Key Points
BAALC expression is significantly lower in APL compared with other subsets of AML and healthy volunteers.
BAALC overexpression can independently predict shorter DFS in patients with high-risk disease.
Abstract
Although overexpression of the brain and acute leukemia, cytoplasmic (BAALC) gene is associated with primary resistant disease and shorter relapse-free, disease-free, and overall survival in different subsets of acute myeloid leukemia (AML), little is known about its clinical impact in acute promyelocytic leukemia (APL). Using real-time reverse transcriptase polymerase chain reaction, we showed that BAALC expression is significantly lower in APL compared with other subsets of AML (P < .001). We also demonstrated that BAALC overexpression was associated with shorter disease-free survival (DFS) (hazard ratio [HR], 4.43; 95% confidence interval [CI], 1.29-15.2; P = .018) in 221 consecutive patients (median age, 35 years; range, 18-82 years) with newly diagnosed APL homogeneously treated with all-trans retinoic acid and anthracycline-based chemotherapy. Cox proportional hazard modeling showed that BAALC overexpression was independently associated with shorter DFS in the total cohort (HR, 5.26; 95% CI, 1.52-18.2; P = .009) and in patients with high-risk disease (ie, those with initial leukocyte counts >10 × 109/L) (HR, 5.3; 95% CI, 1.14-24.5; P = .033). We conclude that BAALC expression could be useful for refining risk stratification in APL, although this needs to be confirmed in independent cohorts.
Introduction
Although clinical trials evaluating outcomes of patients with acute promyelocytic leukemia (APL) treated with all-trans retinoic acid (ATRA) combined with anthracyclines have reported overall remission rates of up to 95% and long-term overall survival (OS) rates higher than 80%,1-6 other studies have demonstrated that the outcomes are less favorable for patients treated outside well-controlled studies or in low- and middle-income countries.7-9 Excluding the well-known differences between low- and middle-income vs high-income countries, outcomes in APL are variable among studies,1,5,7,10 and, in our experience, patients with newly diagnosed APL may present a specific genomic profile important for predicting prognosis.11-14
In line with these findings, overexpression of the brain and acute leukemia, cytoplasmic (BAALC) gene, either alone or in association with the aberrant expression of other genes, is frequently associated with poor prognosis in different subsets of acute myeloid leukemia (AML),15-18 although its functional role in leukemogenesis has not been investigated to the same extent. In contrast, very few studies have investigated the clinical impact of BAALC expression in APL. To date, only 1 German study demonstrated the isolated overexpression of the BAALC gene in APL as an independent prognostic marker for OS in 86 patients treated according to the German AML Cooperative Group (AMLCG) trial, although this independent association was not found for relapse-free survival.19 Corroborating these findings in a cohort treated according to the International Consortium on Acute Promyelocytic Leukemia (IC-APL) study,11 we provide evidence that BAALC overexpression is associated with poor clinical outcomes in APL and may independently predict shorter disease-free survival (DFS) in patients with high-risk disease.
Patients and methods
Patients and treatment protocol
A total of 221 consecutive patients with newly diagnosed APL who were enrolled in the IC-APL study were included. Details about the diagnosis, eligibility criteria, and classification of patients are published elsewhere.11 The treatment protocol was identical to that of the LPA2005 trial reported by the Programa Español de Tratamiento en Hematologia/Dutch-Belgian Hemato-Oncology Cooperative Group (PETHEMA/HOVON) except for the replacement of idarubicin by daunorubicin due to its greater availability and lower cost for the participating centers. Following the tenets of the Declaration of Helsinki, informed written consent was obtained from all patients. The local research ethics board of each participating center approved the study. For comparison purposes, the analysis included total bone marrow (BM) mononuclear cells (BMMCs) from 27 healthy volunteers, along with 53 BM samples from patients with de novo core-binding factor leukemia (CBF leukemia), 51 from patients with cytogenetically normal AML (CN-AML), and 98 from patients with AML not otherwise specified (AML-NOS).
