Key Points
A stem cell graft NK cell dose below 6.3 × 106 cells per kg associates with risk of disease relapse following T-cell–depleted allo-HSCT.
Clinical outcomes of patients undergoing allo-HSCT may be improved by setting an NK cell threshold within donor stem cell grafts.
Abstract
The graft-versus-leukemia (GVL) effect of allogeneic hemopoietic stem cell transplantation (allo-HSCT) is mediated by the donor immune system and acts to decrease the rate of disease relapse. Although studies of posttransplant immune reconstitution have identified correlates of clinical outcome, the number and profile of mature immune cells infused with the stem cell graft is also likely to be an important determinant and has been relatively poorly studied. We characterized immune cells within the stem cell graft of 107 patients who underwent T-cell–depleted allo-HSCT and related this to clinical outcome. The number of natural killer (NK) cells and T cells that were infused varied markedly between patients, but T-cell dose was not an important factor in subsequent outcome. In contrast, the number of NK cells was a powerful determinant of the risk of disease relapse. Patients who received an NK cell dose below the median level of 6.3 × 106 cells per kg had a relapse rate of 40% at 2 years posttransplant compared with only 6% for those whose stem cell graft contained a dose above this value. Analysis of NK subsets showed that this effect was mediated primarily by the CD56dim population of mature effector cells and that high-level expression of the activatory protein DNAM on donor NK cells was also strongly protective. These observations offer important insights into the mechanism of GVL and suggest that optimization studies of the number of NK cells within the stem cell graft should be considered as a means to reduce disease relapse.
Introduction
Allogeneic hemopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment of a range of myeloid and lymphoid malignancies. Successful tumor elimination relies partially on a graft-versus-leukemia (GVL) response that is mediated by the donor immune response and established within the first few weeks following transplantation.1-3 Although many studies have related the clinical outcome of transplantation to features of immune reconstitution4-7 or donor/host genotype,8,9 the potential importance of the cellular composition within the initial stem cell infusion remains poorly defined. Indeed, the number and diversity of immune cells within this product would be expected to influence immune reconstitution and could play a significant role in determining patient outcome. Previous studies have shown that a high CD34+ cell dose in stem cell grafts obtained from peripheral blood is associated with protection from relapse,10 and high CD8+ T-cell doses correlate with improved survival.11 Natural killer (NK) cell activity is controlled by a balance of inhibitory or activatory signaling,12 and there is considerable heterogeneity between individual NK cell repertoires,13 which can predict susceptibility to viral infection.14 Despite this, relatively little is known about the potential importance of the nature of the NK cell infusion given at the time of transplant.
We determined the number and profile of immune cells within the stem cell grafts of 107 patients who underwent allo-HSCT and related this information to subsequent clinical outcome. We demonstrate that the number of CD56dim NK cells infused at the time of transplantation has a profound influence on the risk of subsequent disease relapse, potentially through the expression of the activatory receptor DNAM.
Methods
Patient cohort and sample collection
Samples were obtained from 107 consecutive patients undergoing allo-HSCT for the treatment of hematological malignancies between 2012 and 2015 at the Queen Elizabeth Hospital Birmingham (ethics code: 051Q7071175) following written consent and according to the Declaration of Helsinki. Donors received a 5-day course of granulocyte colony-stimulating factor prior to collection of their apheresis product. Peripheral blood mononuclear cell samples were collected and processed within 24 hours following transplant of the final donor stem cell graft. The number of total nucleated cells and CD34+ cells in the donor stem cell graft was determined by the National Health Service Blood and Transplant Service.
Stem cell graft analysis
Analysis of the peripheral blood mononuclear cell component was conducted using flow cytometric immunophenotyping. Live T and NK cells were identified as part of the lymphocyte gate with exclusion of cells expressing CD14 or CD19 (ECD; Beckman Coulter) and use of a viability dye (propidium iodide solution; Miltenyi). T cells were selected as CD3+CD56− whereas NK cells were selected as CD3−CD56+ (Biolegend; CD3 [HIT3a]; AF700, CD56 [HCD56]; APC-Cy7). Receptor expression on NK cells was studied by use of monoclonal antibodies against KIR proteins (Biolegend; CD158a/h [HP-MA4]; fluorescein isothiocyanate [FITC], CD158b [DX27]; FITC, CD158e [DX9]; FITC), DNAM (Biolegend; DNAM/CD226 [11A8]; APC), NKG2D (Biolegend; NKG2D/CD314 [1D11]; PerCP-Cy5.5), and NKp46 (Biolegend; NKp46/CD335 [9-E2]; Pacific Blue). Cells were washed in magnetic-activated cell sorting buffer and surface stained on ice, in the dark, for 30 minutes. Analysis was performed on a Gallios flow cytometer (Beckman Coulter) and interpreted with Kaluza Analysis Software 1.3 (Beckman Coulter).
