) BM cells and treated with polyIC. (A) Gr1highLy6G+ cells that were isolated from spleens suppressed T-cell proliferation. (B) The numbers of phenotypic granulocytic (CD11b+Ly6G+Ly6Clow) and monocytic (CD11b+Ly6Glow/−Ly6Chi) MDSCs are shown per spleen. (C-D) WT (C) and Pak2-KO (D) splenic CD45.2+Gr1highLy6G+ cells were stained with Giemsa. Inserts show morphology of cells at a high magnification. All scale bars represent 20 mm. Representative data from at least 3 experiments with 3 to 9 mice per genotype are shown.
Pak2-deficient MDSCs are more suppressive than MDSCs from tumor-bearing WT mice
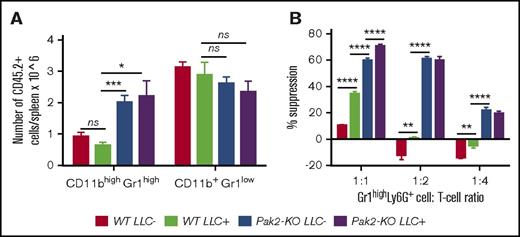
It is well accepted that tumors promote MDSC development. Since Pak2 disruption also induces MDSCs, we compared the suppressive function of Pak2-deficient MDSCs to that of MDSCs from tumor-bearing WT mice. Mice reconstituted with WT and Pak2-KO BM were inoculated with LLC cells or PBS. LLC tumors did not increase the number of splenic CD11bhighGr1high cells in mice with WT BM (WT LLC+ vs WT LLC−) (Figure 2A; representative flow shown in supplemental Figure 2A). However, splenic Gr1highLy6G+ cells from tumor-bearing mice suppressed T-cell proliferation, confirming that LLC tumors induced MDSCs in mice with WT BM (WT LLC+ vs WT LLC−; Figure 2B). More importantly, MDSCs from mice with Pak2-KO BM, even without tumors, displayed significantly more potent T-cell–suppressive function than MDSCs from tumor-bearing mice with WT BM (Pak2-KO LLC− vs WT LLC+, Figure 2B). LLC tumors led to a small but significant increase in Pak2-KO MDSC suppressive function (Pak2-KO LLC+ vs Pak2-KO LLC−, 1:1 ratio) (Figure 2B; representative Modfit analysis shown in supplemental Figure 2B). Together, these data indicate that Pak2 disruption enhances MDSC function.
Pak2-deficient MDSCs are more suppressive than MDSCs from tumor-bearing mice. Mice reconstituted with WT and Pak2-KO BM were inoculated with LLC cells or PBS control subcutaneously. (A) The numbers of CD45.2+CD11b+Gr1high (representing PMNs and MDSCs) and CD45.2+CD11b+Gr1low cells (representing monocytes) are shown per spleen. (B) Effects of splenic Gr1highLy6G+ cells on T-cell proliferation. Representative data from 2 experiments with 5 to 9 mice per genotype are shown. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, not significant.
Pak2-deficient MDSCs are more suppressive than MDSCs from tumor-bearing mice. Mice reconstituted with WT and Pak2-KO BM were inoculated with LLC cells or PBS control subcutaneously. (A) The numbers of CD45.2+CD11b+Gr1high (representing PMNs and MDSCs) and CD45.2+CD11b+Gr1low cells (representing monocytes) are shown per spleen. (B) Effects of splenic Gr1highLy6G+ cells on T-cell proliferation. Representative data from 2 experiments with 5 to 9 mice per genotype are shown. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, not significant.