Gene expression profile of BAALC
All samples used for the gene expression analyses were obtained at diagnosis from BM aspirates and were processed using standard techniques. Following total RNA extraction, real-time quantitative polymerase chain reaction (PCR) assays were performed in duplicate using sample-derived complementary DNA (cDNA) on MicroAmp optical 96-well plates using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) with the ABL FusionQuant Standard kit as the endogenous control. BAALC gene expression was determined by real-time reverse transcriptase PCR using the TaqMan Gene Expression Assay (Hs00227249_m1; Applied Biosystems) following the manufacturer’s instructions. The gene expression of BAALC was calculated relative to a reference cDNA (NB4 cell line, a human APL cell line positive for the PML/RARA fusion gene). Importantly, the same reference cDNA was used as an internal control in all experiments to ensure that the results of different experiments could be comparable. The relative gene expression values for BAALC were quantified using the ∆ cycle threshold (Ct) method, and the results were expressed using 2-ΔΔCt, in which ΔΔCt = ΔCtpatients − ΔCtNB4 cell line.
Statistical analysis
The patients’ baseline characteristics are reported descriptively. The Fisher exact test or the χ2 test, as appropriate, was used to compare categorical variables, and the Kruskal-Wallis test was used to compare continuous variables. Using survival receiver operating characteristic curve analysis20 and the C index,21 we dichotomized patients into 2 groups according to the median value of BAALC expression (low expression, <1.54; high expression, ≥1.54). The clinical end points were described previously.14
Univariable and multivariable proportional hazards regression analysis was performed for potential prognostic factors for OS and DFS. The potential prognostic factors that were examined in multivariable regression analysis were as follows: age at diagnosis, sex, white blood cell (WBC) counts, platelet counts, hemoglobin levels, coagulopathy, French–American–British (FAB) classification, PML breakpoint, creatinine, albumin, uric acid, and fibrinogen. The proportional hazards assumption was tested for each variable of interest. The linearity assumption for all continuous variables was examined using restricted cubic spline estimates of the relationship between the continuous variable and the log relative hazard/risk. All P values were 2-sided with a significance level of .05. All calculations were performed using Stata statistical data analysis software version 14.1 (StataCorp, College Station, TX), Statistical Package for the Social Sciences (SPSS) 19.0, and R 3.3.2 (The CRAN project, www.r-project.org) software.
Results
Figure 1 shows the relative quantification of BAALC expression in BMMCs from healthy volunteers and in samples from patients with newly diagnosed APL, de novo CBF leukemia, CN-AML, and AML-NOS. BAALC expression was significantly lower in APL in comparison with BMMCs from healthy volunteers and to other AML groups (P < .001). Supplemental Table 1 summarizes the central tendency and dispersion measurements of the relative quantification of BAALC expression in all of the enrolled subjects. Next, we restricted our analysis to patients with APL. The baseline features were similar between patients with low and high BAALC expression levels (Table 1).
Differential gene expression of BAALC in BMMCs, APL, and in different subtypes of AML. The expression of the BAALC gene was analyzed by real-time quantitative PCR using the ABL FusionQuant Standard kit as the endogenous control. The horizontal bars represent the median value of BAALC expression relative to expression of the ABL gene. BAALC expression was lower in APL samples as determined by the Kruskal-Wallis test followed by the Dunn posttest.
Differential gene expression of BAALC in BMMCs, APL, and in different subtypes of AML. The expression of the BAALC gene was analyzed by real-time quantitative PCR using the ABL FusionQuant Standard kit as the endogenous control. The horizontal bars represent the median value of BAALC expression relative to expression of the ABL gene. BAALC expression was lower in APL samples as determined by the Kruskal-Wallis test followed by the Dunn posttest.
