TO THE EDITOR:
Lymphoplasmacytic lymphoma (LPL), termed Waldenström macroglobulinemia (WM) when associated with bone marrow infiltration and an immunoglobulin M (IgM) paraprotein, is a low-grade non-Hodgkin B-cell lymphoma characterized by the somatic mutation MYD88L265P in >90% of cases.1,2 Central nervous system (CNS) involvement is termed Bing-Neel syndrome (BNS). Although an infrequent complication, it causes significant morbidity.3 Optimum treatment remains to be established, with traditional CNS-penetrating chemotherapy agents used, as well as Bruton tyrosine kinase inhibitors (BTKis).4 There are limited but emerging data on treatment outcomes. A retrospective study of 28 patients reported an 80% 2-year progression-free survival (PFS) with ibrutinib for both treatment-naïve and relapsed/refractory patients.5 A recent international series of 30 patients treated with zanubrutinib reported >90% clinical and radiological response rates, with median PFS not reached at a median follow-up of 13 months.6 The Dana-Farber group reported successful outcomes with zanubrutinib in 9 patients.7
We describe features and treatment outcomes using a retrospective cohort analysis from the University College London Hospital (UCLH) WM patient registry. Overall survival (OS) was calculated from date of treatment initiation to death, and PFS was calculated from the same starting point to progression, death, or initiation of the next line of therapy, using the Kaplan-Meier method, with censoring at last follow-up. Reverse Kaplan-Meier was used to calculate median follow-up. Progression was defined as per established consensus criteria.4
A total of 474 patients with LPL/WM were registered, and BNS was diagnosed in 59 patients (12%) between 2012 to 2024; 46 had adequate data and were included. The median duration from symptom onset to diagnosis was 5 months (range, 0-60). The median follow-up from diagnosis was 50 months (range, 1-150). A preceding WM/LPL diagnosis was present in 34 of 46 patients (74%), including 31 with WM and 3 with non-IgM LPL. The median time from diagnosis to BNS was 55 months (range, 0-265). Table 1 lists full characteristics.
Leptomeningeal enhancement was present in 33 of 46 patients (72%), with additional parenchymal lesions in 12 patients (26%). No abnormalities were present on magnetic resonance imaging in 7 of 46 patients (15%), despite subsequent cerebrospinal fluid (CSF) confirmation. CSF involvement with LPL/WM was demonstrated in 44 of 46 (96%), using immunophenotyping in 27, MYD88L265P and/or immunoglobulin heavy chain (IgH) clonal rearrangements on allele-specific polymerase chain reaction (AS-PCR) in 16 and cytology alone in 1. Two patients (4%) required brain biopsy for BNS diagnosis.
CSF MYD88L265P was detected in 29 of 30 tested patients; 3 others were not tested for MYD88L265P but had IgH rearrangements. Lack of demonstrable surface immunoglobulin complicated confirmation of clonality in 16 patients, but this was overcome with AS-PCR for MYD88L265 and/or IgH rearrangements.
BNS occurred with systemic progression in 15 of 46 patients (30%), with rising paraprotein and/or evidence of radiological progression; 19 of 46 patients (41%) had CNS-only progression. There was no prior history of WM/LPL in 12 of 46 patients (26%). BNS without systemic disease occurred in 4 patients, with no LPL on bone marrow biopsy and no lymphadenopathy on imaging. One such patient had an IgG kappa paraprotein (2 g/L), whereas the remaining 3 had normal immunofixation. A systemic condition was established in the 8 remaining patients, with clonal B cells and MYD88L265P on bone marrow aspirate. Prior treatment for systemic WM/LPL had been given in 21 of 46 (median, 1 line; range, 0-4).
Systemic therapy for BNS was administered in 42 of 46 patients (95.6%), with high-dose methotrexate (MTX)–based regimens in 37 (80%) and BTKis in 5 patients (11%; zanubrutinib in 4 and ibrutinib in 1; Table 2). Carmustine (BCNU)/thiotepa-conditioned autologous stem cell transplant consolidation was performed in 3 of 46 patients. Intrathecal-only chemotherapy was given in 2 of 46 patients. The median CSF white cell count was 15.5/mm3 (range, 1-153) before treatment and 4/mm3 (range, 0-297) after treatment. The median CSF protein level was 1.40 g/L (range, 0.25-4.69) before treatment and 0.78 g/L (range, 0.34-6.03) after treatment.
Complete resolution of neurological symptoms occurred frontline in 10 of 44 patients, partial resolution in 24 of 44, no response in 7 of 44, and progressive symptoms in 1 of 44; 2 patients died before assessment.
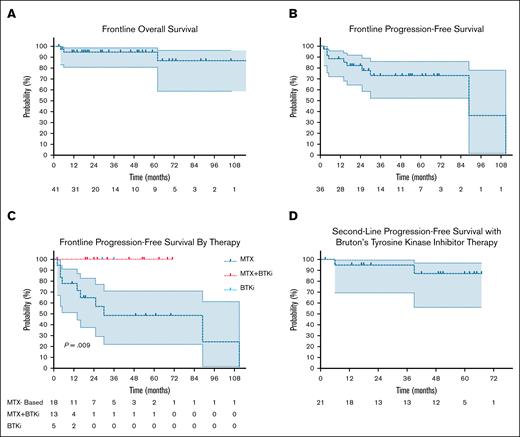
Frontline median OS was not reached; OS at 2 and 4 years were both 93% (95% confidence interval [CI], 84-100). The median PFS was 89 months (range, 70-89), with 1-, 3-, and 5-year PFS of 85% (95% CI, 68-93), 72% (95% CI, 52-86), and 72% (95% CI, 52-86), respectively. PFS after frontline MTX-based therapy was 78% (95% CI, 51-91) at 1 year and 48% (95% CI, 22-71) at 3 years. Symptomatic residual disease was present in 15 of 37 patients (40.6%) who initiated BTKi consolidation. For these patients, PFS was 100% at 1 and 3 years. Compared to patients who commenced to BTKi without preceding chemoimmunotherapy for BNS, PFS was also 100% at 1 year (P = .009), but is limited by smaller patient numbers (n = 5) (Figure 1).
