Key Points
Infection-related hospitalizations were strongly associated with mortality in patients with CLL.
Regular IgRT was not associated with a reduced risk of infection-related hospitalizations.
Visual Abstract
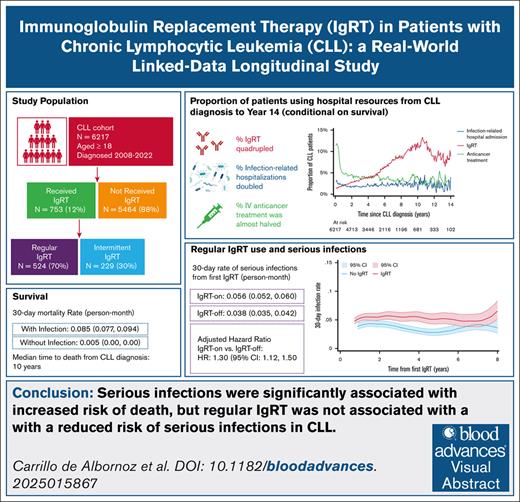
Patients with chronic lymphocytic leukemia (CLL) often experience infections due to immune suppression and/or dysregulation. Hypogammaglobulinemia is a key contributor to immunosuppression in CLL, and immunoglobulin replacement therapy (IgRT) is commonly given to prevent infections. However, the benefit of IgRT in preventing serious infections in CLL remains unclear. This study aimed to describe IgRT treatment patterns in a large, real-world cohort of patients with CLL, and explore the association between IgRT and serious infections. We conducted a retrospective longitudinal study of linked hospital data, including 6217 patients with CLL between 2008 and 2022 in Victoria, Australia. Kaplan-Meier survival analyses were performed to estimate survival, infection incidence, and IgRT use. Cox survival analyses explored associations between infections and IgRT in patients receiving regular prophylactic IgRT. Over the 14-year follow-up, the monthly proportion of patients experiencing serious infections doubled, while the proportion of patients receiving any IgRT quadrupled. The median time to death from CLL diagnosis was 10 years, and patients with serious infections had a higher mortality rate (0.090 [95% confidence interval (CI), 0.074-0.110]) vs those without (0.008 [95% CI, 0.007-0.009]). In total, 753 patients (12.1%) received IgRT, and 524 (8.4%) received IgRT regularly. In patients who received regular IgRT, infection incidence was higher during periods of IgRT (0.056 [95% CI, 0.052-0.060]) compared with periods without IgRT (0.038 [95% CI, 0.035-0.042]). Serious infections were associated with not only IgRT initiation and reinitiation, but also cessation. Further research is needed to evaluate the causal relationship between IgRT and infections in this population.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common hematological malignancy after non-Hodgkin lymphoma and multiple myeloma.1,2 Patients with CLL often experience infections due to immune dysregulation and immunosuppression from a combination of the disease and its treatment, and infections are associated with 30% to 60% of CLL deaths.3-5 Hypogammaglobulinemia, or serum immunoglobulin G (IgG) levels <700 mg/dL,6 is present in 20% to 70% of patients with CLL,7,8 and has been associated with a higher risk of infection.5,9,10 However, this association is not consistent across studies,4,11,12 and hypogammaglobulinemia may also be a marker for more aggressive disease.7,12 Prophylactic immunoglobulin therapy, or immunoglobulin replacement therapy (IgRT), is commonly used with the aim of preventing infections in patients with hypogammaglobulinemia, but its impact on infection prevention and survival in CLL is still uncertain.13
Current international guidelines do not provide strong recommendations, but do suggest the use of IgRT in patients with hypogammaglobulinemia secondary to hematological malignancy and severe or recurrent infections. Nevertheless, requirements for prior use of antibiotics, thresholds for severity of hypogammaglobulinemia, and considerations for IgRT cessation vary across countries.14-16 In Australia, the criteria for clinical use of immunoglobulin15 state that cessation of IgRT should be considered at least after each 12 months of treatment, and IgRT should only be continued if there is a “demonstrated clinical benefit,” but clinical benefit is not defined. Variations in clinical practice among physicians prescribing IgRT are common,17-19 and IgRT patterns of use, its association with serious infections, and other patient outcomes in CLL remain unclear.
Given that immunoglobulin supply is limited and very costly,20 and CLL is one of the hematological malignancies with the highest use of IgRT,18 it is important to understand how IgRT is being used in practice. This study aimed to describe IgRT treatment patterns in a large, real-world cohort of Australian patients with CLL, and to explore the association between IgRT and serious infections.
Methods
Study design
This was a retrospective study of linked longitudinal hospital and death data for adult patients (aged ≥18 years) registered as being diagnosed with CLL between 1 January 2008 and 31 December 2022 in the state of Victoria, Australia.
Data sources
Data on the date of CLL diagnosis were obtained from the Victorian Cancer Registry (VCR), and date of death was obtained from the Victorian Death Index. The VCR and Victorian Death Index record all cancer diagnoses and deaths, respectively, in the state of Victoria. CLL cancer diagnoses in the VCR were identified using the ICD-10-AM (the World Health Organization International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification).21
Data on all inpatient hospital admissions for patients with a CLL diagnosis were obtained from the Victorian Admitted Episodes Dataset (VAED), which collects demographic and clinical data for all admitted episodes in Victorian public and private hospitals. In the VAED, diagnoses are classified using the ICD-10-AM and procedures according to the Australian Classification of Health Interventions (11th edition).21
Data linkage was performed by the Centre for Victorian Data Linkage (CVDL), a specialist data linkage unit that is part of the Victorian Agency for Health Information. The CVDL maintains the Victorian Linkage Map and Integrated Data Resource, which is a collection of almost 40 data sets, which are linked on a monthly basis.22 Data were deidentified by the CVDL, removing all personal identifiable information. Ethical approval was granted by the Monash University Human Research Ethics Committee (ID 40164).