Baseline characteristics
| Characteristic . | All patients . | BAALC expression . | P . | ||||
|---|---|---|---|---|---|---|---|
| Low expression . | High expression . | ||||||
| No. . | % . | No. . | % . | No. . | % . | ||
| Sex | .686 | ||||||
| Female | 119 | 53.8 | 61 | 55 | 58 | 52.3 | |
| Male | 102 | 46.2 | 49 | 45 | 53 | 47.7 | |
| Age, median (range) | 35.3 (18.9, 82.5) | 35.2 (19, 82.5) | 36.1 (18.9, 81.9) | ||||
| ECOG performance status | .88 | ||||||
| 0 | 109 | 55.6 | 52 | 55.3 | 57 | 55.9 | |
| 1 | 46 | 23.5 | 23 | 24.5 | 23 | 22.5 | |
| 2 | 17 | 8.7 | 7 | 7.4 | 10 | 9.8 | |
| ≥3 | 24 | 12.2 | 12 | 12.8 | 12 | 11.8 | |
| Unknown | 25 | — | 16 | — | 11 | — | |
| Relapse risk group | .979 | ||||||
| Low risk | 39 | 19.4 | 19 | 17.3 | 20 | 18 | |
| Intermediate risk | 101 | 44.4 | 50 | 45.5 | 51 | 45.9 | |
| High risk | 81 | 36.3 | 41 | 37.3 | 40 | 36 | |
| Morphologic subtype | .066 | ||||||
| Hypergranular (FAB M3) | 203 | 92.7 | 98 | 89.1 | 105 | 96.3 | |
| Microgranular (FAB M3 variant) | 16 | 7.3 | 12 | 10.9 | 4 | 3.7 | |
| Unknown | 2 | — | — | — | 2 | — | |
| PML breakpoint | .379 | ||||||
| BCR1 | 104 | 58.1 | 48 | 54.5 | 56 | 61.5 | |
| BCR2 | 6 | 3.4 | 2 | 2.3 | 4 | 4.4 | |
| BCR3 | 69 | 38.5 | 38 | 43.2 | 31 | 34.1 | |
| Unknown | 42 | — | 22 | — | 20 | — | |
| WBC counts, median (range), ×109/L | 4.4 (0.8, 134) | 5 (1, 134) | 3.4 (0.8, 128.5) | .907 | |||
| Platelet counts, median (range), ×109/L | 24 (7, 230) | 24 (7, 230) | 25.5 (8, 110) | .87 | |||
| Hemoglobin, median (range), g/dL | 8.7 (3, 14.9) | 8.6 (4.4, 14.9) | 8.75 (3.2, 14.1) | .464 | |||
| Creatinine, median (range), mg/dL | 0.8 (0.3, 4.3) | 0.8 (0.34, 1.8) | 0.8 (0.3, 4.3) | .1 | |||
| Uric acid, median (range), mg/dL | 3.8 (1.1, 10.3) | 3.7 (1.7, 9.2) | 3.8 (1.1, 10.3) | .545 | |||
| Fibrinogen, median (range), mg/dL | 163 (10, 898) | 165.5 (10, 460) | 161 (0.5, 898) | .933 | |||
| Albumin (range), g/dL | 4 (2.2, 5.42) | 4 (2.2, 5.2) | 4 (2.2, 5.4) | .59 | |||
| Characteristic . | All patients . | BAALC expression . | P . | ||||
|---|---|---|---|---|---|---|---|
| Low expression . | High expression . | ||||||
| No. . | % . | No. . | % . | No. . | % . | ||
| Sex | .686 | ||||||
| Female | 119 | 53.8 | 61 | 55 | 58 | 52.3 | |
| Male | 102 | 46.2 | 49 | 45 | 53 | 47.7 | |
| Age, median (range) | 35.3 (18.9, 82.5) | 35.2 (19, 82.5) | 36.1 (18.9, 81.9) | ||||
| ECOG performance status | .88 | ||||||
| 0 | 109 | 55.6 | 52 | 55.3 | 57 | 55.9 | |
| 1 | 46 | 23.5 | 23 | 24.5 | 23 | 22.5 | |
| 2 | 17 | 8.7 | 7 | 7.4 | 10 | 9.8 | |
| ≥3 | 24 | 12.2 | 12 | 12.8 | 12 | 11.8 | |
| Unknown | 25 | — | 16 | — | 11 | — | |