Pak2-KO HPCs display increased sensitivity to GM-CSF signaling
To understand the impact of Pak2 disruption on myeloid lineage commitment and MDSC development at the HPC level, we performed the following experiments. First, we generated MDSCs from BM cells in the presence of GM-CSF, IL-6, and G-CSF as previously described.20,21 Pak2-deficient BM cells yielded ∼1.7- to 1.8-fold more granulocytic and monocytic MDSCs in vitro than WT BM cells (Figure 3A; representative flow shown in supplemental Figure 3A). Since Pak2-KO HPCs yielded fewer colonies than WT HPCs when stimulated with G-CSF,18 we next examined the responsiveness of Pak2-deficient HPCs to GM-CSF signaling. Sorted CD45.2+Pak2-KO or WT BM cells were cultured in methylcellulose culture medium containing GM-CSF alone. Pak2-KO BM cells yielded greater than threefold more colonies than WT cells in response to GM-CSF (Figure 3B). Increased proliferation of Pak2-KO myeloid progenitors was also evidenced by a near fivefold higher number of progenies recovered from the colonies (data not shown). There was no difference in GM-CSF receptor α (Csf2ra) or β (Csf2rb) chain gene expression in these GM-CSF progenies, indicating that increased sensitivity of Pak2-KO HPCs to GM-CSF was not due to upregulation of GM-CSF receptor (Figure 3C). To test the suppressive function of GM-CSF–stimulated myeloid cells, Pak2-KO or WT c-kit+ cells that were enriched for HPCs were maintained in liquid culture in the presence of GM-CSF for 7 days before being cocultured with T cells. Significantly higher numbers of CD11bhighGr1high cells were generated from Pak2-KO HPCs than from WT cells (Figure 3D). Furthermore, Pak2-KO GM-CSF–stimulated myeloid cells displayed greater suppressive function on CD8+ T-cell proliferation in vitro, consistent with an MDSC phenotype (Figure 3E; representative Modfit analysis shown in supplemental Figure 3B). Although there was a trend toward GM-CSF–stimulated Pak2-KO myeloid cells exhibiting more suppressive function on CD4+ T-cell proliferation than WT cells, this difference did not reach statistical significance (data not shown). These data demonstrate that Pak2 disruption increases HPC sensitivity to GM-CSF signaling that directs lineage commitment toward granulocytic lineage, thus promoting MDSC development. Given that Pak2-deficient GM-CSF progenies and spleen Gr1highLy6G+ cells both display an MDSC phenotype and function, either or both cell populations were used in assays described below.
Pak2-KO HPCs display increased sensitivity to GM-CSF signaling. (A) MDSCs were generated from Pak2-KO or WT BM cells in the presence of GM-CSF, IL-6, and G-CSF. (B) Colony formations of sorted CD45.2+ BM cells cultured in methylcellulose with GM-CSF. The numbers of colonies (colony-forming units in response to GM-CSF [CFU-GM]) per femur are shown. (C) GM-CSF receptor α (Csf2ra) and β (Csf2rb) chain gene expression was measured in progenies collected from the GM-CSF colony assay shown in Figure 3B. Pak2-KO or WT BM c-kit+ cells were cultured with GM-CSF for 7 days. (D-E) Cells were stained for CD11b and Gr1 (D) and cocultured with T cells to test their suppressive function (E). The suppressive function on CD8+ T-cell proliferation is shown. Representative data from at least 3 experiments with 3 to 6 mice per genotype are shown. **P < .01; ***P < .001.
Pak2-KO HPCs display increased sensitivity to GM-CSF signaling. (A) MDSCs were generated from Pak2-KO or WT BM cells in the presence of GM-CSF, IL-6, and G-CSF. (B) Colony formations of sorted CD45.2+ BM cells cultured in methylcellulose with GM-CSF. The numbers of colonies (colony-forming units in response to GM-CSF [CFU-GM]) per femur are shown. (C) GM-CSF receptor α (Csf2ra) and β (Csf2rb) chain gene expression was measured in progenies collected from the GM-CSF colony assay shown in Figure 3B. Pak2-KO or WT BM c-kit+ cells were cultured with GM-CSF for 7 days. (D-E) Cells were stained for CD11b and Gr1 (D) and cocultured with T cells to test their suppressive function (E). The suppressive function on CD8+ T-cell proliferation is shown. Representative data from at least 3 experiments with 3 to 6 mice per genotype are shown. **P < .01; ***P < .001.