Clinical outcome
A range of clinical outcomes were measured including relapse incidence (RI), acute graft-versus-host disease (aGVHD), nonrelapse mortality (NRM), and overall survival (OS). RI was defined as any event of relapse after the time of stem cell infusion, with death from a nonrelapse cause acting as a competing risk. NRM was defined as death from any cause other than disease relapse, with death from relapse acting as the competing risk. Incidence of aGVHD was determined by the diagnosis of grade 2+ aGVHD by 100 days posttransplantation and assessed with a competing risk of death before 100 days without aGVHD incidence.15 OS was defined as death, from any cause, posttransplantation.16
Statistical analysis
Statistical analyses were performed using GraphPad Prism (7.0), IBM SPSS Statistics (21), and R (the R project for statistical computing 3.2.2). The effect of all baseline variables on OS was estimated using the Kaplan-Meier method. Log-rank tests compared survival curves. Cox proportional hazard regression analysis was used to identify independent associations with OS.
The effect of all baseline variables on time to event outcomes with competing risks (RI, aGVHD, and NRM) was estimated by cumulative incidence analysis and tested using the Gray method.17 “Death by any other cause” was the competing risk in each instance. The Fine and Gray regression method18 assessed the impact of independent variables in a multivariate model.19,20 Baseline variables with a cutoff point of P < .2 were included in multivariate analyses.
Results
Considerable heterogeneity is observed in the composition of the lymphoid repertoire within the stem cell graft
A total of 107 patients were recruited to the study, and all underwent allo-HSCT as a treatment of a hematological malignancy (Table 1). The median patient and donor ages were 56 years and 48 years, respectively. A total of 70 patients (65%) underwent matched unrelated donor transplant, with a further 10 patients (9%) undergoing a single antigen mismatched transplant from an unrelated donor. Only 27 patients (25%) received transplants from matched sibling donors. Of the entire cohort, 93 patients (87%) received reduced intensity conditioning, whereas only 14 patients (13%) underwent myeloablative conditioning. All patients received in vivo T-cell depletion with either alemtuzumab (n = 79, 74%) or ATG (n = 28, 26%).
Characteristics of patients within the study
| Population characteristics . | Value . | Percentage . |
|---|---|---|
| Total number in cohort | 107 | |
| Patient age, median (range), y | 56 (19-73) | |
| Patients >60 y | 44 | 41 |
| Patient sex (male) | 69 | 64 |
| CMV at risk (−/+) | 39 | 36 |
| Disease | ||
| Myeloid (AML, CML, MDS, MF) | 67 | 63 |
| Lymphoid (ALL) | 14 | 13 |
| Non-Hodgkin lymphoma | 19 | 18 |
| Hodgkin lymphoma | 7 | 7 |
| Conditioning regimen | ||
| Full intensity | ||
| Cyclo/TBI | 14 | 13 |
| Reduced intensity | ||
| Flu/Melph | 53 | 50 |
| FLAMSA/Bu | 14 | 13 |
| Flu/Bu | 12 | 11 |
| BEAM | 11 | 10 |
| Flu/BEAM | 3 | 3 |
| T-cell depletion | ||
| Alemtuzumab | 79 | 74 |
| ATG | 28 | 26 |
| Donor characteristics | ||
| Donor age, median (range), y | 48 (21-66) | |
| Donor sex (male) | 70 | 65 |
| Female donor to male patient | 22 | 21 |
| Donor type | ||
| Matched sibling (10/10) | 27 | 25 |
| Matched unrelated donor (10/10) | 70 | 65 |
| Mismatched unrelated donor (9/10) | 10 | 9 |
| Population characteristics . | Value . | Percentage . |
|---|---|---|
| Total number in cohort | 107 | |
| Patient age, median (range), y | 56 (19-73) | |
| Patients >60 y | 44 | 41 |
| Patient sex (male) | 69 | 64 |
| CMV at risk (−/+) | 39 | 36 |
| Disease | ||