| Relapse risk group | .979 | ||||||
| Low risk | 39 | 19.4 | 19 | 17.3 | 20 | 18 | |
| Intermediate risk | 101 | 44.4 | 50 | 45.5 | 51 | 45.9 | |
| High risk | 81 | 36.3 | 41 | 37.3 | 40 | 36 | |
| Morphologic subtype | .066 | ||||||
| Hypergranular (FAB M3) | 203 | 92.7 | 98 | 89.1 | 105 | 96.3 | |
| Microgranular (FAB M3 variant) | 16 | 7.3 | 12 | 10.9 | 4 | 3.7 | |
| Unknown | 2 | — | — | — | 2 | — | |
| PML breakpoint | .379 | ||||||
| BCR1 | 104 | 58.1 | 48 | 54.5 | 56 | 61.5 | |
| BCR2 | 6 | 3.4 | 2 | 2.3 | 4 | 4.4 | |
| BCR3 | 69 | 38.5 | 38 | 43.2 | 31 | 34.1 | |
| Unknown | 42 | — | 22 | — | 20 | — | |
| WBC counts, median (range), ×109/L | 4.4 (0.8, 134) | 5 (1, 134) | 3.4 (0.8, 128.5) | .907 | |||
| Platelet counts, median (range), ×109/L | 24 (7, 230) | 24 (7, 230) | 25.5 (8, 110) | .87 | |||
| Hemoglobin, median (range), g/dL | 8.7 (3, 14.9) | 8.6 (4.4, 14.9) | 8.75 (3.2, 14.1) | .464 | |||
| Creatinine, median (range), mg/dL | 0.8 (0.3, 4.3) | 0.8 (0.34, 1.8) | 0.8 (0.3, 4.3) | .1 | |||
| Uric acid, median (range), mg/dL | 3.8 (1.1, 10.3) | 3.7 (1.7, 9.2) | 3.8 (1.1, 10.3) | .545 | |||
| Fibrinogen, median (range), mg/dL | 163 (10, 898) | 165.5 (10, 460) | 161 (0.5, 898) | .933 | |||
| Albumin (range), g/dL | 4 (2.2, 5.42) | 4 (2.2, 5.2) | 4 (2.2, 5.4) | .59 | |||
Patients were dichotomized into 2 groups according to the median value of BAALC expression (low expression, <1.54; high expression, ≥1.54). Details can be found in “Statistical analysis.”
—, not taken into account to calculate the frequency; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell.
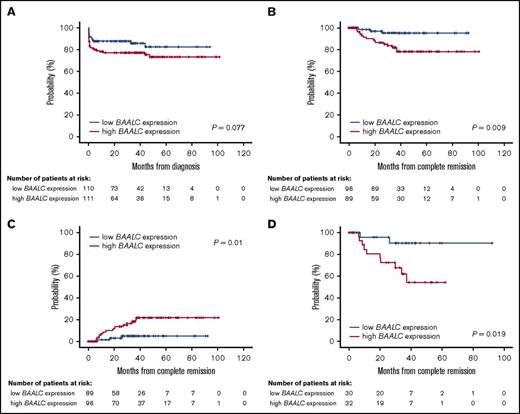
Details for patient outcomes are summarized in Table 2. With a median follow-up of 32 months (range, 1-101 months), the estimated 5-year OS rate was 79% (95% confidence interval [CI], 72-84). Overall, 185 of 221 patients (84%) achieved complete hematological remission (CR). Of 36 patients (16%) who failed to achieve CR, 26 (72%) experienced early mortality (death within 30 days after diagnosis). The main causes of death during induction were hemorrhage (14 patients; 54%), followed by infection (10 patients; 38%), central nervous system thrombosis (1 patient; 4%), and differentiation syndrome (1 patient; 4%). BAALC expression had no impact on CR achievement (P = .067). In contrast, the early mortality rate was significantly higher in patients with high BAALC expression (17% vs 8%; P = .046). Although patients with high BAALC expression had a lower 5-year OS rate (73%; 95% CI, 63-81) than patients with low BAALC expression (82%; 95% CI, 70-90), this difference did not reach statistical significance (P = .077; Figure 2A).
