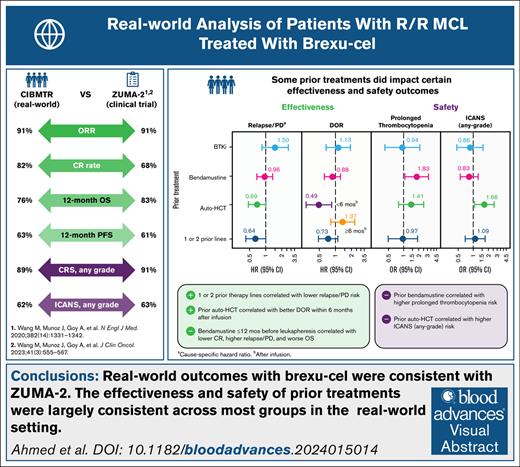
Key Points
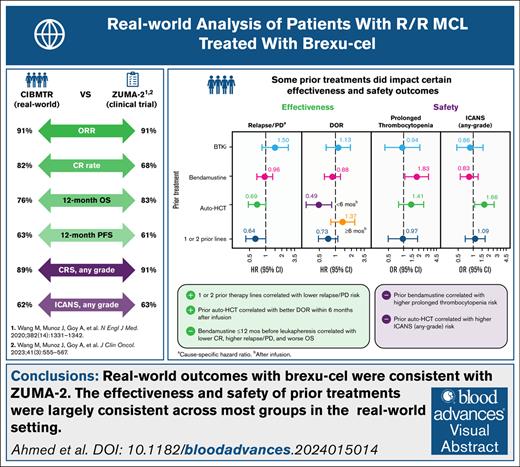
Real-world effectiveness and safety outcomes in patients with R/R MCL treated with brexu-cel are similar to those of the ZUMA-2 trial.
The benefits of brexu-cel extend to patients who have no prior exposure to BTKi therapy or have received few lines of therapy.
Visual Abstract
Brexucabtagene autoleucel (brexu-cel) is a chimeric antigen receptor T-cell therapy approved for relapsed/refractory mantle cell lymphoma (R/R MCL). Here, we report real-world effectiveness and safety outcomes of brexu-cel in a prospective study of patients with R/R MCL, including subgroups based on prior treatment with Bruton's tyrosine kinase inhibitor, bendamustine, or autologous hematopoietic cell transplant (auto-HCT) and number of prior therapy lines, using Center for International Blood and Marrow Transplant Research registry data. A total of 476 patients with R/R MCL who received brexu-cel between July 2020 and December 2022 were included in the analysis. With a median follow-up of 13.5 months, the overall response rate was 91% and complete response rate was 82%. One-year overall survival and progression-free survival rates were 76% and 63%, respectively. One-year cumulative incidence of nonrelapse mortality was 8%. Prior auto-HCT was associated with better duration of response within 6 months after infusion (hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.28-0.85) but greater risk of immune effector cell–associated neurotoxicity syndrome (odds ratio [OR], 1.66; 95% CI, 1.06-2.60). Prior bendamustine was associated with increased risk of prolonged thrombocytopenia (OR, 1.90; 95% CI, 1.13-3.21). In patients with 1 to 2 prior therapy lines, relapse or progression was less frequent compared with those with ≥3 prior lines (HR, 0.64; 95% CI, 0.42-1.00). Collectively, our results suggest that real-world outcomes with brexu-cel were consistent with those of the ZUMA-2 trial, regardless of prior therapy type or number of prior therapy lines.
Introduction
Mantle cell lymphoma (MCL) is a rare and aggressive subtype of B-cell non-Hodgkin lymphoma that accounts for ∼7% of newly diagnosed cases of non-Hodgkin lymphoma and is considered incurable with standard-of-care therapies.1-4 Treatment options for MCL are limited, and most patients are either refractory to frontline chemotherapy or will subsequently relapse.2,3,5,6
Although Bruton's tyrosine kinase inhibitor (BTKi) therapy has greatly improved outcomes in patients with relapsed/refractory (R/R) MCL, prognosis is very poor in those with disease progression after BTKi therapy, and outcomes progressively worsen with each subsequent line of salvage therapy.6-9 In these patients, median overall survival (OS) is 3 to 11 months,1,9-11 necessitating additional therapeutic options.
Brexucabtagene autoleucel (brexu-cel), an autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy, is the first US Food and Drug Administration (FDA)–approved CAR T-cell therapy for adults with R/R MCL.1 In the pivotal phase 2 ZUMA-2 trial, a single infusion of brexu-cel led to durable responses in patients with R/R MCL who were previously treated with a BTKi.12 In the 4-year follow-up analysis of ZUMA-2, median OS was 46.4 months (median OS in patients with a complete response [CR] was 58.7 months).13
The ZUMA-2 eligibility criteria required that patients receive up to 5 lines of prior therapy, including anthracycline- or bendamustine-containing chemotherapy, anti-CD20 monoclonal antibody therapy, and a BTKi (ibrutinib or acalabrutinib), along with additional inclusion criteria based on Eastern Cooperative Oncology Group performance status, as well as renal, hepatic, pulmonary, and cardiac function.12 Initial reports of real-world outcomes with brexu-cel in patients with R/R MCL indicate that favorable outcomes extend to patients beyond the scope of the ZUMA-2 eligibility criteria14,15; however, data on the effect of prior BTKi and other treatments on patient outcomes with CAR T-cell therapy are limited.
The objectives of this study were to assess real-world effectiveness and safety outcomes of brexu-cel in a large population of patients with R/R MCL and to gain a better understanding of how prior treatment history (including prior treatment with BTKi, bendamustine, or autologous hematopoietic cell transplant [auto-HCT], and number of prior lines of systemic therapy) might affect these outcomes.
Methods
Study design and patients
A prospective postmarketing requirement (PMR) study using the Center for International Blood and Marrow Transplant Research (CIBMTR) data infrastructure to capture long-term effectiveness and safety data for brexu-cel in patients with R/R MCL in the United States is ongoing (supplemental Methods). This analysis involved secondary use of data from the PMR study and was conducted in compliance with ethical principles described in the Declaration of Helsinki.