| Myeloid (AML, CML, MDS, MF) | 67 | 63 |
| Lymphoid (ALL) | 14 | 13 |
| Non-Hodgkin lymphoma | 19 | 18 |
| Hodgkin lymphoma | 7 | 7 |
| Conditioning regimen | ||
| Full intensity | ||
| Cyclo/TBI | 14 | 13 |
| Reduced intensity | ||
| Flu/Melph | 53 | 50 |
| FLAMSA/Bu | 14 | 13 |
| Flu/Bu | 12 | 11 |
| BEAM | 11 | 10 |
| Flu/BEAM | 3 | 3 |
| T-cell depletion | ||
| Alemtuzumab | 79 | 74 |
| ATG | 28 | 26 |
| Donor characteristics | ||
| Donor age, median (range), y | 48 (21-66) | |
| Donor sex (male) | 70 | 65 |
| Female donor to male patient | 22 | 21 |
| Donor type | ||
| Matched sibling (10/10) | 27 | 25 |
| Matched unrelated donor (10/10) | 70 | 65 |
| Mismatched unrelated donor (9/10) | 10 | 9 |
“CMV at risk (−/+)” indicates CMV seronegative patient receiving stem cell graft from a CMV seropositive donor. Alemtuzumab dose: 50 mg (10 mg per day for 5 days). ATG dose: FLAMSA/Bu, 1 mg/kg for 1 day, then 2 mg/kg for 2 days; Flu/Bu, 2.5 mg/kg for 2 days.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BEAM, BCNU etoposide Ara-C melphalan; Bu, busulfan; CML, chronic myeloid leukemia; CMV, cytomegalovirus; Cyclo, cyclophosphamide; FLAMSA, fludarabine, Ara-C, and amsacrine; Flu, fludarabine; MDS, myelodysplastic syndrome; Melph, melphalan; MF, myelofibrosis; TBI, total body irradiation.
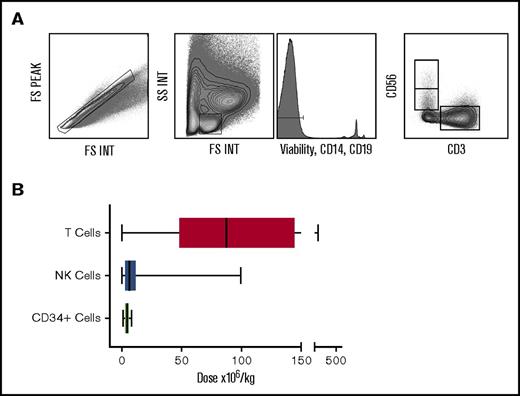
The donor apheresis bag was collected immediately after infusion into the patient, and residual cells were collected for analysis. Mononuclear cells were purified by density centrifugation, and flow cytometry was used to identify subsets for subsequent correlation with patient outcome. NK and T cells were identified by CD56+CD3− and CD56−CD3+ phenotype, respectively (Figure 1A), from a “lymphocyte” gate in which nonviable cells, B cells, and monocytes were removed. The number of lymphocytes, NK cells, and T cells in this gate were calculated as a proportion of the clinically measured absolute number of lymphocytes transferred in the stem cell graft. This number was then corrected for the patient weight in order to determine the “cell dose.”
The number of T cells and NK cells delivered within the stem cell product differs greatly between patients. Plots showing flow gating strategy used to identify lymphocytes, NK and T cells (A). Box and whisker graph displaying dose range of T (range 0.04-458 × 106/kg; median 87.4 × 106/kg), NK (range 0.03-99 × 106/kg; median 6.3 × 106/kg), and CD34+ (range 1.25-8.0 × 106/kg; median 4.7 × 106/kg) cells in the stem cell product received by patients in the cohort (B).
The number of T cells and NK cells delivered within the stem cell product differs greatly between patients. Plots showing flow gating strategy used to identify lymphocytes, NK and T cells (A). Box and whisker graph displaying dose range of T (range 0.04-458 × 106/kg; median 87.4 × 106/kg), NK (range 0.03-99 × 106/kg; median 6.3 × 106/kg), and CD34+ (range 1.25-8.0 × 106/kg; median 4.7 × 106/kg) cells in the stem cell product received by patients in the cohort (B).
The number of CD34+ cells per kg in the graft was relatively consistent across samples and ranged from 1.25 to 8.0 million cells per kg (median 4.72; standard deviation [SD] 1.109), in keeping with the target doses for infusion at our institution. In contrast, significant differences were observed in both the number and proportion of total lymphocytes, T cells, and NK cells between stem cell grafts. In particular, the number of T cells varied from 0.04 to 458 million cells per kg (median 87.41; SD 84.07), whereas the comparable range for NK cells was from 0.03 to 99 million cells per kg with a median of 6.3 million cells per kg (SD 13.57) (Figure 1B).