Study population and definitions
Patients aged ≥18 years at CLL diagnosis in the VCR were considered for inclusion. Those who had undergone a stem cell transplant were excluded, due to small numbers (49 patients) and potential impacts on their infection risk profile.
ICD-10-AM codes used to identify CLL diagnoses, infection episodes, IgRT, anticancer treatment, and stem cell transplant procedures are provided in the supplemental Materials (supplemental Tables 5-8). The diagnostic code for hypogammaglobulinemia was not included in the analysis, as this was not reliably recorded. Comorbidities were classified according to the Charlson Comorbidity Index (CCI), which was already calculated in the VAED for each hospital admission. We used the highest CCI for a hospitalization in the year prior to CLL diagnosis or the first IgRT for each patient. A CCI of 0 to 2 was classified as “mild,” 3 to 4 “moderate,” and ≥5 “severe,” as previously used in other studies.23 No CCI was available for patients who were not admitted to hospital in the year prior to CLL diagnosis (or first IgRT), and for these patients CCI was classified as “unknown.”
IgRT and anticancer treatment
IgRT is fully subsidized by the Australian Government, with no direct cost to the patient. Clinicians decide when and to whom to prescribe IgRT, as long as the patient is eligible for treatment.15 The hospital procedure code for IgRT only included IV immunoglobulin. Administration of subcutaneous immunoglobulin (SCIg) was not recorded in the VAED. SCIg was approved for use in patients with hematological malignancies in Australia in 2013,24 and funded by the SCIg Access Program in Victoria.25 However, in the financial year 2021 to 2022, only 11.9% of the total immunoglobulin grams dispensed in Australia for acquired hypogammaglobulinemia following hematological malignancies (includes all hematological malignancies) were SCIg products, and only 25.5% of those (3% of the total immunoglobulin) were dispensed as SCIg in Victoria.24
Patients with an IgRT procedure code during their follow-up were defined as “received any IgRT,” and these were further classified as regular or intermittent IgRT users. During their follow-up, a “regular IgRT user” had at least 3 IgRT episodes within a 5-month period, with no gap between treatment episodes >3 months. Patients with ≥1 IgRT episode but who did not meet this definition were defined as intermittent. Patients with no IgRT procedure codes were defined as “not received IgRT.” IgRT cessation was defined as a gap between IgRT episodes >3 months, while restarting IgRT included any IgRT episode following cessation. In Australia, IgRT cessation is advised to be considered after 12 months of treatment, and IgRT is only encouraged to continue if there is a “demonstrated clinical benefit.”15
All parenteral anticancer treatments administered in hospital were considered (supplemental Table 4). Oral anticancer treatments were not considered in our analysis, as they were only recorded in our data if administered during a hospitalization. This would not represent real-world utilization as these treatments are mostly given in the out-of-hospital setting.
Serious infections
Serious infections were defined as multi-day infection-related hospitalizations with an ICD-10 and/or Australian-refined diagnosis-related group (AR-DRG) infection code. We used the ICD-10-AM infection codes from a published sepsis study in Victoria,26 and these were further adapted to capture serious infections in our target population based on consultation with clinical experts (supplemental Table 5). AR-DRGs that identified infections were compared with and added to the ICD-10-AM infection codes for completeness (supplemental Table 4).
Statistical analysis
Descriptive statistics were presented according to IgRT use for patients at CLL diagnosis (Table 1) and first IgRT (Table 2). We calculated the proportion of patients (conditional on survival) with infection-related admissions, anticancer treatment and IgRT each month (Figure 1).
Key characteristics of patients with CLL at baseline and outcomes during follow-up
| Key characteristics . | Not received IgRT . | Received any IgRT . |
|---|---|---|
| No. of patients, n (%) | 5464 (87.9) | 753 (12.1) |
| Follow-up, mean (SD), y | 4.7 (3.4) | 6.9 (3.8) |
| Diagnosed before 2015 | 1659 (30.4) | 466 (61.9) |
| Mean age at diagnosis, y | 71.0 (12.0) | 69.5 (10.9) |
| Age at diagnosis, n (%) | ||
| <60 years | 942 (17.2) | 137 (18.2) |
| 60-69 years | 1404 (25.7) | 209 (27.8) |
| ≥70 years | 3118 (57.1) | 407 (54.1) |
| Sex, Female, n (%) | 1919 (36.6) | 262 (34.8) |
| CCI year prior to diagnosis,∗n (%) | ||
| Unknown (no hospitalizations year prior to diagnosis) | 2255 (41.3) | 227 (30.1) |
| Mild | 2677 (49.0) | 457 (60.7) |
| Moderate | 322 (5.9) | 58 (7.7) |
| Severe | 210 (3.8) | 11 (1.5) |
| Key characteristics . | Not received IgRT . | Received any IgRT . |
|---|---|---|
| No. of patients, n (%) | 5464 (87.9) | 753 (12.1) |
| Follow-up, mean (SD), y | 4.7 (3.4) | 6.9 (3.8) |
| Diagnosed before 2015 | 1659 (30.4) | 466 (61.9) |
| Mean age at diagnosis, y | 71.0 (12.0) | 69.5 (10.9) |
| Age at diagnosis, n (%) | ||
| <60 years | 942 (17.2) | 137 (18.2) |
| 60-69 years | 1404 (25.7) | 209 (27.8) |
| ≥70 years | 3118 (57.1) | 407 (54.1) |
| Sex, Female, n (%) | 1919 (36.6) | 262 (34.8) |
| CCI year prior to diagnosis,∗n (%) | ||
| Unknown (no hospitalizations year prior to diagnosis) | 2255 (41.3) | 227 (30.1) |
| Mild | 2677 (49.0) | 457 (60.7) |
| Moderate | 322 (5.9) | 58 (7.7) |
| Severe | 210 (3.8) | 11 (1.5) |
SD, standard deviation.