Attainment of frontline negative CSF MYD88L265 AS-PCR occurred in 13 of 16 evaluable patients, none of whom subsequently relapsed; 9 were treated with MTX-based therapy, 2 with intrathecal and 2 with zanubrutinib.
Second-line therapy for BNS was required in 23 of 46 patients (50%), due to progressive disease in 8 and symptomatic residual disease in 15. A BTKi was used in 21 patients: ibrutinib in 15 and zanubrutinib in 6. The remaining 2 patients received R-bendamustine and R-cladribine. The median time to second-line therapy was 4 months (range, 2-111). The median PFS after second-line BTKi therapy was not reached, with 1- and 3-year PFS both 95% (95% CI, 69-100). Attainment of second-line CSF-negativity by MYD88 or IgH AS-PCR occurred in 6 of 9 evaluable patients, all treated with BTKis. None of these 6 patients relapsed. Third-line therapy for BNS was given in 3 patients.
Zanubrutinib was administered at 320 mg daily doses in 9 of 10, with 1 dose reduction due to febrile neutropenia. Ibrutinib was administered at 420 mg daily doses in all 16 patients.
Our series of 46 patients with BNS is, to our knowledge, the largest published to date. BNS prevalence in our registry was 12%. Presenting symptoms are heterogenous, and although most cases occurred in patients with a known WM/LPL diagnosis, a quarter of them were not previously diagnosed. Four cases were limited to the CNS. This series reinforces that BNS can occur without systemic progression, necessitating the evaluation of neurological symptoms in otherwise stable patients. CSF MYD88L265P was detected in 97% of patients with BNS.
Although MTX-based therapies have historically been used for BNS, the PFS in this cohort is disappointing, with residual disease in half. Frontline PFS was 78% at 12 months, whereas it was 100% with BKTi. BTKi resulted in excellent PFS in both frontline and second-line settings, although it is limited by shorter follow-up. The significant use of high-dose therapy in this cohort reflects practices borrowed from high-grade CNS lymphoma, the aim to obtain rapid disease control in patients with neurological compromise, the pre-BTKi era, and the current unavailability of publicly funded frontline BTKis in England. This analysis adds weight to emerging data in favor of BTKis in BNS.5 Our study is limited by its retrospective nature and potentially differing characteristics in the chemoimmunotherapy and BTKi cohorts.
Patients who were initially positive for MYD88L265 and/or IgH rearrangements on CSF using AS-PCR but negative on posttreatment testing did not subsequently relapse from BNS. Requiring further study, this finding has the potential to guide follow-up, the need for routine disease re-evaluation, and may be a useful surrogate marker for PFS in studies.
We consider BTKi as the therapy of choice for BNS, considering this comparison and other series with encouraging outcomes.6,7 Chemoimmunotherapy still has a role in patients with suspected or proven transformation or potentially in those who are particularly symptomatic disease requiring rapid disease control, but a high proportion require further therapy with a BTKi thereafter. Ultimately, international, multicenter prospective trials are required to inform the optimum treatment approach for BNS, but this will be challenging in a rare disorder.
Overall- and progression-free survival with therapy for Bing-Neel syndrome. Frontline OS (A) and PFS (B) with all therapies. (C) Frontline PFS by therapy type. (D) Second-line PFS with BTKi therapy.
Overall- and progression-free survival with therapy for Bing-Neel syndrome. Frontline OS (A) and PFS (B) with all therapies. (C) Frontline PFS by therapy type. (D) Second-line PFS with BTKi therapy.
Contribution: O.T. conceived the study, collected data, performed analyses, and wrote the draft; S.D. supervised the study; and all authors critically appraised the study and contributed to the final manuscript.
Conflict-of-interest disclosure: J.L. reports membership on an entity's board of director or advisory committees in and other support for attending meetings and/or travel from Bristol Myers Squibb (BMS), Amgen, Takeda, and BeiGene; and honoraria and speakers’ bureau fees from Janssen, Takeda, and BMS. S.D. reports honoraria from, membership on an entity's board of directors or advisory committees in, and research funding from Janssen; honoraria from and membership on an entity's board of directors or advisory committees in Kite Pharma and Sanofi; membership on an entity's board of directors or advisory committees in Cellectar; and membership on an entity's board of directors or advisory committees in and research funding from BeiGene. M.P.L. reports ad hoc advisory boards, particularly on trial design, for Roche, AstraZeneca, Sanofi, UCB, Sanofi, Takeda, Polyneuron, and BeiGene (conference expenses and advisory board); unrestricted speaker fees from BeiGene and Grifols for the production of educational materials; and unrestricted conference expenses from BeiGene and CSL Behring. The remaining authors declare no competing financial interests.
Correspondence: Oliver Tomkins, University College London Hospitals NHS Foundation Trust, 250 Euston Road, London, United Kingdom; email: oliver.tomkins@nhs.net.
References
Author notes
Anonymized data are available on request from the corresponding author, Oliver Tomkins (oliver.tomkins@nhs.net).