Patients who receive high NK cell numbers in the donor graft composition have a substantial reduction in the rate of disease relapse
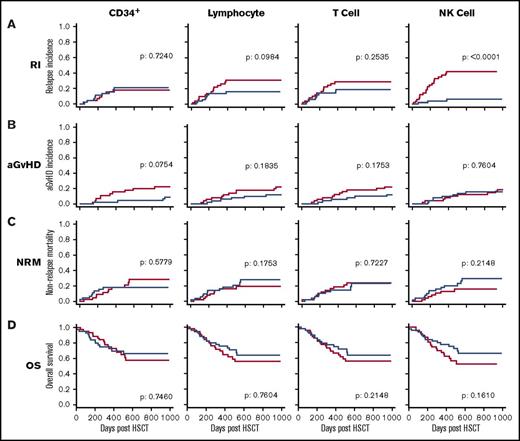
Given the considerable heterogeneity in the number of different leukocytes infused within the stem cell graft, we next examined whether these were related to subsequent clinical outcome. The median doses of CD34+ cells, lymphocytes, T cells, and NK cells within the stem cell graft were 4.7, 186, 87, and 6.3 × 106/kg, respectively. Patients were divided into 2 groups based on receipt of a donor graft infusion with values above or below the median cell dose for each of the 4 cell types. These groups were then assessed, with a univariate analysis, against a range of clinical factors including the incidence of disease relapse or aGVHD, NRM, and OS (Figure 2). A striking positive association was apparent between the number of NK cells infused and protection from disease relapse (P < .001) (Figure 2A). Specifically, the relapse rate among patients who received an infusion of NK cells above the median dose of 6.3 × 106/kg was only 6% at 2 years posttransplant compared with 40% in those who received an NK cell infusion below this value. As such, infusion of the higher number of NK cells was associated with a 6.7-fold decrease in the rate of disease relapse. Receiver operating characteristic curve analysis did not find an improved threshold than the median NK cell dose to distinguish RI (supplemental Figure 1).
The number of NK cells in the stem cell product is protective against disease relapse. Relationship between the number of 4 different cell subsets administered within the stem cell infusion and clinical outcome. The median number of CD34+ cells, lymphocytes, T cells, and NK cells was defined, and transplants were divided into those where the infusion contained a cell dose either above (blue) or below this median (red) value. Clinical outcome measures were RI (A), aGVHD (B), NRM (C), and OS (D). This demonstrates that NK cell dose is the only parameter that influences disease relapse (P < .0001). Cumulative incidence analysis was tested using the Gray method. Log-rank tests were used to analyze the Kaplan-Meier survival curves.
The number of NK cells in the stem cell product is protective against disease relapse. Relationship between the number of 4 different cell subsets administered within the stem cell infusion and clinical outcome. The median number of CD34+ cells, lymphocytes, T cells, and NK cells was defined, and transplants were divided into those where the infusion contained a cell dose either above (blue) or below this median (red) value. Clinical outcome measures were RI (A), aGVHD (B), NRM (C), and OS (D). This demonstrates that NK cell dose is the only parameter that influences disease relapse (P < .0001). Cumulative incidence analysis was tested using the Gray method. Log-rank tests were used to analyze the Kaplan-Meier survival curves.
Patients who received an NK cell dose above the median level had an OS at 2 years posttransplant days of 70% compared with 58% for those in whom the graft composition was below the median. This difference, however, did not reach statistical significance (P = .161). No statistically significant differences were observed in the risk of aGVHD or NRM in association with the NK cell dose in the stem cell graft.
A statistically significant association was not observed between the doses of infused CD34+ cells, lymphocytes, or T cells and the clinical outcomes of aGVHD, RI, NRM, or OS.
Multivariate analysis confirms that infusion of a stem cell graft with an NK cell dose that is above the median value is a determinant of the risk of relapse
In addition to NK cell dose within the stem cell graft, several additional patient characteristics were found to be associated with the risk of disease relapse at a statistical significance of <.2 on univariate analysis (supplemental Table 1). These included patient age, donor type, type of T-cell depletion, patient hemopoietic stem cell transplant comorbidity index (HCT-CI), and total lymphocyte dose. The predictive power of NK cell dose was assessed within multivariate models that included these parameters (Table 2). Unrelated donor transplantation, low HCT-CI, nature of T-cell depletion, and high NK cell dose all remained as independent predictors of protection against disease relapse following multivariate analysis. Importantly, the relative risk of relapse in patients who received an NK cell infusion >6.3 × 106/kg compared with those who received <6.3 × 106/kg was 0.097, representing a 90% reduction in relapse risk.