CCI could not be calculated in patients without any hospitalizations the year before diagnosis (unknown: no hospitalizations year prior to diagnosis); for all other characteristics there were no missing data.
Patient characteristics at first IgRT and treatment duration during follow-up
| Key characteristics . | Intermittent IgRT . | Regular IgRT . | All IgRT . |
|---|---|---|---|
| No. of patients, n (%) | 229 (30.4) | 524 (69.6) | 753 (100.0) |
| Time from CLL diagnosis to first IgRT (years), mean (SD) | 3.5 (3.2) | 3.2 (3.0) | 3.3 (3.1) |
| Follow-up since first IgRT (years), mean (SD) | 2.1 (2.9) | 4.2 (3.1) | 3.6 (3.2) |
| Follow-up since first IgRT (years), n (%) | |||
| <1 year | 127 (55.5) | 73 (13.9) | 200 (26.6) |
| 1-5 years | 66 (28.8) | 276 (52.7) | 342 (45.4) |
| >5 year | 36 (15.7) | 175 (33.4) | 211 (28.0) |
| Mean age at first IgRT (SD) | 73.9 (10.2) | 72.3 (10.3) | 72.8 (10.3) |
| Age at first IgRT, n (%) | |||
| >60 years | 24 (10.5) | 65 (12.4) | 89 (11.8) |
| 60-69 years | 47 (20.5) | 132 (25.2) | 179 (23.8) |
| ≥70 years | 158 (69.0) | 327 (62.4) | 485 (64.4) |
| Sex, n (%) | |||
| Female | 86 (37.6) | 176 (33.6) | 262 (34.8) |
| CCI at first immunoglobulin, n (%) | |||
| Mild | 147 (64.2) | 408 (77.9) | 555 (73.7) |
| Moderate | 54 (23.6) | 91 (17.4) | 145 (19.3) |
| Severe | 28 (12.2) | 25 (4.8) | 53 (7.0) |
| Any serious infections before first IgRT, n (%) | 183 (79.9) | 326 (62.2) | 509 (67.6) |
| Any serious infections 6 months before first IgRT, n (%) | 160 (69.9) | 251 (47.9) | 411 (54.6) |
| No. of serious infections 6 months before first IgRT, n (%) | |||
| None | 69 (30.1) | 273 (52.1) | 342 (45.4) |
| 1-2 | 141 (61.6) | 224 (42.7) | 365 (48.5) |
| 3 or more | 19 (8.3) | 27 (5.2) | 46 (6.1) |
| Total duration of IgRT (including treatment gaps), n (%) | |||
| 1 immunoglobulin only | 177 (77.3) | 0 (0.0) | 177 (23.5) |
| <1 year | 38 (16.6) | 155 (29.6) | 193 (25.6) |
| 1-5 years | 11 (4.8) | 246 (46.9) | 257 (34.1) |
| >5 years | 3 (1.3) | 123 (23.5) | 126 (16.7) |
| Median interval between IgRT, n (%) | |||
| 1 month | 198 (86.5) | 434 (82.8) | 632 (83.9) |
| >1 to 3 months | 14 (6.1) | 90 (17.2) | 104 (13.8) |
| >3 to 12 months | 7 (3.1) | 0 (0.0) | 7 (0.9) |
| >12 months | 10 (4.4) | 0 (0.0) | 10 (1.3) |
| Key characteristics . | Intermittent IgRT . | Regular IgRT . | All IgRT . |
|---|---|---|---|
| No. of patients, n (%) | 229 (30.4) | 524 (69.6) | 753 (100.0) |
| Time from CLL diagnosis to first IgRT (years), mean (SD) | 3.5 (3.2) | 3.2 (3.0) | 3.3 (3.1) |
| Follow-up since first IgRT (years), mean (SD) | 2.1 (2.9) | 4.2 (3.1) | 3.6 (3.2) |
| Follow-up since first IgRT (years), n (%) | |||
| <1 year | 127 (55.5) | 73 (13.9) | 200 (26.6) |
| 1-5 years | 66 (28.8) | 276 (52.7) | 342 (45.4) |
| >5 year | 36 (15.7) | 175 (33.4) | 211 (28.0) |
| Mean age at first IgRT (SD) | 73.9 (10.2) | 72.3 (10.3) | 72.8 (10.3) |
| Age at first IgRT, n (%) | |||
| >60 years | 24 (10.5) | 65 (12.4) | 89 (11.8) |
| 60-69 years | 47 (20.5) | 132 (25.2) | 179 (23.8) |
| ≥70 years | 158 (69.0) | 327 (62.4) | 485 (64.4) |
| Sex, n (%) | |||
| Female | 86 (37.6) | 176 (33.6) | 262 (34.8) |
| CCI at first immunoglobulin, n (%) | |||
| Mild | 147 (64.2) | 408 (77.9) | 555 (73.7) |
| Moderate | 54 (23.6) | 91 (17.4) | 145 (19.3) |
| Severe | 28 (12.2) | 25 (4.8) | 53 (7.0) |
| Any serious infections before first IgRT, n (%) | 183 (79.9) | 326 (62.2) | 509 (67.6) |
| Any serious infections 6 months before first IgRT, n (%) | 160 (69.9) | 251 (47.9) | 411 (54.6) |
| No. of serious infections 6 months before first IgRT, n (%) | |||
| None | 69 (30.1) | 273 (52.1) | 342 (45.4) |
| 1-2 | 141 (61.6) | 224 (42.7) | 365 (48.5) |
| 3 or more | 19 (8.3) | 27 (5.2) | 46 (6.1) |
| Total duration of IgRT (including treatment gaps), n (%) | |||
| 1 immunoglobulin only | 177 (77.3) | 0 (0.0) | 177 (23.5) |
| <1 year | 38 (16.6) | 155 (29.6) | 193 (25.6) |
| 1-5 years | 11 (4.8) | 246 (46.9) | 257 (34.1) |
| >5 years | 3 (1.3) | 123 (23.5) | 126 (16.7) |
| Median interval between IgRT, n (%) | |||
| 1 month | 198 (86.5) | 434 (82.8) | 632 (83.9) |
| >1 to 3 months | 14 (6.1) | 90 (17.2) | 104 (13.8) |
| >3 to 12 months | 7 (3.1) | 0 (0.0) | 7 (0.9) |
| >12 months | 10 (4.4) | 0 (0.0) | 10 (1.3) |
Descriptive data, not adjusted for survival. Intermittent IgRT also includes patients who only had 1 IgRT episode. Serious infection was defined as an infection-related hospitalization.