Summary of outcomes of patients with APL according to BAALC expression
| . | No. (%) . | CR % . | OS . | DFS . | CIR . | |||
|---|---|---|---|---|---|---|---|---|
| No. . | 5-y % (95% CI) . | No. . | 5-y % (95% CI) . | No. . | 5-y % (95% CI) . | |||
| All patients | 221 (100) | |||||||
| Low BAALC expression | 89 | 110 | 82 (70, 90) | 89 | 95 (86, 98) | 87 | 5 (1, 10) | |
| High BAALC expression | 79 | 111 | 73 (63, 81) | 96 | 78 (66, 86) | 91 | 21 (11, 30) | |
| P | .067 | .077 | .009* | .01* | ||||
| Low-risk patients | 39 (17) | |||||||
| Low BAALC expression | 87 | 19 | 93 (63, 99) | 14 | 100 | 13 | 0 | |
| High BAALC expression | 82 | 20 | 77 (54, 90) | 19 | 91 (54, 99) | 18 | 7 (0, 21) | |
| P | 1.00 | .225 | .48 | .5 | ||||
| Intermediate-risk patients | 101 (46) | |||||||
| Low BAALC expression | 95 | 51 | 97 (85, 99) | 44 | 96 (79, 99) | 43 | 3 (0, 9) | |
| High BAALC expression | 84 | 50 | 90 (79, 96) | 46 | 87 (71, 94) | 45 | 11 (2, 23) | |
| P | .062 | .143 | .158 | .15 | ||||
| High-risk patients | 81 (37) | |||||||
| Low BAALC expression | 81 | 40 | 59 (35, 76) | 31 | 90 (66, 97) | 31 | 9 (0, 20) | |
| High BAALC expression | 72 | 41 | 47 (25, 66) | 31 | 53 (28, 72) | 28 | 41 (11, 62) | |
| P | .432 | .237 | .019* | .019* | ||||
| . | No. (%) . | CR % . | OS . | DFS . | CIR . | |||
|---|---|---|---|---|---|---|---|---|
| No. . | 5-y % (95% CI) . | No. . | 5-y % (95% CI) . | No. . | 5-y % (95% CI) . | |||
| All patients | 221 (100) | |||||||
| Low BAALC expression | 89 | 110 | 82 (70, 90) | 89 | 95 (86, 98) | 87 | 5 (1, 10) | |
| High BAALC expression | 79 | 111 | 73 (63, 81) | 96 | 78 (66, 86) | 91 | 21 (11, 30) | |
| P | .067 | .077 | .009* | .01* | ||||
| Low-risk patients | 39 (17) | |||||||
| Low BAALC expression | 87 | 19 | 93 (63, 99) | 14 | 100 | 13 | 0 | |
| High BAALC expression | 82 | 20 | 77 (54, 90) | 19 | 91 (54, 99) | 18 | 7 (0, 21) | |
| P | 1.00 | .225 | .48 | .5 | ||||
| Intermediate-risk patients | 101 (46) | |||||||
| Low BAALC expression | 95 | 51 | 97 (85, 99) | 44 | 96 (79, 99) | 43 | 3 (0, 9) | |
| High BAALC expression | 84 | 50 | 90 (79, 96) | 46 | 87 (71, 94) | 45 | 11 (2, 23) | |
| P | .062 | .143 | .158 | .15 | ||||
| High-risk patients | 81 (37) | |||||||
| Low BAALC expression | 81 | 40 | 59 (35, 76) | 31 | 90 (66, 97) | 31 | 9 (0, 20) | |
| High BAALC expression | 72 | 41 | 47 (25, 66) | 31 | 53 (28, 72) | 28 | 41 (11, 62) | |
| P | .432 | .237 | .019* | .019* | ||||
Summary of outcomes of patients with APL according to BAALC expression (in total cohort, and according to PETHEMA/GIMEMA criteria for relapse).22
CI, confidence interval; CIR, cumulative incidence of relapse; CR, complete remission.
Statistically significant differences (P < .05).
Patient survival. The probability of OS (A), DFS (B), and cumulative incidence of relapse (C) in patients with APL according to BAALC expression (entire cohort). DFS (D) in patients with a high risk of relapse according to BAALC expression.
Patient survival. The probability of OS (A), DFS (B), and cumulative incidence of relapse (C) in patients with APL according to BAALC expression (entire cohort). DFS (D) in patients with a high risk of relapse according to BAALC expression.