NK cell dose remains an independent predictor of RI in a multivariate analysis
| . | P . | Hazard ratio . | CI (2.5%) . | CI (97.5%) . |
|---|---|---|---|---|
| RI | ||||
| >56 years old | .069 | 0.477 | 0.22 | 1.06 |
| Alemtuzumab T-cell depletion | .018 | 0.424 | 0.21 | 0.87 |
| Sibling transplant | <.001 | 4.588 | 1.99 | 10.60 |
| High HCT-CI | <.001 | 4.097 | 1.82 | 9.24 |
| Lymphocyte dose > median | .730 | 1.148 | 0.52 | 2.55 |
| NK dose > median | <.001 | 0.097 | 0.03 | 0.33 |
| aGVHD | ||||
| Female to male transplant | .320 | 2.049 | 0.49 | 8.52 |
| Mismatched unrelated donor (9/10) | .030 | 7.988 | 1.23 | 51.96 |
| Sibling transplant | .720 | 0.649 | 0.06 | 7.02 |
| Full-intensity conditioning | .006 | 11.414 | 2.01 | 64.73 |
| CD34 dose > median | .130 | 0.341 | 0.08 | 1.39 |
| Lymphocyte dose > median | .860 | 1.176 | 0.19 | 7.21 |
| T-cell dose > median | .820 | 1.207 | 0.23 | 6.30 |
| NRM | ||||
| >56 years old | .360 | 1.530 | 0.61 | 3.84 |
| Mismatched unrelated donor (9/10) | .014 | 2.970 | 1.24 | 7.10 |
| OS | ||||
| Mismatched unrelated donor (9/10) | .012 | 3.449 | 1.32 | 9.02 |
| Alemtuzumab T-cell depletion | .127 | 0.560 | 0.27 | 1.18 |
| High HCT-CI | .008 | 2.525 | 1.27 | 5.03 |
| NK dose > median | .102 | 0.547 | 0.27 | 1.13 |
| . | P . | Hazard ratio . | CI (2.5%) . | CI (97.5%) . |
|---|---|---|---|---|
| RI | ||||
| >56 years old | .069 | 0.477 | 0.22 | 1.06 |
| Alemtuzumab T-cell depletion | .018 | 0.424 | 0.21 | 0.87 |
| Sibling transplant | <.001 | 4.588 | 1.99 | 10.60 |
| High HCT-CI | <.001 | 4.097 | 1.82 | 9.24 |
| Lymphocyte dose > median | .730 | 1.148 | 0.52 | 2.55 |
| NK dose > median | <.001 | 0.097 | 0.03 | 0.33 |
| aGVHD | ||||
| Female to male transplant | .320 | 2.049 | 0.49 | 8.52 |
| Mismatched unrelated donor (9/10) | .030 | 7.988 | 1.23 | 51.96 |
| Sibling transplant | .720 | 0.649 | 0.06 | 7.02 |
| Full-intensity conditioning | .006 | 11.414 | 2.01 | 64.73 |
| CD34 dose > median | .130 | 0.341 | 0.08 | 1.39 |
| Lymphocyte dose > median | .860 | 1.176 | 0.19 | 7.21 |
| T-cell dose > median | .820 | 1.207 | 0.23 | 6.30 |
| NRM | ||||
| >56 years old | .360 | 1.530 | 0.61 | 3.84 |
| Mismatched unrelated donor (9/10) | .014 | 2.970 | 1.24 | 7.10 |
| OS | ||||
| Mismatched unrelated donor (9/10) | .012 | 3.449 | 1.32 | 9.02 |
| Alemtuzumab T-cell depletion | .127 | 0.560 | 0.27 | 1.18 |
| High HCT-CI | .008 | 2.525 | 1.27 | 5.03 |
| NK dose > median | .102 | 0.547 | 0.27 | 1.13 |
Patient characteristics or stem cell product cell dose with a univariate P < .2 association with a clinical outcome were included in a multivariate model to assess independent predictors in a regression model. (P < .2 in bold.) Fine and Gray regression method was used for RI, aGVHD, and NRM to take competing risk into account. Cox proportional hazard regression analysis was used to identify independent associations with OS.
CI, confidence interval.
Several variables were associated with risk of developing aGVHD of grade 2 or greater but only HLA mismatch and full-intensity conditioning remained significant upon multivariate analysis. HLA mismatch was also the only independent predictor of NRM. Finally, the use of HLA mismatch between patient and donor, HCT-CI, type of T-cell depletion, and NK dose were also found to be significant in univariate analysis of predictors for OS, but only HLA mismatch and HCT-CI remained as independent predictive risk factors following multivariate analysis.