Proportion of patients with CLL with infection-related hospital admissions, immunoglobulin, and chemotherapy treatment during follow-up, conditional on survival. Monthly proportion of patients with admission episodes related to IgRT, anticancer treatment, or infections.
Proportion of patients with CLL with infection-related hospital admissions, immunoglobulin, and chemotherapy treatment during follow-up, conditional on survival. Monthly proportion of patients with admission episodes related to IgRT, anticancer treatment, or infections.
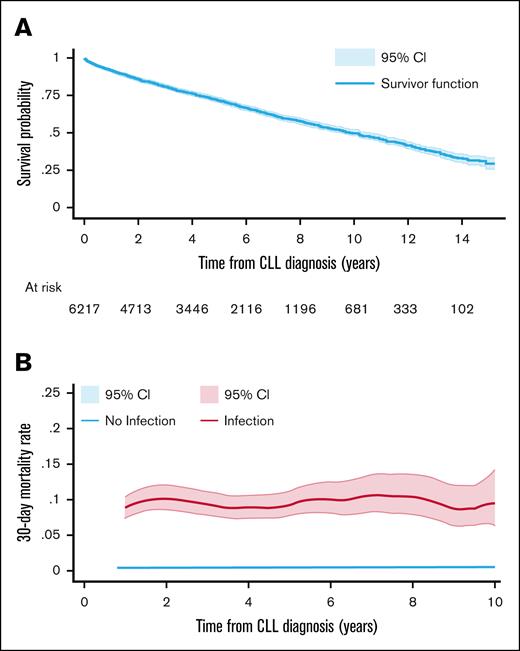
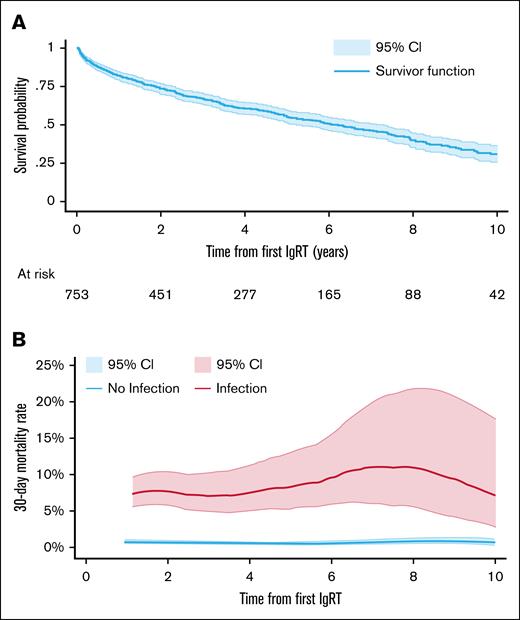
Kaplan-Meier survival analysis was used to estimate time-to-event outcomes with censoring on 31 December 2022, the last follow-up date. Overall survival to death from any cause was estimated from the date of CLL diagnosis in the full cohort (Figure 2), and from first IgRT (Figure 3) in patients who had received IgRT at some stage. Time to first IgRT was calculated from diagnosis to first IgRT in the full cohort, conditional on survival. In regular IgRT users, time to IgRT cessation was calculated from first IgRT to the cessation date, and time to restart IgRT was calculated from first IgRT cessation to the next IgRT, conditional on survival. Smoothed hazard rates27 were estimated for mortality according to serious infection (Figure 2B) in the full population and patients who received IgRT (Figure 3B), and for incidence of serious infections according to IgRT periods (on-IgRT: use in the previous 30 days, off-IgRT: no use in the previous 30 days) in patients who received regular IgRT (Figure 5).
Overall survival and mortality rate per person per month from CLL diagnosis. (A) Overall survival Kaplan-Meier curve from CLL diagnosis in the full patient cohort. (B) Smoothed hazard mortality rate with 95% CI from CLL diagnosis, according to whether a serious infection had occurred in the prior 30 days.
Overall survival and mortality rate per person per month from CLL diagnosis. (A) Overall survival Kaplan-Meier curve from CLL diagnosis in the full patient cohort. (B) Smoothed hazard mortality rate with 95% CI from CLL diagnosis, according to whether a serious infection had occurred in the prior 30 days.