Of the 185 patients who achieved CR, 19 patients (10%) relapsed at a median time of 40 days (range, 1-389 days). The estimated 5-year DFS rate was 87% (95% CI, 80-92), and the cumulative incidence of relapse (CIR) rate was 14% (95% CI, 8-20). Patients with high BAALC expression had a lower 5-year DFS rate (78%; 95% CI, 66-86) than those with low BAALC expression (95%; 95% CI, 86-98) (P = .009) (Figure 2B). Cox proportional hazards modeling showed that high expression of BAALC was independently associated with shorter DFS (hazard ratio [HR], 5.26; 95% CI, 1.52-18.2) (P = .009) (Table 3). In addition, the CIR rate was higher in patients with high BAALC expression (21%; 95% CI, 11-30) than in patients with low BAALC expression (5%; 95% CI, 0-10) (P = .01) (Figure 2C).
Multivariable Cox model for OS and DFS
| . | OS . | DFS . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |||
| All patients | ||||||||
| Age: continuous variable | 1.01 | 0.99 | 1.03 | .187 | 0.98 | 0.95 | 1.02 | .578 |
| Sex: male vs female | 0.93 | 0.5 | 1.73 | .824 | 1.07 | 0.4 | 2.5 | .987 |
| WBC, ×109/L: continuous variable | 2.3 | 1.7 | 5.03 | <.001 | 3.44 | 1.54 | 7.66 | .002 |
| BAALC expression: high vs low | 1.69 | 0.88 | 3.24 | .11 | 5.26 | 1.52 | 18.2 | .009 |
| Low-risk patients | ||||||||
| Age: continuous variable | 0.95 | 0.88 | 1.03 | .236 | —* | —* | —* | |
| Sex: male vs female | 1.08 | 0.19 | 6.05 | .928 | —* | —* | —* | |
| BAALC expression: high vs low | 3.51 | 0.4 | 30.7 | .256 | —* | —* | —* | |
| Intermediate-risk patients | ||||||||
| Age: continuous variable | 0.99 | 0.93 | 1.05 | .845 | 0.96 | 0.9 | 1.02 | .248 |
| Sex: male vs female | 1.29 | 0.26 | 6.42 | .752 | 2.24 | 0.41 | 12.2 | .35 |
| BAALC expression: high vs low | 4.29 | 0.49 | 36.8 | .184 | 4.38 | 0.51 | 37.6 | .177 |
| High-risk patients | ||||||||
| Age: continuous variable | 1.03 | 1.01 | 1.05 | .025 | 1.09 | 0.96 | 1.05 | .684 |
| Sex: male vs female | 1.06 | 0.51 | 2.2 | .87 | 0.87 | 0.26 | 2.83 | .819 |
| BAALC expression: high vs low | 1.26 | 0.6 | 2.64 | .53 | 5.3 | 1.14 | 24.5 | .033 |
| . | OS . | DFS . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |||
| All patients | ||||||||
| Age: continuous variable | 1.01 | 0.99 | 1.03 | .187 | 0.98 | 0.95 | 1.02 | .578 |
| Sex: male vs female | 0.93 | 0.5 | 1.73 | .824 | 1.07 | 0.4 | 2.5 | .987 |
| WBC, ×109/L: continuous variable | 2.3 | 1.7 | 5.03 | <.001 | 3.44 | 1.54 | 7.66 | .002 |
| BAALC expression: high vs low | 1.69 | 0.88 | 3.24 | .11 | 5.26 | 1.52 | 18.2 | .009 |
| Low-risk patients | ||||||||
| Age: continuous variable | 0.95 | 0.88 | 1.03 | .236 | —* | —* | —* | |
| Sex: male vs female | 1.08 | 0.19 | 6.05 | .928 | —* | —* | —* | |
| BAALC expression: high vs low | 3.51 | 0.4 | 30.7 | .256 | —* | —* | —* | |
| Intermediate-risk patients | ||||||||
| Age: continuous variable | 0.99 | 0.93 | 1.05 | .845 | 0.96 | 0.9 | 1.02 | .248 |
| Sex: male vs female | 1.29 | 0.26 | 6.42 | .752 | 2.24 | 0.41 | 12.2 | .35 |
| BAALC expression: high vs low | 4.29 | 0.49 | 36.8 | .184 | 4.38 | 0.51 | 37.6 | .177 |
| High-risk patients | ||||||||
| Age: continuous variable | 1.03 | 1.01 | 1.05 | .025 | 1.09 | 0.96 | 1.05 | .684 |
| Sex: male vs female | 1.06 | 0.51 | 2.2 | .87 | 0.87 | 0.26 | 2.83 | .819 |
| BAALC expression: high vs low | 1.26 | 0.6 | 2.64 | .53 | 5.3 | 1.14 | 24.5 | .033 |
Multivariable Cox modeling was hampered by the small number of events (relapses) in the low-risk group; therefore, no statistical analysis was performed.