The target enrollment of 500 patients for the PMR study was completed in December 2022; CIBMTR patient registry data, collected up to 26 December 2023, were used as the basis for this analysis. Eligible patients aged ≥18 years with R/R MCL received brexu-cel as standard-of-care treatment between July 2020 and December 2022 and provided informed consent for participation in the CIBMTR research database. Patients who were enrolled in clinical trials were excluded. Patients with prior nontransplant cellular therapy (including prior CAR T-cell therapy), effectiveness and/or safety data not due or not reported, or incomplete baseline data were also excluded. Patient subgroups based on prior therapy (before leukapheresis and not including bridging therapy) were not mutually exclusive. Bridging therapy comprised any anticancer treatment (including bendamustine and BTKis) given between leukapheresis and lymphodepletion, including treatments initiated before leukapheresis.
End points and assessments
Effectiveness end points assessed included objective response rate (ORR), CR and partial response (PR) rates, time to CR (TtCR), duration of response (DOR), relapse or progressive disease (relapse/PD), progression-free survival (PFS), and OS. Responses were evaluated by positron emission tomography or computerized tomography assessments (using the 2014 Lugano classification16) obtained after the first dose of brexu-cel and before any subsequent treatment given for relapse/PD. Definitions of effectiveness end points are detailed in the supplemental Methods.
Safety end points included the incidence, severity, time to onset, and duration of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS); prolonged cytopenia (including neutropenia and thrombocytopenia); clinically significant infections; subsequent neoplasms; nonrelapse mortality (NRM); and causes of death. CRS and ICANS grading was based on the American Society for Transplantation and Cellular Therapy consensus criteria.17 CRS and ICANS durations were defined as time from onset of symptoms to resolution, with death without resolution treated as a competing risk. Patients with ongoing CRS or ICANS at the data cutoff date were censored at date of last contact or retreatment with brexu-cel, whichever occurred first. Additional safety end points are described in the supplemental Methods.
Statistical analysis
Descriptive analyses for outcomes were conducted for the overall population, subgroups of interest, and prior BTKi type (among patients who received only 1 type of BTKi). Percentages and 95% Clopper-Pearson exact confidence intervals (CIs) were calculated for dichotomous outcomes (ORR, CR and PR rates, CRS, ICANS, and prolonged cytopenia). DOR, PFS, and OS were estimated using the Kaplan-Meier method. Cumulative incidence functions were used to estimate CRS and ICANS duration, TtCR, relapse/PD, and NRM. Statistical analyses used for subgroup comparisons are described in the supplemental Methods.
For the multivariable analyses, biological and clinical judgments were made to preselect clinically relevant exposure variables, followed by a stepwise empirical method to determine the final selection of covariates. To avoid collinearity, the main variables of interest were forced into 2 separate models (model 1: prior BTKi, bendamustine, and auto-HCT; model 2: number of prior therapy lines). To assess the impact of the timing of bendamustine use before leukapheresis, a sensitivity analysis of model 1 was conducted, categorizing prior bendamustine timing as ≤12 months before leukapheresis, >12 months before leukapheresis, or no prior bendamustine (including prior BTKis and auto-HCT). Additional details regarding the variable selection process and other key variables for stepwise selection in both models are described in the supplemental Methods. To assess the impact of prior treatment patterns, a multivariable logistic regression model was used to estimate odds ratios (ORs) with 95% CIs for ORR, CR rate, CRS, ICANS, and prolonged cytopenia. Hazard ratios (HRs) for DOR, PFS, and OS, or cause-specific HRs for TtCR, relapse/PD, and NRM were estimated with 95% CIs using a multivariable Cox proportional hazards model. For analysis of DOR, a time-dependent covariate was used to estimate the effect of prior auto-HCT, with 6 months identified as the optimal cutoff point. All statistical tests were 2-sided with a significance level of 0.05. All analyses were conducted using SAS 9.4 (M1).
Results
Patient disposition and baseline characteristics
Data from 500 patients with R/R MCL treated with commercial brexu-cel between July 2020 and December 2022 across 84 centers in the United States (n = 83) and Canada (n = 1) who were registered in the CIBMTR database for the US PMR study were available for this prospective, observational investigation; 476 patients with their first follow-up form completed by day 100 were included in the full analysis (supplemental Figure 1). A total of 24 patients were excluded owing to clinical trial enrollment (n = 1), prior history of non-HCT cellular therapy (eg, CAR T-cell therapy; n = 7), patient baseline data forms not submitted (n = 14), or effectiveness and/or safety follow-up data not reported (n = 2). The subgroup analysis set included 469 patients; 7 patients were excluded from the full analysis set due to missing data on prior treatment (supplemental Figure 1). At the data cutoff date (26 December 2023), the median follow-up among all patients was 13.5 months (range, 0-37.3).
Baseline characteristics for the overall population (N = 476) and subgroups are summarized in Table 1 and supplemental Table 2. In the overall population, the median age was 67.0 years (range, 34.1-84.9) and 76% were male. Before infusion, 5% of patients had an Eastern Cooperative Oncology Group performance status score of ≥2, 76% had clinically significant comorbidities (defined in the supplemental Methods), and 4% had active central nervous system involvement. At diagnosis, 184 of 271 patients (68%) had Ki-67 proliferation index of ≥30%, 117 of 271 (43%) had Ki-67 proliferation index of ≥50%, and 49 of 253 (19%) had TP53 or 17p deletion status. Patients received a median of 4 prior therapy lines (range, 1-12), with 78% having received ≥3 prior therapy lines. Before leukapheresis, 88% of patients had received ≥1 BTKi (ibrutinib, 47%; acalabrutinib, 37%; zanubrutinib, 15%; and other BTKis, <1%), 55% had received bendamustine, and 30% were prior auto-HCT recipients. Among patients with reported timing of prior bendamustine use (n = 225), the median time from bendamustine treatment to leukapheresis was 16.8 months (interquartile range [IQR], 5.5-40.0), with 91 of 225 patients (40%) having received bendamustine within 12 months before leukapheresis and 134 of 225 (60%) at >12 months before leukapheresis. The median time from leukapheresis to infusion was 28 days (IQR, 26-34); 45% of patients received bridging therapy (including BTKi [20%] or bendamustine [4%]), and 9% of patients were planned to receive their infusions in the outpatient setting. Among all 476 patients included in the analysis population, up to ∼70% were potentially ineligible for ZUMA-2, primarily due to pulmonary or cardiovascular comorbidities or inadequate hematologic parameters (supplemental Table 3).