Overall survival and mortality rate per person per month from first IgRT. (A) Overall survival Kaplan-Meier curve from first immunoglobulin in patients who received IgRT. (B) Smoothed hazard mortality rate with 95% CI from first IgRT, according to whether a serious infection had occurred in the prior 30 days.
Overall survival and mortality rate per person per month from first IgRT. (A) Overall survival Kaplan-Meier curve from first immunoglobulin in patients who received IgRT. (B) Smoothed hazard mortality rate with 95% CI from first IgRT, according to whether a serious infection had occurred in the prior 30 days.
Cox proportional hazard models were used to explore factors (ie, serious infections, anticancer treatment, age, CCI, and sex) associated with: time from CLL diagnosis to first IgRT, time from first IgRT to cessation, and time from cessation to restarting IgRT. The proportional hazard test was performed for all of these models (supplemental Table 1). We used Cox proportional hazard models if the proportional hazards assumption held (supplemental Table 2), otherwise we conducted Cox survival analyses including those variables for which the proportional hazard assumption failed as time-varying covariates (supplemental Table 3).
The partial association between IgRT use in the prior month and future serious infections in patients who received regular IgRT, conditional on survival, was explored using: 1) Andersen-Gill multiple-failure survival model,28,29 controlling for time-varying (serious infection and anticancer treatment in the prior month) and fixed covariates (age, sex, and CCI at first IgRT); 2) Andersen-Gill with prior monthly infections, anticancer treatment, and IgRT as time-varying covariates, while also controlling for fixed hazard ratios (HRs) of age, sex, and comorbidities. Andersen-Gill models have been previously used to assess the risk of IgRT and recurrent infections.10
Exploratory analyses were conducted in patients diagnosed before 2015, and those diagnosed in 2015, and afterward to consider changes in immunoglobulin use following the introduction of the National Immunoglobulin Governance program in Australia in 2014.30 Data analyses used deidentified data, and were conducted in the Victorian Department of Health Microsoft Azure secure virtual environment using STATA v.18.0.
Results
We included 6217 patients diagnosed with CLL between January 2008 and December 2022 (supplemental Figure 1). A total of 5464 patients (87.9%) had not received IgRT, and 753 (12.1%) were treated with ≥1 IgRT dose during their follow-up. Baseline characteristics and follow-up differed significantly between the 2 groups, except for sex and age (Table 1). Patients treated with any IgRT had longer follow-up than patients who had not received IgRT (mean 6.9 vs 4.7 years), and a lower comorbidity burden (1.5% vs 3.8% with severe CCI), but had more hospital admissions in the year prior to diagnosis (69.9% vs 58.7%).
Over the 14-year follow-up period (Figure 1), conditional on survival, the proportion of patients receiving IgRT each month increased from 2.0% in the first year after diagnosis to 8.8% by year 14, with a monthly peak of 12.0% at 10 years after diagnosis. The corresponding proportion of patients undergoing in-hospital anticancer treatment decreased from 4.8% in year 1% to 2.9% by year 14, while the proportion of patients experiencing serious infections increased over time from 1.9% to 3.9%. When split by year of diagnosis, patients diagnosed before 2015 had higher IgRT use and anticancer treatment during their first 8 years of diagnosis compared with patients diagnosed after 2015 during the equivalent period, while the proportion of patients experiencing serious infections was similar (supplemental Figure 2).
Overall survival
During the follow-up period, 2191 of 6217 patients (35.2%) died. The median time to death from CLL diagnosis was 121.5 months, ∼10 years (Figure 2A). Patients with serious infections had a higher mortality rate per person-month (0.085 [95% confidence interval (CI), 0.077-0.094]) than patients without serious infections (0.005 [95% CI, 0.005-0.005]); that is, for every 100 patients with a serious infection 8.5 would die within a month, compared with 0.5 of 100 patients without infections (Figure 2B).
Among the 753 patients who started IgRT, 346 (45.9%) died during their follow-up. The median survival from first immunoglobulin was 72.7 months, ∼6 years (Figure 3A). Similar to the full CLL cohort, patients who received IgRT (Figure 3B) who had an infection-related admission in the last month had a higher 30-day mortality rate per person-month than those without infections (0.090 [95% CI, 0.074-0.110] vs 0.008 [95% CI, 0.007-0.009]).
Immunoglobulin treatment patterns
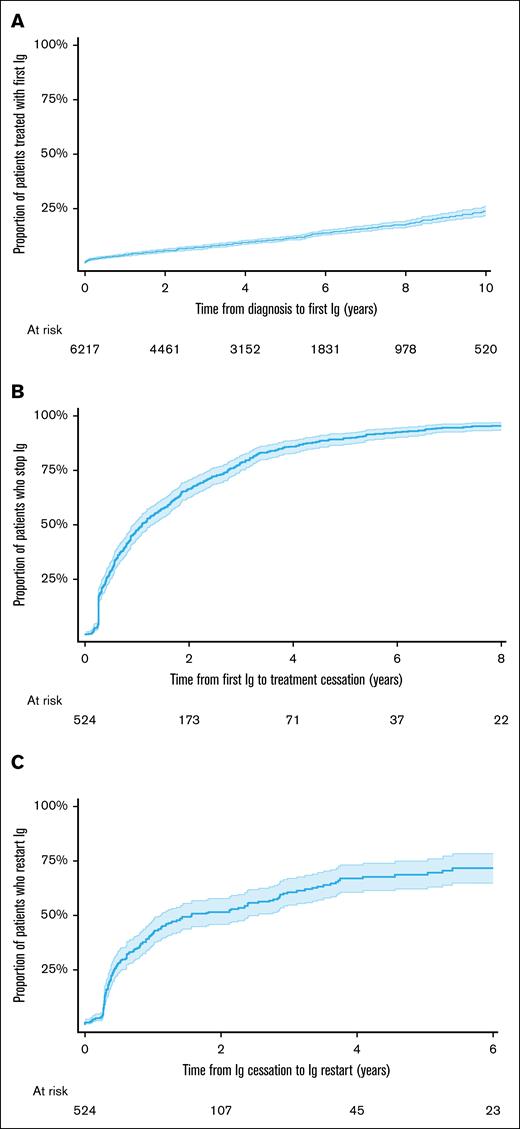
The probability of receiving IgRT increased with time since diagnosis. Ten years after diagnosis, conditional on survival, 25% of all patients with CLL had received their first IgRT (Figure 4A).