The number of CD56dim NK cells within the stem cell graft is the predominant determinant of the risk of disease relapse
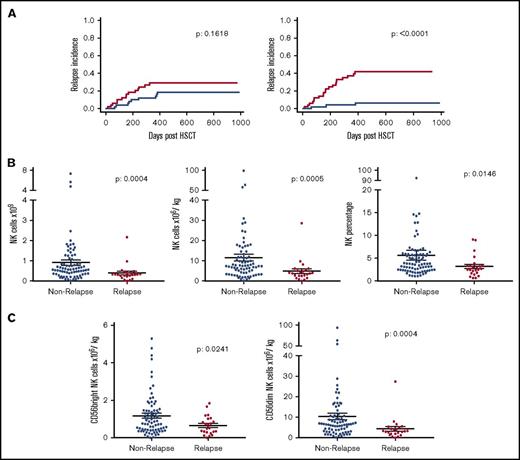
In order to investigate the mechanisms underlying the association between infusion of a high NK cell dose and relative protection from disease relapse, we next went on to examine if a specific NK cell subset was associated with this effect. The mean NK cell dose that had been given in the stem cell graft in patients who went on to suffer disease relapse was 4.9 × 106/kg compared with an average of 11.5 × 106/kg in patients who did not relapse within 2 years following allo-HSCT (Figure 3B). This was mirrored by the lower average total NK cell number (0.39 × 109) and frequency of NK cells (3.2% of all lymphocytes) received in the stem cell graft by patients who relapsed compared with those who did not (0.91 × 109 and 5.7% of all lymphocytes, respectively).
CD56dimNK cells within the stem cell product are the major contributing factor to the association with disease relapse risk. Relationship between the number of CD56bright and CD56dim NK cells administered within the stem cell infusion and RI. The median dose of both subsets was defined, and transplants were divided into those where the infusion contained a cell dose either above (blue) or below this median (red) value (A). The cohort was then split into patients who relapsed or not within 2 years posttransplant to assess absolute number (n = 100), dose (n = 100), and percentage (n = 107) of all NK cells within the stem cell product (B). This was also shown in CD56bright and CD56dim NK cells (n = 100) (C). All NK groups proved to be significantly reduced in relapse patients.
CD56dimNK cells within the stem cell product are the major contributing factor to the association with disease relapse risk. Relationship between the number of CD56bright and CD56dim NK cells administered within the stem cell infusion and RI. The median dose of both subsets was defined, and transplants were divided into those where the infusion contained a cell dose either above (blue) or below this median (red) value (A). The cohort was then split into patients who relapsed or not within 2 years posttransplant to assess absolute number (n = 100), dose (n = 100), and percentage (n = 107) of all NK cells within the stem cell product (B). This was also shown in CD56bright and CD56dim NK cells (n = 100) (C). All NK groups proved to be significantly reduced in relapse patients.
The expression level of CD56 was distinguishable through flow cytometry (Figure 1A) and was used to divide NK cells into immature CD56bright and mature effector CD56dim NK cells (Figure 3A,C). We therefore examined how the number of these 2 subsets within the stem cell infusion was related to relapse risk. The risk of disease relapse was not influenced by infusion of doses of CD56bright NK cells above or below the median value. Importantly, however, the RI at 2 years posttransplant for patients who received at least the median dose of 5.7 × 106/kg CD56dim NK cells was only 8.3% compared with 43% in those who received a dose below this value (P < .0001). In relation to average values, patients who went on to relapse had received lower doses of both CD56bright (0.65 × 106/kg) and CD56dim cells (4.3 × 106/kg) compared with those who did not relapse (1.2 × 106/kg and 10.4 × 106/kg, respectively). These observations indicate that the number of mature cytotoxic effector CD56dim NK cells infused within the stem cell graft is a powerful determinant of the strength of the GVL effect following allogeneic transplantation.
Low expression of DNAM on CD56dim NK cells within the stem cell graft is associated with an increased risk of disease relapse
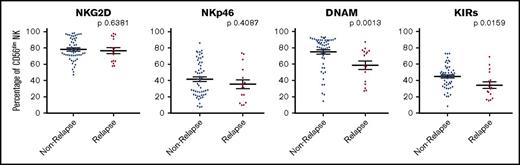
We next examined if features related to the phenotype of infused CD56dim NK cells could further stratify the risk of disease relapse within the patient cohort. Cell surface expression of the dominant activatory receptors NKG2D, NKp46, and DNAM, and the inhibitory KIR receptors CD158a/h, CD158b, and CD158e were examined by flow cytometry (supplemental Figure 2). The expression of NKG2D and NKp46 on CD56dim NK cells did not vary between patients who relapsed compared with those who did not relapse. However, lower expression levels of both the KIR proteins (P = .0159) and, even more strikingly, the transmembrane activatory glycoprotein DNAM (P = .0013) on CD56dim NK cells were associated with increased risk of disease relapse (Figure 4). These data suggest that the protective effect of high NK cell numbers within the stem cell graft may be partially dependent on the DNAM+ CD56dim subset.