Most baseline characteristics were consistent across prior therapy subgroups (Table 1; supplemental Table 2). BTKi-naive patients had a lower median number of prior therapy lines, and lower proportions of these patients had a Ki-67 proliferation index of ≥50%, had been treated with bendamustine, or received bridging therapy vs patients with prior BTKi exposure. A lower proportion of patients who received ≥3 prior therapy lines had TP53 or 17p deletion at diagnosis, and a higher proportion were previously treated with a BTKi or bendamustine or had received prior auto-HCT or bridging therapy, compared with patients who received 1 to 2 prior therapy lines. Among patients who received bridging therapy, a greater proportion of those who received 1 to 2 prior therapy lines (48%) experienced a CR or PR to bridging therapy vs those who received ≥3 prior therapy lines (26%). Baseline characteristics for the bendamustine and auto-HCT subgroups are described in the supplemental Results.
Univariate analysis of effectiveness outcomes
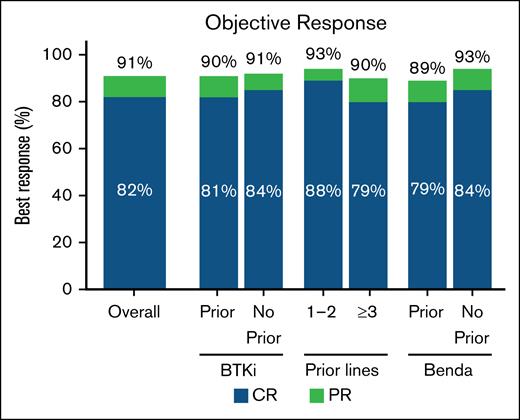
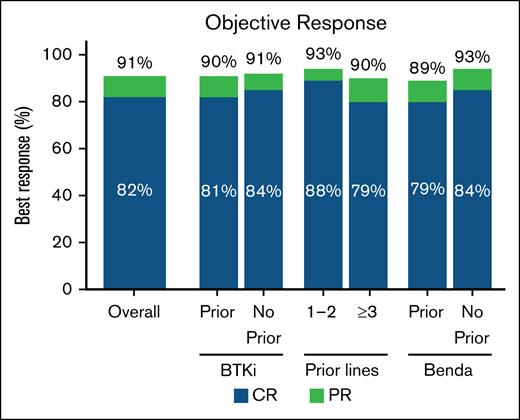
In the overall population, the ORR with brexu-cel was 91% (95% CI, 87-93); the CR rate was 82% (95% CI, 78-85; Figure 1; supplemental Table 4).
ORR in the overall population and BTKi, number of prior therapy lines, and bendamustine subgroups. Best response rate (CR or PR) in the overall population (n = 455), and in prior BTKi (n = 393), no prior BTKi (n = 55), 1 to 2 prior therapy lines (n = 99), ≥3 prior therapy lines (n = 349), prior Benda (n = 244), and no prior Benda (n = 204) subgroups. Benda, bendamustine.
ORR in the overall population and BTKi, number of prior therapy lines, and bendamustine subgroups. Best response rate (CR or PR) in the overall population (n = 455), and in prior BTKi (n = 393), no prior BTKi (n = 55), 1 to 2 prior therapy lines (n = 99), ≥3 prior therapy lines (n = 349), prior Benda (n = 244), and no prior Benda (n = 204) subgroups. Benda, bendamustine.
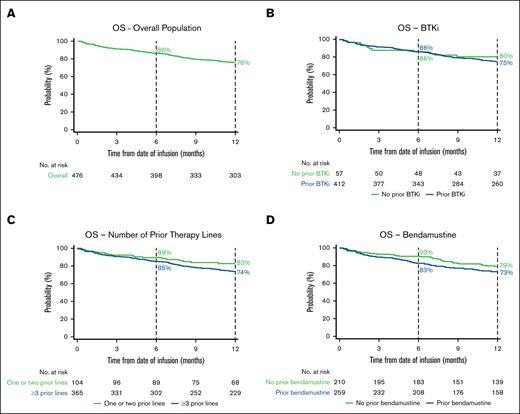
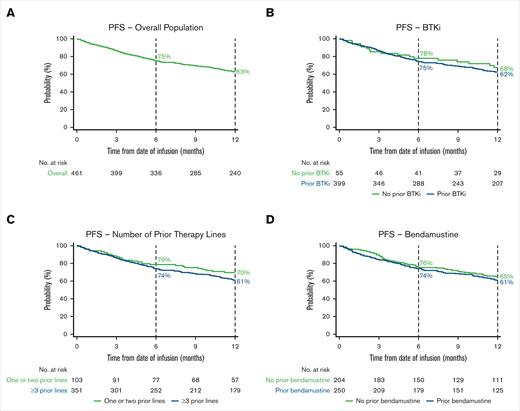
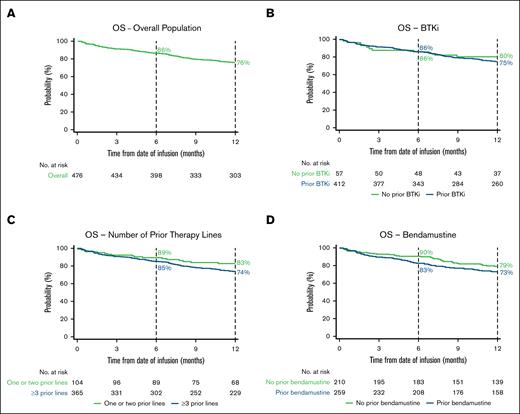
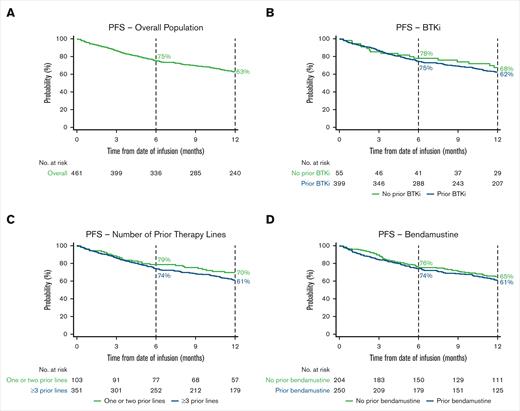
The 6-month OS rate was 86% (95% CI, 83-89), and the 12-month OS rate was 76% (95% CI, 72-80; Figure 2A; supplemental Table 4). The 6-month PFS rate was 75% (95% CI, 71-79), and the 12-month PFS rate was 63% (95% CI, 58-67; Figure 3A; supplemental Table 4).