IgRT patterns in patients with CLL. (A) Time to first IgRT in the full CLL cohort. (B) Time from first IgRT to cessation in regular IgRT users. (C) Time from IgRT cessation to restart in regular IgRT users.
IgRT patterns in patients with CLL. (A) Time to first IgRT in the full CLL cohort. (B) Time from first IgRT to cessation in regular IgRT users. (C) Time from IgRT cessation to restart in regular IgRT users.
Of the 753 patients who received IgRT during their follow-up (Table 2), 524 (69.6%) had at least 1 period of regular IgRT. A total of 229 patients (30.4%) in this IgRT cohort received immunoglobulin treatment intermittently, and 177 (77.3%) of them only had 1 immunoglobulin treatment episode.
At first IgRT, patients who received regular IgRT were younger, had a lower comorbidity burden, fewer serious infections before their first IgRT, and longer follow-up than intermittent users. Among patients who received regular IgRT, the mean follow-up was 4.2 years. During their follow-up period (conditional on survival and follow-up duration), 29.6% had IgRT for <1 year, 46.9% for 1 to 5 years, and 23.5% received IgRT for >5 years. IgRT cessation and recommencement of IgRT was common; half stopped IgRT 13 months after their first IgRT episode (Figure 4B). Of those who stopped IgRT, half restarted after 18 months of cessation (Figure 4C).
Associations between IgRT and serious infections
We observed an association between serious infections and IgRT initiation. In the full CLL cohort, patients who had a serious infection were more likely to start IgRT in the next 30 days, at a rate of 0.075 (95% CI, 0.067-0.838) per person-month, compared with those without serious infections, at a rate of 0.001 (95% CI, 0.001-0.001) per person-month.
Among the 524 patients who received regular IgRT, the IgRT cessation rate per person-month was higher in those who experienced a serious infection in the previous month compared with those who did not (0.091 [95% CI, 0.067-0.125] vs 0.026 [95% CI, 0.023-0.029]). Similarly, IgRT reinitiation rate per person-month was 0.225 (95% CI, 0.167-0.303) for those who had a serious infection in the prior month, and 0.020 (95% CI, 0.017-0.234) in those without a serious infection.
After adjusting for anticancer treatment, age, sex, and comorbidities at first IgRT, patients who received regular IgRT with serious infections in the previous month were significantly more likely to stop treatment than those who did not have a serious infection (HR, 3.81; 95% CI, 2.71-5.35), and significantly more likely to restart IgRT (HR, 11.61; 95% CI, 8.00-16.85). Anticancer treatment in the last month also increased the likelihood of stopping (HR, 1.87; [95% CI, 1.37-2.54) and restarting (HR, 2.25; 95% CI, 1.50-3.38) IgRT (supplemental Table 2). In the full CLL cohort, patients who had serious infections (HR, 31.91; 95% CI, 24.94-40.82) and anticancer treatment (HR, 2.51; 95% CI, 2.05-3.07) were also significantly more likely to start IgRT than those who did not (supplemental Table 3).
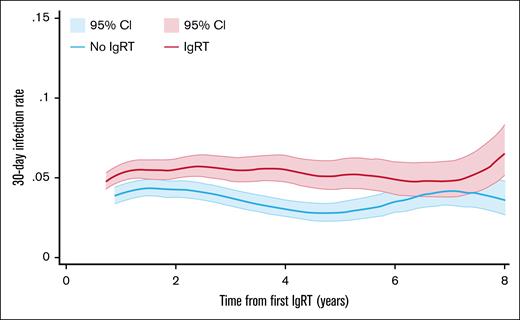
After their first IgRT, patients who received regular IgRT had an incidence rate for serious infections of 0.0472 per person-month (95% CI, 0.045-0.050). This incidence rate was higher during periods of IgRT compared with periods of treatment cessation (0.056 [95% CI, 0.052-0.060] vs 0.038 [95% CI, 0.035-0.042] per person-month; Figure 5). After adjusting for baseline covariates, serious infection, and anticancer treatment in the previous month, the risk of serious infections was significantly higher in patients who continued IgRT compared with those who ceased IgRT (HR, 1.30; 95% CI, 1.12-1.50]; supplemental Table 4; supplemental Figure 3). Other factors associated with 30-day risk of serious infection were anticancer treatment, previous infection-related admission, age, and severe comorbidity burden at first IgRT (supplemental Table 4). Patients who experienced a serious infection had a significantly higher incidence of a subsequent infection in the next 30 days compared with patients who did not; with incidence rates of 0.260 per person-month (95% CI, 0.228-0.296) vs 0.040 per person-month (95% CI, 0.038-0.043), respectively.