Patients receiving higher doses of DNAM+CD56dim NK cells per kg within stem cell product demonstrate a reduced incidence of disease relapse. The patient cohort was divided into patients who relapsed or remained in remission within 2 years posttransplant. Populations of CD56dim NK cells from the stem cell product expressing NKG2D and NKp46 did not associate with relapse, but DNAM- and KIR-expressing cells did (n = 71 for all phenotypic markers).
Patients receiving higher doses of DNAM+CD56dim NK cells per kg within stem cell product demonstrate a reduced incidence of disease relapse. The patient cohort was divided into patients who relapsed or remained in remission within 2 years posttransplant. Populations of CD56dim NK cells from the stem cell product expressing NKG2D and NKp46 did not associate with relapse, but DNAM- and KIR-expressing cells did (n = 71 for all phenotypic markers).
Discussion
Our findings demonstrate a strong association between the infusion of high numbers of donor NK cells in the stem cell graft and relative protection from disease relapse within the patient. This was shown both in univariate and multivariate models. Mature effector CD56dimDNAM+ NK cells transferred within the graft were found to be the phenotype most associated with this disease relapse protection.
These findings indicate that the number of NK cells infused at the time of transplant is a strong determinant of the potency of the GVL effect of allogeneic transplantation. NK cells have a half-life of ∼2 weeks and undergo active homeostatic proliferation, following transfer into a T-cell–depleted host, to become the dominant lymphocyte population at 1 month posttransplant (supplemental Figure 3).21,22 Interestingly we found no correlation between the dose of CD34+ or NK cells in the stem cell graft and number of NK cells in the peripheral blood at 1 month posttransplant (supplemental Figure 4). However, only a minority of the total lymphoid pool is present within peripheral blood, and as such, the NK cell count does not necessarily reflect the total NK cell population within patient tissue.23 Relatively modest differences in the initial “inoculum” of donor NK cells might translate into relatively profound differences in tumor cell lysis within the first few weeks following transplantation. It is noteworthy that the number of invariant natural killer T cells within the graft has also been shown to be associated with improved progression free survival.24
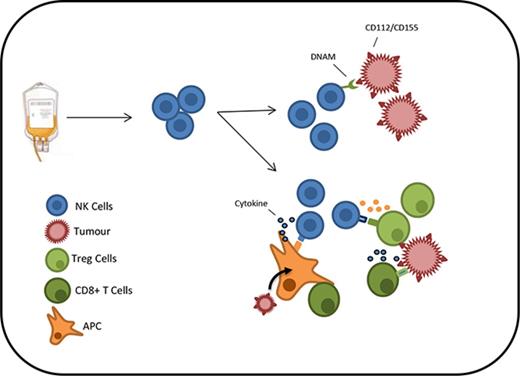
The mechanisms that might underlie this association are unclear at the current time. The most straightforward explanation is that donor NK cells have a direct lytic activity against residual tumor cells within the patient.2,25 NK cells have potent activity against transformed cells, and purified NK cell infusions have been shown to demonstrate antitumor efficacy. However, an alternative possibility is that NK cells might exert an indirect effect on the priming, expansion, or activity of the alloreactive T-cell immune response that develops in the early posttransplant period (Figure 5). As such, larger numbers of NK cells might act to boost T-cell activation, perhaps through mechanisms such as activation of host dendritic cells, or serve to suppress inhibitory cell subsets.26,27 It should also be remembered that the transplant conditioning regimen included T-cell depletion,28-30 and as such, NK cells dominate the lymphoid repertoire during the early phase of immune reconstitution; this may serve to accentuate the clinical impact of the NK cell infusion at the time of transplant.31
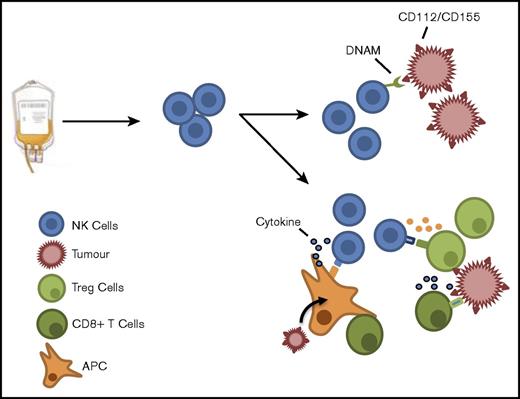
NK cells from the stem cell product may act in either a direct or indirect manner to produce a GVL effect. Residual tumor cells may potentially be eliminated via engagement of DNAM on NK cells with ligands on the tumor cell. In addition, NK cells may act indirectly to boost the alloreactive T-cell response by influencing the immune environment through cytokine release or modulation of host regulatory cell function.