Kaplan–Meier estimates of the proportion of patients with OS over time. OS in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. Vertical dashed lines indicate 6- and 12-month landmarks.
Kaplan–Meier estimates of the proportion of patients with OS over time. OS in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. Vertical dashed lines indicate 6- and 12-month landmarks.
Kaplan–Meier estimates of the proportion of patients with PFS over time. PFS in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. Vertical dashed lines indicate 6- and 12-month landmarks.
Kaplan–Meier estimates of the proportion of patients with PFS over time. PFS in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. Vertical dashed lines indicate 6- and 12-month landmarks.
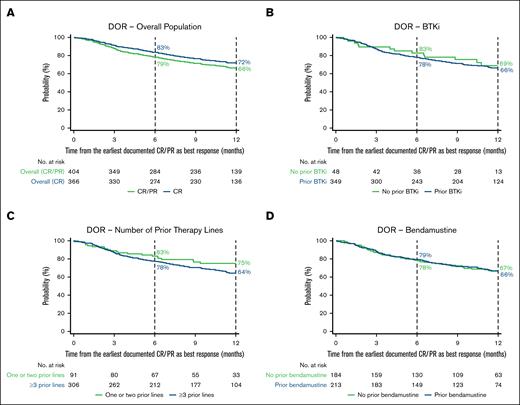
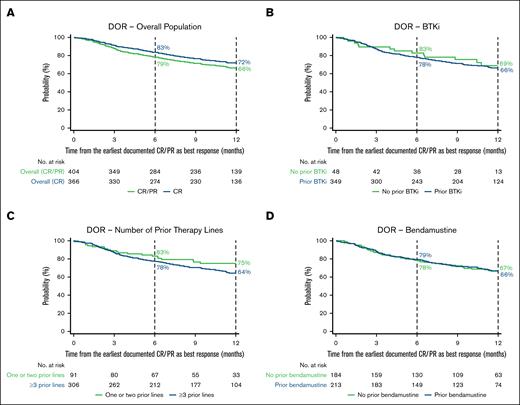
Among patients who achieved CR or PR as a best response (n = 412), the proportion of patients with an ongoing response 6 and 12 months after initial response was 79% (95% CI, 74-82) and 66% (95% CI, 61-71), respectively (Figure 4A; supplemental Table 4). In patients who achieved CR as a best response (n = 371), the proportion of patients remaining in response at 6 and 12 months after initial response was 83% (95% CI, 79-87) and 72% (95% CI, 67-76), respectively. In patients who achieved PR as a best response (n = 41), the proportion with an ongoing response 6 and 12 months after initial response was 31% (95% CI, 17-46) and 13% (95% CI, 4-28), respectively.
Kaplan–Meier estimates of the proportion of patients with an ongoing response over time. DOR in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. In panel A, CR and CR/PR denote best responses achieved. Vertical dashed lines indicate 6- and 12-month landmarks.
Kaplan–Meier estimates of the proportion of patients with an ongoing response over time. DOR in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. In panel A, CR and CR/PR denote best responses achieved. Vertical dashed lines indicate 6- and 12-month landmarks.
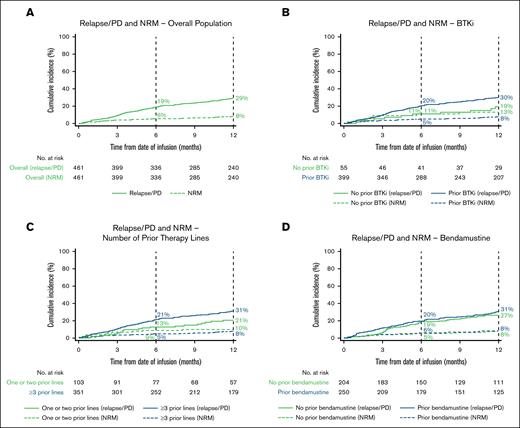
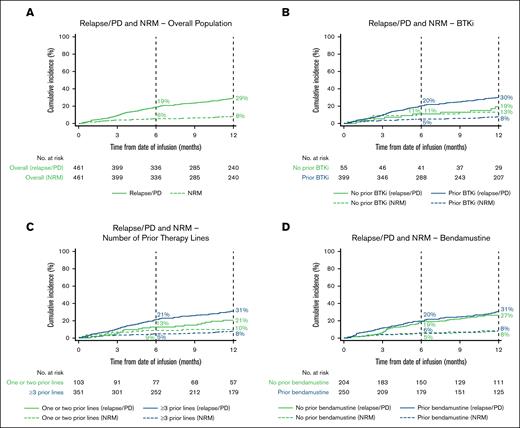
The estimated cumulative incidence of relapse/PD at 12 months was 29% (95% CI, 25-33; Figure 5A; supplemental Table 4).
Estimated cumulative incidence of the proportion of patients with relapse/PD and NRM over time. Relapse/PD and NRM in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. Vertical dashed lines indicate 6- and 12-month landmarks.