Pak2-KO MDSCs are more proliferative than WT cells
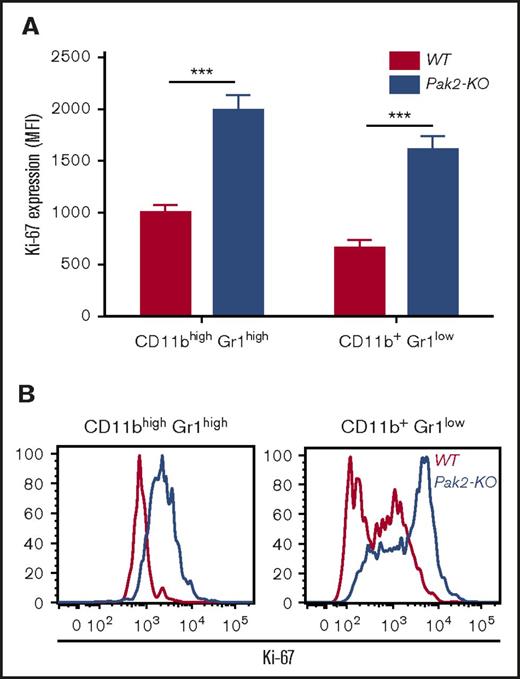
To better understand the underlying mechanisms of Pak2-deficient MDSC expansion in the spleen, we examined the proliferation of Pak2-KO and WT splenic CD11bhighGr1high cells by Ki-67 staining. Mean fluorescence intensity (MFI) of Ki-67 in CD11bhighGr1high cells (representing PMNs and MDSCs in mice with WT and Pak2-KO BM, respectively) was determined. Pak2-KO cells displayed a two- to threefold higher proliferation than WT cells (Figure 4A-B), indicating that Pak2 disruption provides a proliferative advantage to MDSCs, thus contributing to their splenic expansion. Similarly, Pak2-KO CD11b+Gr1low cells (monocytes) were also more proliferative than WT cells (Figure 4A-B).
Pak2-KO spleen MDSCs are more proliferative than WT cells Freshly isolated splenocytes from mice reconstituted with Pak2-KO or WT BM were stained for CD11b, Gr1, and intracellular Ki-67. (A) MFI of Ki-67 in gated CD45.2+CD11bhighGr1high and CD11b+Gr1low populations is shown. (B) Representative flow histograms are shown. Representative data from 2 experiments with 3 to 6 mice per genotype are shown. ***P < .001.
Pak2-KO spleen MDSCs are more proliferative than WT cells Freshly isolated splenocytes from mice reconstituted with Pak2-KO or WT BM were stained for CD11b, Gr1, and intracellular Ki-67. (A) MFI of Ki-67 in gated CD45.2+CD11bhighGr1high and CD11b+Gr1low populations is shown. (B) Representative flow histograms are shown. Representative data from 2 experiments with 3 to 6 mice per genotype are shown. ***P < .001.
Pak2 disruption results in decreased spontaneous and Fas-FasL–induced apoptosis in MDSCs
We next evaluated whether resistance to apoptosis contributes to the increased accumulation of MDSCs in mice with Pak2-deficient BM. Spontaneous late apoptosis (Annexin V+/PI+) of spleen MDSCs in mice with Pak2-KO and WT BM was examined. As myeloid cell turnover is regulated by the Fas-FasL apoptosis pathway,22,23 we also examined the responsiveness of Pak2-KO MDSCs to FasL-induced apoptosis. Splenic MDSCs from mice with Pak2-KO BM exhibited a near 40% and 60% reduction in spontaneous and Fas-FasL–mediated apoptosis as compared with WT CD11bhighGr1high cells, respectively, indicating that Pak2-KO MDSCs are less sensitive to both spontaneous and FasL-induced apoptosis (Figure 5A; representative flow shown in supplemental Figure 4A). Accordingly, Pak2-KO MDSCs expressed ∼60% less Fas compared with WT CD11bhighGr1high cells. In contrast, the expression levels of Fas in Pak2-KO CD11b+Gr1low monocytes, CD3+ T cells, and B220+ B cells was comparable to or higher than that found in WT cells (Figure 5B; representative flow shown in supplemental Figure 4B). There was no significant difference in spontaneous or Fas-FasL–mediated apoptosis in CD11b+Gr1low monocytes between the 2 genotypes (supplemental Figure 4C).