Considering the importance of the PETHEMA/Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) criteria for predicting relapse,22 we opted to evaluate the impact of BAALC expression separately in patients with low-, intermediate-, and high-risk disease. For low-risk patients (39 patients; 17%), BAALC expression had no impact on CR (P = 1.00), OS (P = .225), DFS (P = .48), or CIR (P = .5). Similar results were observed for intermediate-risk patients (101 patients; 46%; CR, P = .062; OS, P = .143; DFS, P = .158; CIR, P = .15). In contrast, high-risk patients (81 patients; 37%) with high BAALC expression had a lower 5-year DFS rate (53%; 95% CI, 28-72) than those with low BAALC expression (90%; 95% CI, 66-97) (P = .019; Figure 2D). BAALC expression retained its significant prognostic value in multivariable analysis (HR, 5.3; 95% CI, 1.14-24.5; P = .033) (Table 3). Also, patients with high BAALC expression had a higher CIR rate (41%; 95% CI, 11-62) than patients with low BAALC expression (9%; 95% CI, 0-20) (P = .019). The CR (P = .432) and OS (P = .237) rates were similar in patients with low vs high BAALC expression (Table 2).
Discussion
Although the overexpression of BAALC is widely accepted as a predictor of inferior outcomes in AML,15,17,18 and, more recently, has been used as a marker to detect minimal residual disease in CN-AML,23 the biological mechanisms underlying these findings await clarification. Eisfeld et al showed that BAALC overexpression is closely related to a heritable polymorphism in the promoter region of the gene.24 This polymorphism, rs62527607, is thought to create a high-affinity binding site for the activating RUNX1 transcription factor, which in turn leads to high constitutive BAALC expression.24 Of note, the methylation status of the promoter regions of the BAALC gene in primary APL blasts at diagnosis and relapse seems to be unrelated to its overexpression (V.M.d.D.W., unpublished data). In terms of its biological significance, BAALC expression may reflect functional differences in normal hematopoiesis and leukemogenesis. Although constitutive activation of BAALC does not appear to alter the proliferation/survival rates of healthy hematopoietic stem or progenitor cells,25 its induced expression promotes cell-cycle progression of leukemia cell lines by sustaining extracellular signal-regulated kinase activity.26 This is mediated by an interaction with the scaffold protein MEK kinase-1, which inhibits the interaction between extracellular signal-regulated kinase and MAPK phosphatase 3 (MKP3/DUSP6). In addition, there is evidence that BAALC can block myeloid differentiation and thereby promote leukemogenesis when combined with the self-renewal promoting oncogene HOXA9.25 The functional role of BAALC in the induction of an APL-leukemic phenotype remains unknown.
Here, we demonstrated that BAALC expression is significantly lower in APL than in other subsets of AML. We also described the prognostic importance of BAALC expression in a cohort of patients homogeneously treated with ATRA and anthracycline-based chemotherapy and addressed these findings in a large (at least in countries of Latin ancestry7 ) and clinically heterogeneous subset of APL, such as patients with high-risk disease. Most importantly, BAALC overexpression could be used to identify patients with poor prognosis regardless of leukocyte count and regardless of the treatment regimens we and others19 have conducted. Although our results are in accordance with those reported by Nolte et al,19 in our cohort, BAALC overexpression was independently associated with lower DFS in high-risk APL, which was not found by Nolte et al. It is important to emphasize that the studies have some methodological differences. Specifically, the Nolte et al study had longer follow-up, a lower frequency of patients with high-risk disease, a higher median patient age, and a higher frequency of the microgranular subtype. However, the most important difference between the AMLCG trial and IC-APL studies was the adopted treatment protocol. The AMLCG treatment protocol included a more intensive chemotherapy regimen with higher doses of cytarabine compared with the IC-APL study; this may lead to differences in prognostic markers.