Thirty-day rate of infection-related hospital admissions in regular IgRT users according to IgRT in the previous month. Smoothed hazard infection rates with 95% CI from first IgRT in regular IgRT users. The pink line indicates the infection rate per person-month in periods of IgRT use (on-IgRT), and the blue line indicates the infection rate per person-month in periods without IgRT use (off-IgRT), conditional on survival.
Thirty-day rate of infection-related hospital admissions in regular IgRT users according to IgRT in the previous month. Smoothed hazard infection rates with 95% CI from first IgRT in regular IgRT users. The pink line indicates the infection rate per person-month in periods of IgRT use (on-IgRT), and the blue line indicates the infection rate per person-month in periods without IgRT use (off-IgRT), conditional on survival.
Discussion
To our knowledge, this is the first study to examine long-term, real-world IgRT patterns, serious infections, and survival in a large cohort of patients with CLL. Our study highlighted the burden of infections in patients with CLL and the impact on survival. Over the 14-year follow-up period, serious infections increased from 1.9% to 3.9%, while IgRT use increased from 2.0% to 8.8%. Among patients receiving IgRT at regular intervals, we found a higher incidence of serious infections while on IgRT compared with off-treatment periods. Serious infections were associated with IgRT initiation, cessation, and reinitiation.
Serious infections in the previous month were significantly associated with an increased risk of death in the full cohort and in those receiving IgRT. This is consistent with the previously described role of infections as a leading cause of death in patients with CLL.3-5 A recent retrospective study of patients with CLL diagnosed in Italy between 1988 and 2018 (N = 210) reported a median overall survival of 10.5 years in patients who experienced severe infections, and lower overall survival if the severe infection occurred within the first year of diagnosis (median 4.6 years).5
In our cohort, 12.1% of patients with CLL received IgRT at some time during the follow-up, almost double the proportion of IgRT use in patients with CLL recently reported in a United States longitudinal study.31 The higher uptake in our study might be influenced by the public funding of IgRT in Australia. Among patients with CLL who received IgRT, 69.6% of them had their infusions at regular intervals, which follows the Australian criteria for IgRT,15 and these patients continued regular IgRT for many years. Over 20% of patients receiving IgRT only had 1 treatment episode. One possibility could be that some of these patients may have received immunoglobulin for indications other than infection prevention, such as treatment of immune-mediated cytopenias.
Our results indicated that in patients who received regular IgRT, periods of IgRT were associated with an increased risk of serious infections compared with periods without IgRT. This may reflect a combination of patient selection (ie, it is possible that patients who stopped IgRT had lower risk of infection and therefore no longer needed IgRT) and treatment effects (ie, the protective effect of IgRT was not large enough to prevent higher rates of serious infections in these selected patients who remained on IgRT). Siffel et al32 reported similar results in a retrospective analysis of United States claims data, including 118 patients with CLL with hypogammaglobulinemia treated with IgRT and 118 matched controls who had not received IgRT; after 12 months of follow-up, patients treated with IgRT experienced a significantly higher mean number of infections and severe infections compared with non-IgRT–treated controls. Conversely, Soumerai et al31 reported a significantly lower odds ratio for infections and severe infection in patients with CLL after IgRT. Their study analyzed data from 196 patients with CLL who received IgRT in 8 United States hospitals; the odds ratio for infections at 3-months (N = 196), 6 months (N = 175), and 12 months (N = 137) before and after IgRT indicated a significant decrease in all infections and severe infections following IgRT. Both studies were retrospective database analyses including patients with CLL, but their methods differed considerably. Siffel et al32 compared IgRT with non-IgRT matched controls over a short period of time, and the authors highlighted that selection bias and lack of clinical prognostic factors in their data were key limitations. The main limitation in Soumerai et al31 was the before/after approach in patients who had just received their first IgRT, which may have favored IgRT due to regression to the mean. In our study, we found that previous infection plays a key role on the decision to start a patient on IgRT, and subsequently they may be more likely to experience a decrease in infections due to regression to the mean after treatment. Soumerai et al31 acknowledged this as a source of bias, which limits the interpretation of their odds ratio.