Estimated cumulative incidence of the proportion of patients with relapse/PD and NRM over time. Relapse/PD and NRM in the overall population (A) and BTKi (B), number of prior therapy lines (C), and bendamustine (D) subgroups. Vertical dashed lines indicate 6- and 12-month landmarks.
Safety and univariate analysis of safety outcomes
In the overall population, the rate of any-grade CRS was 89% (95% CI, 86-92) and the rate of grade ≥3 CRS was 11% (95% CI, 8-14). Median time to CRS onset was 5 days (IQR, 2-7); median duration of CRS was 6 days (IQR, 4-8). The rate of any-grade ICANS was 62% (95% CI, 57-66) and the rate of grade ≥3 ICANS was 30% (95% CI, 26-34; Table 2). Median time to ICANS onset was 7 days (IQR, 6-10); median duration of ICANS was 8 days (IQR, 4-15). CRS and ICANS events resolved by 3 weeks from onset in 95% (95% CI, 92-96) and 76% (95% CI, 71-81) of patients, respectively. Grade 5 CRS occurred in 9 of 473 patients (2%) and grade 5 ICANS occurred in 9 of 459 (2%); 2 patients experienced both grade 5 CRS and ICANS. Medications for management of CRS and/or ICANS included tocilizumab (76%), corticosteroids (61%), anakinra (16%), and siltuximab (5%). In patients who received prophylaxis for CRS and/or ICANS (n = 426), antiepileptic agents were used for ICANS prophylaxis in 55% of patients; tocilizumab and corticosteroids were used for CRS and/or ICANS prophylaxis in 7% and 3% of patients, respectively.
In patients who were alive at day 30 after infusion (n = 457), prolonged neutropenia (present on, or after, day 30) occurred in 14% (95% CI, 11-18) of patients and prolonged thrombocytopenia occurred in 19% (95% CI, 16-23; Table 2). Clinically significant infections occurring at any time point were reported in 50% of patients. Viral, bacterial, and fungal infections occurred in 35%, 24%, and 5% of patients, respectively (Table 2); types of infection were not mutually exclusive. Of 164 patients with reported viral infection, 104 (63%) had COVID-19. Among patients with available data, 204 of 275 (74%) had received a COVID-19 vaccine. Subsequent neoplasms (not including disease progression/primary disease; predominantly basal cell and squamous cell skin cancers and myelodysplasia) developed in 9% of all patients (Table 2).
The estimated cumulative incidence rate of NRM was 4% (95% CI, 3-6) at day 100, 6% (95% CI, 4-8) at day 180, and 8% (95% CI, 6-11) at year 1 (Figure 5A; Table 2). In patients who experienced NRM (n = 46), the leading cause of NRM during the first 100 days after brexu-cel infusion (n = 19) was bacterial infection (5/19 [26%]) and after ≥100 days (n = 27) was COVID-19 infection (11/25 reported cases [44%]), which was the only viral infection that led to NRM. The leading cause of death in patients who died at any time during follow-up was primary disease (81/150 [55%]; supplemental Table 6).
Multivariable analysis of effectiveness and safety outcomes
In multivariable analyses adjusted for key prognostic factors, effectiveness and safety outcomes were consistent across most subgroups (supplemental Table 9).
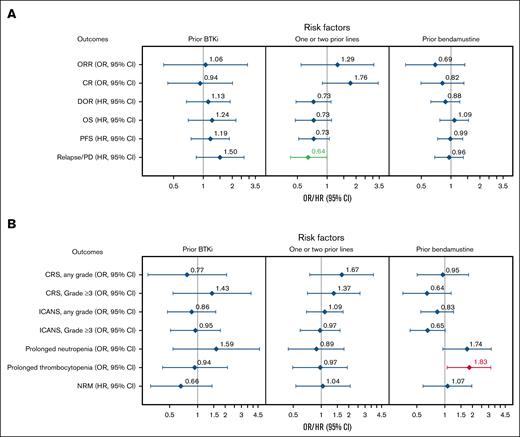
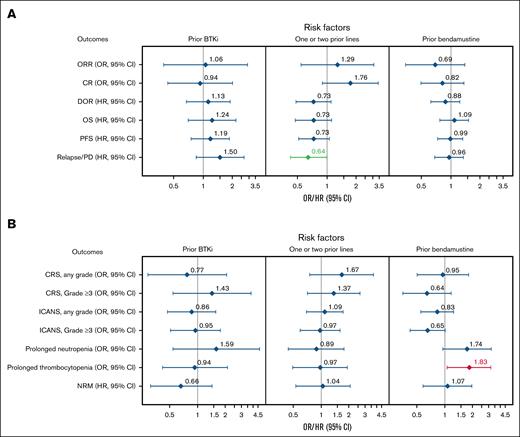
In multivariable analyses for effectiveness outcomes, patients with 1 to 2 prior therapy lines had a lower risk of relapse/PD compared with those who received ≥3 prior lines (HR, 0.64; 95% CI, 0.42-1.00; Figure 6A). The CR rate trended higher, and DOR and OS were numerically better in patients with 1 to 2 prior therapy lines vs ≥3 prior lines. Prior auto-HCT recipients had a better DOR within the first 6 months after infusion (supplemental Figure 3A), with an associated reduced risk of relapse (model 1 [HR, 0.49; 95% CI, 0.28-0.85] and model 1 sensitivity analysis [HR, 0.50; 95% CI, 0.28-0.89]), compared with those who did not receive prior auto-HCT. Similar effectiveness outcomes were observed regardless of prior BTKi or bendamustine treatment (Figure 6A). Notably, although there was no overall difference in outcomes when comparing patients who received prior bendamustine with those who did not, patients who received bendamustine therapy within 12 months before leukapheresis had a lower CR rate, a higher risk of relapse/PD, and worse OS compared with those who received bendamustine >12 months before leukapheresis (supplemental Table 10).
Subgroup analysis of effectiveness and safety outcomes with multivariate adjustment. Forest plots of effectiveness (A) and safety (B) outcomes adjusted for key baseline and clinical covariates in BTKi, number of prior therapy lines, and bendamustine subgroups. Variables with multivariate P value < 0.05 are highlighted in green (favorable) or red (unfavorable).