Pak2 disruption results in decreased spontaneous and Fas-FasL–induced apoptosis in MDSCs. (A) Freshly isolated splenocytes from mice reconstituted with Pak2-KO or WT BM were cultured in the presence or absence of FasL for 2 hours prior to CD11b, Gr1, Annexin V, and propidium iodide (PI) staining. The percentage of Annexin V+PI+ cells in the gated CD45.2+CD11bhighGr1high population is shown. (B) Freshly isolated splenocytes from mice reconstituted with Pak2-KO or WT BM were stained for CD45.2, Fas, CD11b, Gr1, CD3, and B220. The MFI of Fas in gated CD45.2+ populations is shown. (C) Bcl2, (D) Mcl1, (E) Bcl-xL, (F) Bak1, and (G) Bax expression in Pak2-KO MDSCs or WT PMNs (progenies from the GM-CSF colony assays shown in Figure 3B) was determined by quantitative real-time PCR. Representative data from 3 or 4 experiments with 3 to 10 mice per genotype are shown. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Pak2 disruption results in decreased spontaneous and Fas-FasL–induced apoptosis in MDSCs. (A) Freshly isolated splenocytes from mice reconstituted with Pak2-KO or WT BM were cultured in the presence or absence of FasL for 2 hours prior to CD11b, Gr1, Annexin V, and propidium iodide (PI) staining. The percentage of Annexin V+PI+ cells in the gated CD45.2+CD11bhighGr1high population is shown. (B) Freshly isolated splenocytes from mice reconstituted with Pak2-KO or WT BM were stained for CD45.2, Fas, CD11b, Gr1, CD3, and B220. The MFI of Fas in gated CD45.2+ populations is shown. (C) Bcl2, (D) Mcl1, (E) Bcl-xL, (F) Bak1, and (G) Bax expression in Pak2-KO MDSCs or WT PMNs (progenies from the GM-CSF colony assays shown in Figure 3B) was determined by quantitative real-time PCR. Representative data from 3 or 4 experiments with 3 to 10 mice per genotype are shown. *P < .05; **P < .01; ***P < .001; ****P < .0001.
We next determined the expression levels of several genes that regulate myeloid cell survival. The expression levels of 2 antiapoptotic genes, Bcl2 (Figure 5C) and Mcl1 (Figure 5D), were significantly decreased and increased, respectively, in Pak2-KO MDSCs when compared with WT CD11bhighGr1high cells. There was no significant difference in the expression of Bcl-xL, another antiapoptotic gene, between these 2 cell types (Figure 5E). However, mRNA levels of Bak1 and Bax, 2 proapoptotic genes, were significantly decreased in Pak2-KO MDSCs (Figure 5F-G). Taken together, our data demonstrate that loss of Pak2 decreases MDSC sensitivity to apoptosis through downregulation of Fas and differential regulation of multiple pro- and antiapoptotic genes.
Loss of Pak2 activates STAT5 and downregulates IRF8 expression in MDSCs
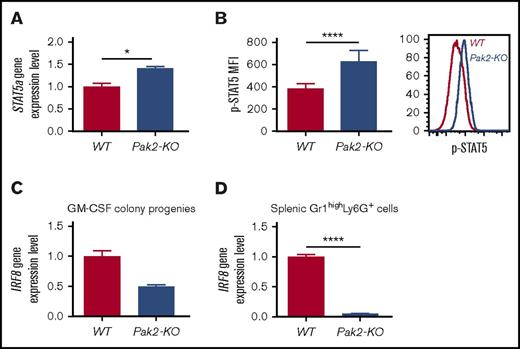
IRF8 is a well-recognized essential transcription factor that governs myeloid lineage commitment and MDSC development in mice and humans.6,24-26 IRF8-deficient mice exhibit splenomegaly and accumulation of MDSCs, ultimately developing a chronic myeloid leukemia–like disease.6,25 Mice with Pak2-KO BM display a phenotype that shares some commonalities with that of IRF8-deficient mice, including hypersensitivity to GM-CSF, MDSC resistance to apoptosis, and MDSC expansion. However, these mice do not develop leukemia.18 STAT5 represses IRF8 transcription in chronic myeloid leukemia cells and MDSCs.25,27 We thus examined STAT5 activation and IRF8 mRNA expression in Pak2-KO MDSCs. Pak2-deficient GM-CSF colony progenies displayed higher levels of STAT5a mRNA expression (Figure 6A) and STAT5 phosphorylation (Figure 6B), consistent with reports that GM-CSF activates STAT5 during myeloid cell differentiation.25,28 Furthermore, decreased IRF8 expression was observed in both Pak2-deficient GM-CSF colony progenies (Figure 6C) and spleen MDSCs (Figure 6D). These data reveal a possible functional interaction between the Pak2 and STAT5/IRF8 axis in regulating myeloid lineage commitment and MDSC development.