Several findings over the last 2 decades show that highly effective treatment strategies can overcome characteristics that were previously associated with an adverse outcome, thereby making those prognostic markers less relevant.27-29 This may also be true in patients with APL who are treated with the state-of-the-art combination of ATRA and arsenic trioxide (ATO). Recent clinical trials show that the ATRA-ATO combination is less toxic and more effective than ATRA plus chemotherapy. Consequently, patients with low- or intermediate-risk APL30,31 and with high-risk APL show higher long-term and leukemia-free survival with ATRA-ATO.32 In addition, the advantages of ATRA-ATO over ATRA chemotherapy increase over time, showing significantly greater and more sustained antileukemic efficacy.30,32 It remains unknown whether prognostic markers that were previously related to inferior outcomes in APL will retain their clinical relevance in the ATRA-ATO era or whether they will be needed considering the encouraging results with this regimen. For example, Cicconi et al demonstrated that fms-related tyrosine kinase 3–internal tandem duplication mutations had no impact on either event-free survival or CIR in low-risk and intermediate-risk patients receiving ATRA and ATO regimens as first-line therapy. These results suggested that ATRA-ATO treatment abrogates the negative prognostic impact of fms-related tyrosine kinase 3–internal tandem duplication mutations.33 In agreement, Powell et al showed that the addition of ATO on consolidation therapy significantly improved the prognosis (event-free, DFS. and OS) of adults with newly diagnosed APL and negated all previously identified prognostic factors, including initial leukocyte count.34 Although further studies are needed to confirm all of these findings, it is possible that the prognostic influence of BAALC expression and other important markers for predicting outcomes in APL35 may be different or even irrelevant for patients treated with ATRA-ATO. It is important to note that even in light of these findings, most of the patients in countries in Latin America still rely on the publicly funded health care system and arsenic treatment is still not available in most of them. In particular, in Brazil, the use of arsenic for APL treatment is not authorized by health surveillance agencies, most likely because of its cost. Apparently, there is still a long way to go before the ATRA-ATO combination will be implemented as the therapy of choice for APL in these countries.
Although initial leukocyte and platelet counts are currently used as markers to predict relapse in APL,22 these parameters can change significantly in a short period of time. Based on our experience12-14 and on the experiences of others,36-38 the use of genetic data could improve risk stratification in APL. This could help clinicians make decisions about consolidation strategies and also provide the possibility of early intervention if molecular relapse occurs. However, the findings of all of these studies should be interpreted with caution because most of them, including our own, lack validation in independent cohorts and are limited by their sample sizes. Furthermore, we cannot rule out the possibility that methodological differences between studies could lead to different results and conclusions. More importantly, it is very likely that all of the molecular markers described so far will not remain clinically valid if the ATRA-ATO regimen becomes the most used protocol worldwide. Meanwhile, alternative strategies for improving prognostication in APL should be tested, particularly in countries in which the clinical use of arsenic has not yet been authorized.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors gratefully acknowledge the members of the International Consortium on Acute Leukemia of the American Society of Hematology.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2013/08135-2). A.R.L.-A. received a fellowship from FAPESP (grant 2007/55067-1).
Authorship
Contribution: A.R.L.-A. conceived and designed the study, performed experiments, analyzed and interpreted data, performed the statistical analyses, and drafted the article; D.A.P.-M. and L.C.K. updated the clinical data, performed experiments, and collected data; P.L.F.-N., V.M.d.D.W., and J.L.C.-S. performed experiments and collected data; R.A.M.M., R.B., K.P., R.P., C.S.C., E.M.F., and M.d.L.C. provided samples and updated the clinical data; S.L.S., M.S.T., R.C.R., D.G., A.G., B.L., F.L.-C., M.A.S., N.B., and E.M.R. designed the treatment protocol; M.A.S. performed and reviewed the statistical analyses; E.M.R. approved the final version of the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eduardo M. Rego, Department of Internal Medicine and Center for Cell Based Therapy, Medical School of Ribeirao Preto, University of Sao Paulo. Av. Bandeirantes, 3900, Ribeirao Preto SP 14048-900, Brazil; e-mail: edumrego@hotmail.com or emrego@hcrp.usp.br.