The incidence of serious infections in our study was much higher than those reported by Murru et al,5 where patients with low IgG levels had an infection incidence of 7.56 per 100 patient-years. This may be related to differences in study populations and methods, as IgRT was not mentioned, the incidence of severe infection was measured from diagnosis rather than first IgRT, and only 50 patients with CLL had IgG levels <700 mg/dL.5
Factors associated with increased infection risk identified in our study were older age, higher comorbidity burden at first IgRT, and serious infection or anticancer treatment in the prior 30 days. CCI, hypogammaglobulinemia, and third-line or subsequent anticancer treatment have previously been identified as independent risk factors for severe infections.10 It is possible that patients who continued IgRT had more severe hypogammaglobulinemia, which in turn may have increased their risk of recurrent infections. In our analyses of IgRT patterns, having an infection-related admission in the previous month not only increased the likelihood of starting IgRT, but also of stopping treatment and restarting IgRT following a period of cessation. Anticancer treatment also increased the likelihood of starting, stopping, and restarting IgRT, but the rationale behind this is unclear. In Australia, ongoing reviews are required to request ongoing supply of IgRT, and IgRT is only continued if there is a demonstrated clinical benefit. Given that IgRT is used to prevent infections, it is possible that a serious infection during IgRT treatment may be interpreted as lack of clinical benefit. In a survey of hematologists in Australia and New Zealand, 21% stated they would continue IgRT unless an adverse event occurred, 24% said they would stop after a fixed duration, and 15% would stop if the patient was in clinical remission and IgG levels were back to normal. However, this survey did not include lack of effectiveness as a response category.17
The proportion of patients with CLL using IgRT increased over time after diagnosis, largely driven by low rates of IgRT cessation, whereas the proportion of patients experiencing serious infections remained relatively constant during the 14-year follow-up. This highlights a disconnect between infection risk and IgRT use over the disease journey. Our results indicate higher use of IgRT in patients diagnosed with CLL before 2015 compared with those diagnosed after this date, indicating a potential reduction in IgRT initiations in patients with CLL since the immunoglobulin governance program was introduced.30 Keegan et al analyzed IgRT use in patients with CLL in Australia, and found a sustained average annual growth in IgRT use of 5.5% during the period 2008 to 2013.3 Since then, there has been an average annual growth of 7.6% in the number of patients with CLL given IgRT in Australia, which peaked in 2018 to 2019 (N = 1753), and a decrease in subsequent years up to 2021 to 2022 (N = 1696).33
Our study has several limitations. Among them, the retrospective design and potential selection bias. The lack of some key clinical prognostic factors, disease severity, and cancer treatment-related data was another limitation. We could not stratify patients or adjust for infection risk, particularly underlying hypogammaglobulinemia, and the presence of unknown confounding factors cannot be ignored. There were significant differences at baseline between patients treated with IgRT and those who did not receive IgRT, and between IgRT regular users and those who received IgRT once or intermittently, which suggests patients who received regular IgRT may have a different risk profile that puts them at higher risk of infection. To minimize these issues, we limited our analysis of the association between IgRT and serious infections to patients who received regular IgRT, who received IgRT as recommended for prophylactic use. Our analysis only included serious infections, and outpatient anticancer treatment, infections outside of hospital, and oral antibiotic treatments outside of hospital were not captured. In particular, we could capture the impact of targeted therapies. It is possible that changes in therapy from chemotherapy-based treatment to immunotherapy and targeted therapies may have impacted on the use of IgRT and infection risk.
Additional limitations include the potential for missing data and misclassification due to coding errors, as variables were identified through ICD-10, AR-DRGs, and Australian Classification of Health Interventions procedure codes. Despite our efforts to only capture serious infections, and reduce the potential overcounting of the same infection, the possibility of having overestimated infections remains as ICD-10 codes and AR-DRGs were used to identify infections. Results on the association between serious infections and IgRT are limited to patients receiving regular IgRT, and may not be generalizable to the wider CLL population.
We could not include data on SCIg use, although this is unlikely to have a big impact given the small proportion of patients with hematological malignancies receiving SCIg in Victoria during the study period,24 and the slow uptake of SCIg since its approval for the treatment of this patient population in Australia in 2013.34
Despite these limitations, this is the first study to compare the risk of serious infections from first IgRT over a long time period, according to IgRT use in patients who receive IgRT at regular intervals as recommended. Our large sample size and 14-year follow-up period provide valuable evidence on IgRT long-term use in CLL, from treatment initiation to cessation and reinitiation, and factors affecting these patterns, such as infections and anticancer treatment.
Conclusions
In this real-world cohort of patients with CLL, hospital admissions due to infection were associated with increased risk of death, but regular IgRT was not associated with a reduced incidence of infection admissions. Infection episodes influenced IgRT initiation, cessation, and reinitiation. Most patients who started IgRT regularly, continued treatment for many years, and the proportion of patients who were having IgRT at any given month increased over time. Given the increasing use and costs of IgRT, these results highlight the need to evaluate the causal association between IgRT and infections, and determine which patient subgroup, and at what point during their disease course, may benefit the most from IgRT treatment. Further research, particularly randomized controlled trials, is needed to fully understand the effectiveness and cost-effectiveness of IgRT in this patient population.
Acknowledgments
The authors acknowledge the Victorian Department of Health as the source of the Victorian Admitted Episodes Dataset for this study, and the Centre for Victorian Data Linkage for the provision of data linkage.
This study is funded by the Medical Research Future Fund (MRFF) Value-Ig Study (MRF2017480). S.C.d.A. is supported by National Health and Medical Research Council (NHMRC) Blood Synergy grant (1189490). A.M. is supported by an AustralianNHMRC postgraduate scholarship (GNT2022415), and a Tour de Cure Postgraduate Grant (RSP-237-2024). A.M.H. is supported by an NHMRC Emerging Leader Fellowship (GNT2008447). E.M.W. is funded by an NHMRC Investigator grant (GNT1177784). Z.K.M. is funded by an NHMRC Emerging Leader Investigator grant (GNT1194811).
Authorship
Contribution: S.C.d.A., Z.K.M., A.M.H., and D.P. designed the study; S.C.d.A. conducted the data analysis, interpreted results, and wrote the manuscript draft; A.I. and D.P. contributed to data acquisition; X.Z. and R.A. contributed to the data analysis; A.M., Z.K.M., and E.M.W. provided key clinical input and advice; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.M.W. and Z.K.M. received grant funding from CSL Behring not related to this study; and research funding to institution from AbbVie, Amgen, AstraZeneca, BeiGene, Celgene, Janssen, New Zealand Blood Service, Novartis, Sanofi, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Sara Carrillo de Albornoz, School of Public Health and Preventive Medicine, Monash University, 553 St Kilda Rd, Melbourne VIC 3004, Australia; email: sara.carrillo@monash.edu.
References
Author notes
Z.K.M. and D.P. are joint senior authors.
The data included in this article were provided via an application process by the Centre for Victorian Data Linkage (CVDL) and are not publicly available. An online application can be made to CVDL at https://vahi.vic.gov.au/ourwork/data-linkage/apply.
The full-text version of this article contains a data supplement.