Loss of Pak2 activates STAT5 and downregulates IRF8 expression in MDSCs. (A) STAT5a gene expression and (B) STAT5 protein phosphorylation in Pak2-KO MDSCs or WT PMNs (progenies from the GM-CSF colony assays shown in Figure 3B) were determined by quantitative real-time PCR and flow cytometry, respectively. (C-D) IRF8 gene expression in Pak2-KO MDSCs or WT PMNs (progenies from the GM-CSF colony assays shown in Figure 3B) (C) and Pak2-KO or WT Gr1highLy6G+ cells (MDSCs and PMNs, respectively) (D) isolated from spleen are shown. Representative data from 2 or 3 experiments with 3 to 6 mice per genotype are shown. *P < .05; ****P < .0001.
Loss of Pak2 activates STAT5 and downregulates IRF8 expression in MDSCs. (A) STAT5a gene expression and (B) STAT5 protein phosphorylation in Pak2-KO MDSCs or WT PMNs (progenies from the GM-CSF colony assays shown in Figure 3B) were determined by quantitative real-time PCR and flow cytometry, respectively. (C-D) IRF8 gene expression in Pak2-KO MDSCs or WT PMNs (progenies from the GM-CSF colony assays shown in Figure 3B) (C) and Pak2-KO or WT Gr1highLy6G+ cells (MDSCs and PMNs, respectively) (D) isolated from spleen are shown. Representative data from 2 or 3 experiments with 3 to 6 mice per genotype are shown. *P < .05; ****P < .0001.
Pak2-deficient T cells induce MDSC generation in vitro
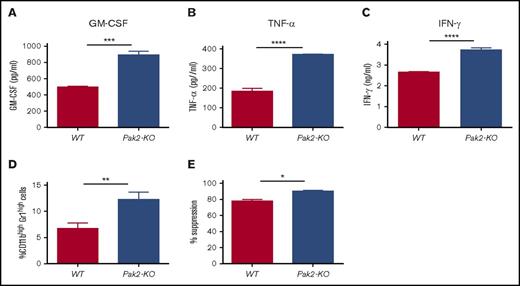
IRF8 also affects myeloid cell lineage differentiation indirectly by repressing GM-CSF expression in T cells.6 IRF8-deficient T cells produce more GM-CSF, thereby activating STAT5 and inducing MDSC expansion in mice.6 Given that loss of Pak2 leads to STAT5 activation and IRF8 downregulation in MDSCs, we next examined whether Pak2 deficiency also enhances GM-CSF production by T cells. We found a near twofold increase in GM-CSF secretion from Pak2-KO splenic CD4+ T cells when compared with WT cells (Figure 7A). In addition, Pak2-KO CD4+ T cells produced significantly more TNF-α and IFN-γ (Figure 7B-C), both of which have been shown to promote MDSC expansion and suppressive function.29-32 We then cultured BM cells from naive C57BL/6 mice in the presence of supernatant from Pak2-KO or WT splenic CD4+ T cells for 5 days. Cells generated with Pak2-KO CD4+ T-cell supernatant displayed an almost twofold increase in the percentage of CD11bhighGr1high cells and were more suppressive of T-cell proliferation (Figure 7D-E). These findings indicate that Pak2-deficient T cells induce MDSC generation in a cell-contact–independent manner, possibly through cytokine production including GM-CSF, TNF-α, and IFN-γ.
Pak2-deficient T cells induce MDSC generation in vitro. Purified splenic CD4+ T cells from mice reconstituted with Pak2-KO or WT BM were stimulated with CD3/CD28 beads for 3 days before supernatant was collected. (A-C) Amounts of GM-CSF (A), TNF-α (B), and IFN-γ (C) in the supernatant were determined by enzyme-linked immunosorbent assay. Naive C57BL/6 mice BM cells were cultured in the presence of supernatant from Pak2-KO or WT splenic CD4+ T cells for 5 days, collected, and then cocultured with purified splenic T cells from naive C57BL/6 mice to measure their suppressive function on T-cell proliferation. (D-E) The percentage of the CD11bhighGr1high population in panel D and the percentage suppression of T-cell proliferation by BM cells cultured in CD4+ T-cell supernatant (E) are shown. Representative data from 2 or 3 experiments with 3 to 6 mice per genotype are shown. *P < .05; **P < .01; ***P < .001; ***P < .0001.