Subgroup analysis of effectiveness and safety outcomes with multivariate adjustment. Forest plots of effectiveness (A) and safety (B) outcomes adjusted for key baseline and clinical covariates in BTKi, number of prior therapy lines, and bendamustine subgroups. Variables with multivariate P value < 0.05 are highlighted in green (favorable) or red (unfavorable).
In multivariable analyses for safety outcomes, prior bendamustine was associated with an increased risk of prolonged thrombocytopenia vs no prior bendamustine (OR, 1.90; 95% CI, 1.13-3.21; Figure 6B). Prior auto-HCT was associated with a greater risk of any-grade ICANS compared with no prior auto-HCT (model 1 [OR, 1.66; 95% CI, 1.06-2.60] and model 1 sensitivity analysis [OR, 1.63; 95% CI, 1.02-2.59]; supplemental Figure 3B). Comparable safety profiles were observed regardless of prior BTKi or number of prior therapy lines (Figure 6B).
Baseline variables that were significant for effectiveness and safety outcomes, along with additional multivariable analyses data, are described in the supplemental Results.
Discussion
This CIBMTR study represents, to our knowledge, the largest real-world analysis to date of the effectiveness and safety of brexu-cel in patients with R/R MCL. Here, we show that outcomes with brexu-cel in the real-world setting are consistent with those reported in the ZUMA-2 trial, similar in patients regardless of BTKi treatment status, and may be improved in patients who receive brexu-cel in earlier lines. Moreover, this study complements other real-world studies demonstrating therapeutic benefit with brexu-cel in R/R MCL, with clinically meaningful effectiveness and a consistent safety profile.14,15
Although up to ∼70% of patients in our study were potentially ineligible for ZUMA-2 (based on comorbidities and hematologic parameters), real-world outcomes with brexu-cel were consistent with those of ZUMA-2. The ORRs both here and in ZUMA-2 were 91%; the CR rate was 82% in this study and 68% in ZUMA-2.18 The estimated 12-month OS and PFS rates were 76% and 63%, respectively, in this analysis (median follow-up, 13.5 months), and 83% and 61%, respectively, in ZUMA-2 (median follow-up, 12.3 months).12 Here, the incidence of any-grade CRS was 89% (grade ≥3; 11%) and the incidence of any-grade ICANS was 62% (grade ≥3; 30%). In ZUMA-2, the incidence of any-grade CRS (per Lee 2014 criteria19) was 91% (grade ≥3; 15%) and the incidence of any-grade neurologic events (per Common Terminology Criteria for Adverse Events) was 63% (grade ≥3; 31%).12
Notably, the 8% NRM rate at 1 year in this study was similar to the 9.1% NRM rate observed in the US Lymphoma CAR T Consortium analysis that evaluated the safety and effectiveness of brexu-cel in patients with R/R MCL in standard-of-care practice, with infection being the primary cause of NRM in both studies.15 COVID-19 and bacterial infections remain a significant cause of NRM after CAR T-cell therapy, with COVID-19 being the leading cause of death from infection after day 100 in this study. Hence, improved strategies are needed to mitigate infection in patients.20,21 In our study, patients underwent brexu-cel treatment in the initial years of the COVID-19 pandemic. The CIBMTR began capturing COVID-19 vaccination status in July 2021. In this study, among patients with reported data since then, 74% had been administered a COVID-19 vaccination. The long-term trajectory of COVID-19–related mortality remains uncertain, particularly with improvements in prognosis over time.
The large sample size of this study allowed for a robust analysis of subgroup outcomes. In subgroups defined by prior treatment with BTKi, bendamustine, or auto-HCT, and number of prior therapy lines, we found that effectiveness and safety outcomes were generally similar to those of the overall population and largely consistent, regardless of prior therapy type or number of prior therapy lines. Prior auto-HCT was associated with improved DOR for the first 6 months after infusion, although this effect was not sustained beyond 6 months. Patients who had prior auto-HCT were younger than those who did not; hence, durable responses may be a reflection of their lower median age. Additionally, given that patients who receive auto-HCT typically have disease that is responsive to treatment, they may have different disease biology compared with those without prior auto-HCT. Patients with prior auto-HCT had an increased risk of any-grade ICANS compared with patients without. The relationship between prior auto-HCT and increased risk of ICANS is likely very complex and may involve a combination of disease- and treatment-related factors (eg, prior bendamustine use had a nonsignificant trend with increased ICANS), as well as immunological considerations.
BTKis are oral therapies that are well tolerated and highly effective for certain hematologic malignancies. Some studies suggest that combining a BTKi with CAR T-cell therapy can improve CAR T-cell proliferation, engraftment, and effector function, and may provide a more efficacious treatment than either agent alone.22 Given that the FDA approval of commercial brexu-cel is irrespective of prior BTKi use, it was important to determine whether outcomes with brexu-cel in the real-world setting differed based on prior BTKi use. In BTKi-naive patients, we showed that the ORR and CR rate were high (91% and 84%, respectively) and 12-month OS and PFS rates were 80% and 68%, respectively. Moreover, there were no significant differences in effectiveness or safety outcomes in BTKi-naive patients vs those who were BTKi exposed after adjusting for differences in other clinical factors. Notably, real-world outcomes among patients who were BTKi naive and those who had previously received a BTKi were similar to those observed in the pivotal ZUMA-2 cohort 1 study (which required prior treatment with a BTKi) and the recent ZUMA-2 cohort 3 study (including only patients with R/R MCL who had not received prior BTKi therapy),12,23 suggesting agreement between clinical trial results and real-world data. Although there may be differences in how distinct BTKi agents affect CAR T-cell efficacy,22,24 we found no significant differences in outcomes between patients who received ibrutinib, acalabrutinib, or zanubrutinib in our analysis.