NK cells from the stem cell product may act in either a direct or indirect manner to produce a GVL effect. Residual tumor cells may potentially be eliminated via engagement of DNAM on NK cells with ligands on the tumor cell. In addition, NK cells may act indirectly to boost the alloreactive T-cell response by influencing the immune environment through cytokine release or modulation of host regulatory cell function.
NK cells demonstrate a range of cytotoxic and immunomodulatory functions, and our data on the critical importance of the CD56dim subset indicate that the cytotoxic activity of NK cells is a predominant factor in providing protection against relapse. Further support is provided by the observation that high-level expression of DNAM on donor CD56dim NK cells and the overall dose of DNAM+CD56dim NK cells within the stem cell graft are protective against disease relapse (supplemental Figure 5). DNAM is a dominant activatory molecule on NK cells and serves to trigger cytotoxic activity against a range of targets. CD112 and CD155 are the 2 ligands of DNAM and are expressed by AML blast cells.32 Moreover, DNAM-dependent killing of AML cell lines has been shown in vitro,33 and decreased expression of DNAM has been demonstrated on NK cells taken from patients with AML and is believed to act as a mechanism of tumor evasion.34 Interestingly, the expression of DNAM was increased on CD56dim NK cells in CMV seropositive donors (supplemental Figure 6) and may potentially contribute toward the association between CMV reactivation following allo-HSCT and protection from relapse.35,36 These observations provide a potential mechanism for our findings as “fresh” donor NK cells, which express high levels of DNAM, would be expected to have the potential to directly lyse CD112- or CD155-expressing tumor cells. Previous studies have used donor NK cell infusions as a treatment of acute leukemia, and Miller et al37 reported that 5 out of 19 patients with very high-risk AML were able to obtain a complete response after infusion of haploidentical NK grafts.
The magnitude of the impact of NK dose on risk of disease relapse was striking, and our findings could have considerable impact for clinical practice. Stem cell grafts that contain at least 6.3 × 106 NK cells per kg are associated with 6.7-fold reduction in the risk of relapse without increasing the risk of clinically significant aGVHD or NRM. CD34+ cell dose is already measured within stem cell donations, and it would be relatively straightforward to also assess the number of the CD3−CD56dim cells. Where the NK dose is inadequate, it is likely to be possible to add more cells from processing of residual apheresis product. Alternatively, up to 2 × 107 NK cells per kg can be obtained following a single donor lymphapheresis.37
These findings indicate the profound influence that the initial NK cell infusion has on determining the clinical features following allogeneic stem cell transplantation. Further investigation of the mechanism that underlies this association has the potential to uncover new insights into NK cell biology and may have the potential to greatly improve the clinical outcome of patients undergoing this procedure.
The full-text version of this article contains a data supplement.
Acknowledgments
Mohammad Ashab Uddin and Phil Jenkins collected clinical data as part of Stem Cell Immunotherapies (National Health Service Blood and Transplant, Birmingham, United Kingdom).
This study was funded by Bloodwise (Grant Code: 12052) and the Medical Research Council.
Authorship
Contribution: L.M. designed research, performed research, performed statistical analysis, analyzed and interpreted data, and wrote the manuscript; F.K. designed research, interpreted data, and wrote the manuscript; Y.L.T.C. designed research and assisted in data interpretation; S.E. performed research assisted in data interpretation; D.M. contributed analytical tools and performed statistical analysis; J.N. collected clinical data; J.B. collected clinical samples; C.C. assisted in data interpretation; R.M. assisted in data interpretation and manuscript preparation; J.Z. designed research, interpreted data and wrote the manuscript; and P.M. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Moss, University of Birmingham, Institute of Immunology and Immunotherapy, Dennis Howell Building, Vincent Dr, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: p.moss@bham.ac.uk.
References
Author notes
L.M. and F.K. are joint first authors.
J.Z., R.M., and P.M. are joint senior authors.