Pak2-deficient T cells induce MDSC generation in vitro. Purified splenic CD4+ T cells from mice reconstituted with Pak2-KO or WT BM were stimulated with CD3/CD28 beads for 3 days before supernatant was collected. (A-C) Amounts of GM-CSF (A), TNF-α (B), and IFN-γ (C) in the supernatant were determined by enzyme-linked immunosorbent assay. Naive C57BL/6 mice BM cells were cultured in the presence of supernatant from Pak2-KO or WT splenic CD4+ T cells for 5 days, collected, and then cocultured with purified splenic T cells from naive C57BL/6 mice to measure their suppressive function on T-cell proliferation. (D-E) The percentage of the CD11bhighGr1high population in panel D and the percentage suppression of T-cell proliferation by BM cells cultured in CD4+ T-cell supernatant (E) are shown. Representative data from 2 or 3 experiments with 3 to 6 mice per genotype are shown. *P < .05; **P < .01; ***P < .001; ***P < .0001.
Discussion
Knowledge on the role of Pak2 in regulating normal hematopoiesis, particularly myelopoiesis, remains limited.15-17 We have previously shown that Pak2 disruption in HSPCs induces myeloid lineage skewing and CD11bhighGr1high cell expansion in mice.18 In this study, we further characterized that Pak2-deficient CD11bhighGr1high cells suppressed T-cell proliferation, consistent with an MDSC phenotype. Our data demonstrated that loss of Pak2 function in HSPCs leads to (1) increased HPC sensitivity to GM-CSF signaling, (2) increased MDSC proliferation, (3) decreased MDSC sensitivity to apoptosis, and (4) promotion of MDSC by Pak2-deficient CD4+ T cells that produce more IFN-γ, TNF-α, and GM-CSF. In summary, we have provided evidence that loss of Pak2 function enhances MDSC development, expansion, and suppressive function through both cell-intrinsic and extrinsic mechanisms.
Studies by O’Hagan et al have shown that T-cell–specific deletion of Pak2 resulted in a marked reduction in both thymus- and peripherally derived Tregs, accompanied by the development of spontaneous colitis.33 These mice developed splenomegaly with MDSC expansion, likely in response to colitis.33 Following Pak2 deletion, there was an early increase in the number of peripheral blood CD45.2+CD4+FoxP3+ Tregs in mice with Pak2-KO BM. This difference subsided by 6 months (supplemental Figure 6A). There was no difference in the number of splenic Tregs between mice with Pak2-KO BM and WT controls (supplemental Figure 6B). Moreover, mice with Pak2-KO BM did not exhibit signs of spontaneous colitis clinically or histologically (supplemental Figure 6C). These data demonstrated that MDSC expansion in mice with Pak2-KO BM is not secondary to a deficiency in the number or the function of Tregs.
The selective sensitivity of Pak2-deficient HSPCs to GM-CSF is intriguing, and its underlying mechanisms remain elusive. This increased sensitivity is not due to increased GM-CSF receptor expression in MDSCs (Figure 3C). GM-CSF induces STAT5 activation during monocyte/macrophage differentiation28 and MDSC expansion,25,34 concordant with our finding of increased STAT5 phosphorylation in Pak2-deficient MDSCs (Figure 6B). In addition to increased sensitivity to GM-CSF, Pak2-deficient CD4+ T cells produce more GM-CSF (Figure 7A), further promoting MDSC expansion via cell-extrinsic mechanisms.
Studies in cell lines have shown that Pak2 can be both pro- and antiapoptotic depending on the cellular milieu.35-40 Disruption of Pak2 in T cells in Pak2flox/flox CD4Cre+ mice leads to increased apoptosis and cell death in semimature CD4 single-positive thymocytes.17 Our data demonstrate that Pak2 disruption results in decreased sensitivity to apoptosis in CD11bhighGr1high MDSCs (Figure 5A), but not CD11b+Gr1low monocytes (supplemental Figure 4C), indicating that Pak2 fosters apoptosis specifically in CD11bhighGr1high MDSCs. This finding is further supported by our observation that Pak2 deficiency results in decreased Fas expression in CD11bhighGr1high cells but increased Fas expression in T and B cells and no difference in CD11b+Gr1low monocytes (Figure 5B). Our data, along with reports by others,17 indicate that the role of Pak2 in cell survival and apoptosis is cell-type specific.