Recent studies suggest that bendamustine, an agent commonly used for the treatment of MCL,25 could attenuate T-cell fitness and consequently affect the manufacturing and function of CAR T cells, thereby negatively affecting treatment outcomes.26,27 These observations are consistent with findings from the 3-year follow-up of ZUMA-2, in which lower peak CAR T-cell levels and product doubling times were seen in some patients exposed to bendamustine therapy within 6 months of leukapheresis.18 Moreover, data from the US Lymphoma CAR T Consortium showed higher rates of manufacturing failure and failure to infuse in patients exposed to bendamustine within the 6-month period before leukapheresis compared with those who received bendamustine therapy much earlier or not at all, suggesting that the timing of bendamustine therapy relative to brexu-cel manufacturing and infusion could be important.15 In our study, based on a much larger patient population, patients who were exposed to bendamustine within 12 months before leukapheresis had lower CR rates, a greater likelihood of relapse/PD, and shorter OS compared with those who received bendamustine >12 months before leukapheresis. However, overall outcomes did not differ between patients who received prior bendamustine therapy vs those who did not. In contrast to the US Lymphoma CAR T Consortium study, in which a large proportion of patients in the bendamustine cohort had high-risk disease features, in our study, baseline disease characteristics were similar in patients who received prior bendamustine compared with those who did not, which may explain why effectiveness outcomes were similar. The incidence of prolonged thrombocytopenia was higher in patients with prior bendamustine therapy compared with those who did not receive bendamustine, likely due to bendamustine-induced myelosuppression.28
The National Comprehensive Cancer Network recommends brexu-cel as a second-line or later treatment option for patients with R/R MCL after prior treatment with induction chemotherapy.29 Combined American Society for Transplantation and Cellular Therapy/CIBMTR/European Society for Blood and Marrow Transplantation guidelines recommend brexu-cel for patients with R/R MCL who are intolerant to, or relapse after, treatment with at least 1 BTKi.30,31 By comparison, the FDA approval for brexu-cel includes all patients with R/R MCL, regardless of prior therapy.32 As such, in the real-world setting, patients may receive brexu-cel as early as the second treatment line; however, there has been a paucity of data describing the effectiveness and safety in patients who receive brexu-cel in early lines of therapy. Notably, we found that patients with 1 to 2 prior therapy lines had less relapse/PD compared with those who received ≥3 prior therapy lines. Whether reduced relapse/PD in patients with 1 to 2 prior therapy lines is an indication of better T-cell fitness/quality or that patients who receive more prior therapy lines have more refractory or higher risk disease is not known. Moreover, the observed numerical, but not statistically significant, improvements in CR rate, DOR, and OS in patients with 1 to 2 prior therapy lines could have been affected by the small sample size of this subgroup compared with that of patients who received ≥3 prior lines.
Although the large number of patients included in the CIBMTR analysis set is a major strength of this study, its relatively short 13.5-month follow-up is a limitation (eg, longer follow-up is required to estimate longer-term OS and PFS rates). Moreover, the US Lymphoma CAR T Consortium study and a recent UK intention-to-treat analysis found that 11% and 20% of patients with R/R MCL who underwent leukapheresis, respectively, did not proceed to infusion.15,33 Given that the CIBMTR collected data only on patients who received brexu-cel, results of this study only represent effectiveness and safety outcomes in patients who actually received a brexu-cel infusion. Additionally, due to the observational nature of this study, there may be confounding variables for which data cannot be fully adjusted using statistical methods. Subgroups based on BTKi exposure were not balanced in terms of patient numbers, which could potentially affect the statistical power of the analysis. Moreover, patients with missing data were excluded and data regarding the timing and exposure to prior bendamustine and BTKis, as well as high-risk subgroups of interest (eg, TP53 mutation status, complex karyotype), were not available. This analysis did not specifically focus on effectiveness and safety of brexu-cel in high-risk patients; however, these will be a focus of future subgroup analyses of the CIBMTR data set.
In conclusion, this study is an important contribution to the growing body of real-world data on the use of brexu-cel therapy for patients with R/R MCL. Here, we have provided evidence that the effectiveness and safety of brexu-cel in a real-world population are similar to those seen in ZUMA-2 and that the high response rates and clinically meaningful benefit of brexu-cel extend to patients with R/R MCL who have no prior exposure to BTKi therapy or have received few lines of therapy.
Acknowledgments
The authors thank all investigators, coordinators, study-site personnel, and patients and their families for participating in this study. Medical writing support was provided by Laurie Baggio, of Avalere Health, with funding provided by Kite, a Gilead company.
This study was funded by the National Cancer Institute (CIDR [U24 CA233032]) and Kite, a Gilead Company. The Center for International Blood and Marrow Transplant Research is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; Cellular Immunotherapy Data Resource (NCI, U24 CA233032); 75R60222C00011 from the Health Resources and Services Administration; and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research. Support is also provided by the Medical College of Wisconsin, National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc; Amgen, Inc; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; BioLineRx; Blue Spark Technologies; bluebird bio, inc; Blueprint Medicines; Bristol Myers Squibb Co; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; Deutsche Knochenmarkspenderdatei; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research and Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miller Pharmacal Group, Inc; Miltenyi Biotec, Inc; MorphoSys; Management Science Associates-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie Company; PPD Development, LP; REGiMMUNE; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi, Inc; Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
Kite, a Gilead company, was involved in the development of the study protocol and in data collection, analysis, and interpretation.