Apoptosis is tightly regulated by 2 main pathways: (1) the intrinsic or mitochondrial pathway and (2) the extrinsic or death receptor pathway.41 BCL-2 family members are key mediators of the mitochondria-dependent intrinsic apoptosis pathway, with Bcl2, Mcl1, and Bcl-xL displaying antiapoptotic function and Bax and Bak1 exerting proapoptotic activity.41,42 Upregulation of Bcl-xL and downregulation of Bax confers apoptotic resistance to MDSCs and contributes to their persistence in cancer.43 Significantly lower levels of Bcl2, Bax, and Bak1 expression were observed in Pak2-deficient MDSCs than in WT CD11bhighGr1high PMNs (Figure 5C,F-G). In contrast, there was no difference in Bcl-xL expression between the 2 genotypes (Figure 5E). These data indicate that Pak2 positively regulates Bcl2, Bax, and Bak1 expression and that Bax and Bak1 play a relatively more important role than Bcl2 in modulating apoptosis in Pak2-deficient MDSCs. Indeed, the lower level of Bcl2 expression is in line with our previous finding that Pak2 disruption upregulates c-Myc expression18 and the report that c-Myc overexpression suppresses Bcl2 protein level in myeloid cells.44 The elevated expression of Mcl1, a potent prosurvival modulator of neutrophil development,45,46 further contributes to the resistance to apoptosis seen in Pak2-deficient MDSCs.
IRF8 is a well-described regulator of myeloid cell apoptosis. It mediates myeloid cell apoptosis through upregulation of Bcl-xL43 and downregulation of Bax23,43 and Bcl-2.47 Respectively decreased and increased levels of Bax and Bcl-xL expression were observed in IRF8-deficient MDSCs from tumor-bearing mice than in myeloid cells with the same phenotypes from tumor-free mice.43 Downregulation of IRF8 and Bax expression in Pak2-deficient MDSCs is consistent with the finding in MDSCs from tumor-bearing mice43 and the report that IRF8-KO CD11b+ primary myeloid cells display lower Bax expression level than WT cells.23 On the other hand, Pak2-deficient MDSCs, while having reduced IRF8 expression (Figure 6C-D), had a comparable level of Bcl-xL expression when compared with WT CD11bhighGr1high PMNs (Figure 5E). The discrepancy in Bcl-xL expression between Pak2- and IRF8-deficient MDSCs suggests that Pak2 may also regulate transcription factors other than IRF8, which downregulate Bcl-xL expression, thus offsetting the impact of IRF8 on Bcl-xL expression.
The death receptor Fas and its physiological ligand FasL mediate the extrinsic apoptosis-signaling pathways.48 Deregulation of the Fas-mediated apoptosis pathway confers increased MDSC resistance to apoptosis in tumor-bearing hosts.43 Pak2-deficient MDSCs, with diminished IRF8 expression and cell-surface Fas protein levels, display resistance to Fas-FasL–mediated apoptosis (Figure 5A). Our data are in line with the reported decreased sensitivity of IRF8-deficient MDSCs to spontaneous and Fas-mediated apoptosis.23,43 Collectively, our data demonstrate that loss of Pak2 function results in deregulated intrinsic and extrinsic anti- and pro apoptotic pathways, thus conferring apoptosis resistance to MDSCs.
Mice with Pak2-KO BM share some common (although milder) phenotypes with IRF8-deficient mice, suggesting that Pak2 may regulate myelopoiesis and MDSC development at least partially through IRF8. IRF8 expression in myeloid cells is controlled by multiple mechanisms, including the myeloid master regulator PU.1.49 Given that loss of Pak2 does not alter PU.1 expression in HSPCs,18 it is possible that Pak2 regulates IRF8 expression through alternate pathways. Activation of STAT5 by GM-CSF represses IRF8 transcription in MDSCs and other myeloid cells.25,34 Our findings that Pak2-deficient HPCs are more sensitive to GM-CSF signaling (Figure 3B), Pak2-deficient CD4+ T cells produce higher amounts of GM-CSF and induce MDSC generation in vitro (Figure 7A,D-E), and Pak2-deficient MDSCs display higher levels of STAT5 activation (Figure 6B) suggest that Pak2 may regulate IRF8 expression through GM-CSF/STAT5 signaling. Cytokines such as IFN-γ also induce IRF8 expression.50 Although Pak2-deficient CD4+ T cells produce significantly higher amounts of IFN-γ (Figure 7C), lower rather than higher levels of IRF8 expression were observed in Pak2-deficient MDSCs (Figure 6C-D). This finding indicates that GM-CSF/STAT5 signaling plays a more prominent role than IFN-γ signaling in regulating IRF8 expression in Pak2-deficient MDSCs. Further studies are warranted to delineate the functional interactions between Pak2 and IRF8.