Authorship
Contribution: N.A., S.K.T., M.H., Z.-H.H., N.G., M.S., H.X., M.C.P., and A.F.H. contributed to the concept and design of the study; M.B. was responsible for data preparation; Z.-H.H. performed the statistical analyses; and all authors contributed to the interpretation of the data and writing and critical review of the manuscript, provided final approval for publication submission, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: N.A. has received fees for consulting or advisory role from Kite/Gilead and Bristol Myers Squibb (BMS); and has received research funding from Kite/Gilead. M.H. has received fees for consulting or advisory role from Incyte, MorphoSys, Kite/Gilead, Genmab, Seagen, Gamida Cell, Novartis, Legend Biotech, Kadmon, ADC Therapeutics, Omeros, Caribou, BMS, CRISPR, and AbbVie; has been paid to participate in a speakers bureau by AstraZeneca, BeiGene, Kite, and ADC Therapeutics; and has received research funding from Takeda, ADC Therapeutics, Spectrum Pharmaceutical, and Astellas Pharma. Z.-H.H. owns stock in Gilead and is an employee of Kite, a Gilead Company. N.G. has received fees for consulting or advisory role from Kite, Ono Pharma, BMS, Janssen, Seagen Inc, Caribou Biosciences, Novartis, Genentech, and ADC Therapeutics; has received research funding from Tessa Therapeutics; and owns stock in Sangamo. M.S. has an immediate family member employed by BMS; has received fees for consulting or advisory role from AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, BeiGene, BMS, MorphoSys, Incyte, TG Therapeutics, Innate Pharma, Kite, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate Therapeutics, MEI Pharma, and Atara Biotherapeutics; and has received research funding from Mustang Bio, Celgene, BMS, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, MorphoSys, and Incyte. F.L. has received fees for consulting or advisory role from A2 Bio, Allogene, Amgen, bluebird bio, BMS/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group, Iovance, Kite/Gilead, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; has received research funding from Kite/Gilead, Allogene, CERo Therapeutics, Novartis, bluebird bio, BMS, National Cancer Institute, Leukemia and Lymphoma Society; has several patents held by the institution (unlicensed) in the field of cellular immunotherapy; has had travel expenses paid by A2 Bio; and has served in an education or editorial role for Aptitude Health, the America Society of Hematology, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, and Society for Immunotherapy of Cancer. J.G. has received fees for consulting or advisory role from AbbVie, Genentech, and Kite/Gilead; and has received research funding from Loxo. M.J.F. has an immediate family member who owns stock in Roche/Genentech; has received fees for consulting or advisory role from Allogene Therapeutics, Kite/Gilead, and Adaptive Biotechnologies; and has received research funding as a site principal investigator from Cargo Therapeutics and Kite/Gilead. L.E.B. has received fees for consulting or advisory role from ADC Therapeutics, AstraZeneca, BeiGene, Kite/Gilead, Loxo/Lilly, Novartis, Nurix, and Roche/Genentech; has had travel expenses paid by Kite/Gilead and Roche/Genentech; holds patents for CCR4 chimeric antigen receptor (CAR) T cells for treatment of patients with CCR4-positive cancer and CD33-CAR for treatment of patients with CD33+ acute myeloid leukemia; and has received research funding from Amgen, Merck, AstraZeneca, and Mustang Bio. M.W. has received fees for consulting or advisory role from AbbVie, Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Deciphera, InnoCare, Janssen, Kite/Gilead, Leukemia & Lymphoma Society, Lilly, Merck, Milken Institute, Oncternal, Parexel, PeproMene Bio, Pharmacyclics, and VelosBio; has been paid honoraria from AbbVie, Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Dava Oncology, Eastern Virginia Medical School, IDEOlogy Health, Janssen, Kite/Gilead, Leukemia and Lymphoma Society, TS Oncology LLC, Medscape, Meeting Minds Experts, MJH Life Sciences, Merck, Moffit Cancer Center, Oncology Specialty Group, OncLive, Pharmacyclics, Physicians’ Education Resource, Practice Point Communications, and Studio ER Congressi; and has received research funding from Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genmab, Genentech, Innocare, Janssen, Juno Therapeutics, Kite/Gilead, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, VelosBio, and Vincerx. M.A.K.-D. has received fees for consulting or advisory role from Kite Pharma; and has received research funding as a site principal investigator from Novartis, BMS and Pharmacyclics. C.S. has received fees for consulting or advisory role from Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/Gilead, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, and GlaxoSmithKline (GSK); and has received research funding for clinical trials from Juno Therapeutics, Celgene/BMS, BMS, Precision Biosciences, Actinium Pharmaceuticals, and Sanofi-Genzyme. C.J.T. has received fees for consulting or advisory role from Caribou Biosciences, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics, Kyverna Therapeutics, Century Therapeutics, AbbVie, Differentia Bio, Advesya, and IGM Biosciences; has been paid honoraria from BMS; has received research funding from Juno Therapeutics and Nektar Therapeutics; has the right to receive royalties as an inventor on patents related to CAR T-cell therapy; and has stock options for Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, Arsenal Bio, eGlint, and Cargo Therapeutics. S.A. has served in a consultancy or advisory role for ADC Therapeutics, Kite/Gilead, and Chimagen; has received research funding from Nektar Therapeutics, Merck, Xencor, Chimagen, and Genmab; and has served on the data safety monitoring board for Myeloid Therapeutics. B.L. has received fees for consulting or advisory role from Enlivex. A.N. was an employee of Gilead Sciences Europe at the time of study conception; owns stock in Autolus, Iovance, Gilead, and Amgen; and has been paid honoraria from Gilead and Autolus. D.D. owns stock in Gilead and BMS. I.K. is an employee of Kite, a Gilead company; and owns stock in Gilead Sciences, Inc. D.L. and H.X. are employees of Kite, a Gilead company. M.C.P. has received fees for consulting or advisory role from BMS and Novartis; and has received research funding from Kite/Gilead, Novartis, BMS, Janssen, and GlaxoSmithKline. A.F.H. has received fees for consulting or advisory role from Karyopharm, Takeda, Tubulis, Regeneron, Genmab, Pfizer, Caribou, Adicet Bio, AbbVie, BMS, Genentech, AstraZeneca, ADC Therapeutics, Seagen Inc, and Merck; and has received research funding from BMS, Genentech, AstraZeneca, ADC Therapeutics, Merck, Seagen Inc, and Kite/Gilead. The remaining authors declare no competing financial interests.
Correspondence: Nausheen Ahmed, The University of Kansas Cancer Center, 2330 Shawnee Mission Pkwy, Kansas City, KS 66205; email: nahmed5@kumc.edu; and Marcelo C. Pasquini, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Milwaukee, WI 53226; email: mpasquini@mcw.edu.
References
Author notes
N.A. and S.K.T. contributed equally to this study and are joint first authors.
Kite is committed to sharing clinical data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The full-text version of this article contains a data supplement.