We have previously shown that Pak2 suppresses both phenotypic PMN (CD11bhighGr1high) and monocyte (CD11b+Gr1low) differentiation, with a more significant impact on the former.18 In contrast, IRF8 promotes monocyte differentiation51 while suppressing PMN differentiation.7 Such differential effect of Pak2 and IRF8 on monocytopoiesis again signifies that Pak2 also regulates myeloid lineage transcription factors other than IRF8. Indeed, we have previously reported that Pak2 disruption in HSPCs results in decreased and increased JunB and c-Myc expression, respectively.18
Pak kinases, as downstream effectors of Ras, are potential therapeutic targets in malignancies with hyperactive Ras, which account for ∼30% of human cancers and are common in myeloid malignancies.52-55 However, the reports that Pak2 depletion suppresses β-catenin activity56 and downregulation of β-catenin promotes human MDSC activation and expansion in cancer57 raise safety concerns regarding the pharmacological inhibition of group I Paks that target Pak1, Pak2, and Pak3. Our findings provide further evidence that the efficacy of targeting Paks may be undermined by tumor escape from immune control and/or acceleration of tumorigenesis through MDSC expansion. In the case of myeloid leukemia, Pak2 inhibition may further promote leukemogenesis through downregulation of IRF8, a well-described leukemia suppressor gene. These safety concerns regarding Pak2 inhibition warrant further investigation in both human and murine systems.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Nicolas Larmonier and Catherine Smith for helpful discussions and Vanessa Frisinger for administrative assistance.
This work was supported by the National Institutes of Health, National Cancer Institute (grants R01 CA104926 [E.K.] and R01 CA142928 [J.C.]), Hyundai Hope on Wheels (Y.Z. and E.K.), Tee Up for Tots, Angel Charity for Children, and People Acting Now Discover Answers (E.K.).
Authorship
Contribution: Y.Z. designed and performed research, analyzed and reviewed data, and wrote the manuscript; S.H. performed experiments, analyzed data. and edited the manuscript; J.S. and E.A.H. performed experiments and edited the manuscript; M.S. and M.P. analyzed data; J.C. provided the Pak2flox/flox mice; and E.K. advised on the study, reviewed and discussed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zeng, Department of Pediatrics, University of Arizona, 1501 North Campbell Ave, PO Box 245073, Tucson, AZ 85724-5073; e-mail: yzeng@peds.arizona.edu.

![Figure 3. Pak2-KO HPCs display increased sensitivity to GM-CSF signaling. (A) MDSCs were generated from Pak2-KO or WT BM cells in the presence of GM-CSF, IL-6, and G-CSF. (B) Colony formations of sorted CD45.2+ BM cells cultured in methylcellulose with GM-CSF. The numbers of colonies (colony-forming units in response to GM-CSF [CFU-GM]) per femur are shown. (C) GM-CSF receptor α (Csf2ra) and β (Csf2rb) chain gene expression was measured in progenies collected from the GM-CSF colony assay shown in Figure 3B. Pak2-KO or WT BM c-kit+ cells were cultured with GM-CSF for 7 days. (D-E) Cells were stained for CD11b and Gr1 (D) and cocultured with T cells to test their suppressive function (E). The suppressive function on CD8+ T-cell proliferation is shown. Representative data from at least 3 experiments with 3 to 6 mice per genotype are shown. **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/22/10.1182_bloodadvances.2017007435/3/m_advances007435f3.jpeg?Expires=1767719820&Signature=NHm2My~deLMldBIL2gtabbzLtNgOHWnXojVuo8AbRTneYtMOxhdbW5IvVxUbt-qsTg5DW0xil0X~OhnWthkP-MPvc2-XmhTqqx3WeXLayz6xf8vxTcJ7zKfwPFHFT6L83yJaeu45cDiDv-dtfycwPYiVdFYhPpmgd4b3x251DJwoKcIHQxUAh7t8xYLw~KEnAmVeJh4mLn3yIs7Cbqd-gIy3ZBHR7KxfNiHoT842xlUerk4zH0cLFFAinSvock6SsWDqJJEeEqtx6evAvemqy6FMWqHLh8IW3ZYXclbieIXr9pxtxCnrOLGDKd1oNDs9MAN7a84m8pj~cd5tbA~OxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)