TO THE EDITOR:
Infant acute lymphoblastic leukemia (iALL) is an aggressive disease that remains a major clinical challenge.1-3 In 70% to 80% of iALL, translocations of KMT2A gene, most commonly with AFF1 [t(4;11) (q21;q23)], produce an oncogenic fusion protein which recruits a large protein complex, "rewriting" epigenetic marks to alter expression of target genes.4-6 Understanding the KMT2A fusion protein complex has been vital for identifying targets such as menin for novel therapies.7 Investigation of genes regulated by KMT2A::AFF1 also remains an important goal.
Previously, we showed that one of the most profoundly dysregulated genes in KMT2A::AFF1 leukemia was PROM1, which encodes the cell surface glycoprotein CD133.8 Proliferation of KMT2A::AFF1 ALL cell lines was highly dependent on CD133 expression and PROM1/CD133 was expressed at significantly higher levels in KMT2Ar ALL than in KMT2A germ line ALL.8-10
CD133 has been identified as a marker of stem cells in many cancers11,12 including leukemia,13,14 and is also expressed on normal hematopoietic stem and progenitor cells.15 Expression of CD133 in samples from patients with KMT2A::AFF1 ALL, is often heterogenous and the mechanisms by which CD133 contributes to leukemia biology are unclear.16
Here we investigate the function of CD133 in KMT2A::AFF1 iALL using a primary human fetal liver–derived model of KMT2Ar leukemia17 that recapitulates the pattern of CD133 expression observed in patient samples. We found that CD133 marks an aggressive population of blasts with a stem cell-like signature in KMT2Ar ALL. This provides a rationale for targeting CD133 by pharmacological or immunotherapy-based approaches alongside other treatment modalities.
Single guide RNA (Synthego) triplets targeting the PROM1 start codon were used for PROM1 knockout (PROM1 KO) (supplemental Table 1). Cells were electroporated with Cas9-only or Cas9-single guide RNA complexes using a Neon Transfection System (Thermo Fisher) at 1600 V, 10 milliseconds, 3 pulses and recovered overnight in SFEM II (Stemline) supplemented with 10% fetal bovine serum (Invitrogen), 10 nM interleukin-3 and 5 nM interleukin-7 (Peprotech).
For CRISPRKMT2A::AFF1 blast coculture, MS5 stromal layers were prepared as described previously.17 About 2000 CRISPRKMT2A::AFF1 blasts were seeded/well in StemSpan SFEM II (serum free medium for culture and expansion of hematopoietic cells) supplemented as above and incubated at 37°C per 5% CO2 with twice-weekly half-volume medium changes. Flow-cytometric readouts were performed weekly with replating onto fresh stromal layers (supplemental Table 2).
For CRISPRKMT2A::AFF1 blast xenograft assays, CD19+ blasts from the bone marrow of mice that developed CRISPRKMT2A::AFF1 ALL were used for xenotransplantation assays, either after sorting for CD133+ and CD133– fractions, or after PROM1 KO. About 30 000 to 60 000 blasts were injected via tail vein into sublethally irradiated NSG mice, and the mice were monitored as described previously.17
Additional methods are described in the supplemental Material.
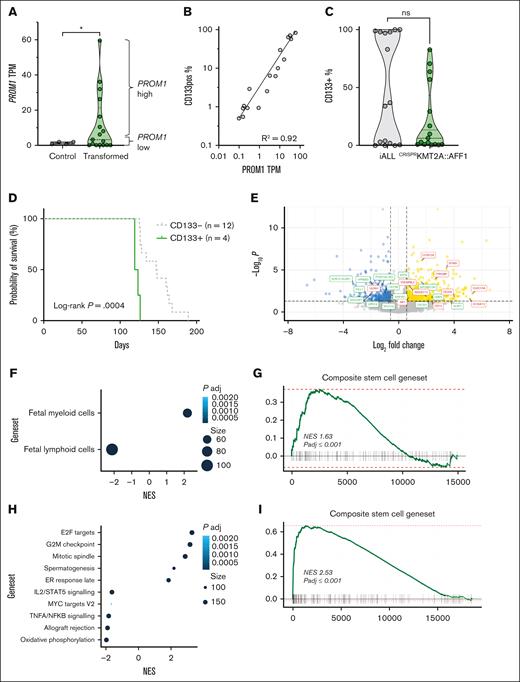
We examined expression of PROM1 and CD133 in the human fetal liver–derived CRISPRKMT2A::AFF1 iALL model.17PROM1 expression was significantly increased in CD19+ bone marrow blasts from mice with CRISPRKMT2A::AFF1 leukemia compared to Cas9-only controls (Figure 1A; P = .02), and correlated strongly with CD133 cell surface protein expression as measured by flow cytometry (Figure 1B). The pattern of CD133 expression in CRISPRKMT2A::AFF1 leukemias was variable, as in patient samples (Figure 1C).
The CRISPRKMT2A::AFF1 iALL model recapitulates variation in CD133 expression, and CD133 status correlates with differences in functional and molecular signatures. (A) PROM1 TPM from hCD45+CD19+ cells from bone marrow of NSG mice transplanted with Cas9-only (control, n = 4) and CRISPRKMT2A::AFF1 leukemias (transformed, n = 17) fetal liver CD34+ cells (P = .02). PROM1 high (above median) and low (below median) leukemias are indicated by brackets. (B) Correlation of PROM1 gene expression and CD133 protein expression by flow cytometry for CRISPRKMT2A::AFF1 leukemias (n = 17, R2 = 0.92). (C) CD133 positivity in CD19+ blasts in samples of patients with iALL at diagnosis (n = 11) and relapse (n = 5) compared to CRISPRKMT2A::AFF1 leukemias (n = 17). (D) Survival curves of NSG mice with CD133+ (defined as >20% of blasts CD133+) or CD133– primary CRISPRKMT2A::AFF1 leukemias (n = 4 CD133+, n = 12 CD133–, log-rank P = .0004). (E) Volcano plot of differential gene expression between PROM1 high and low CRISPRKMT2A::AFF1 leukemias. Genes with adjusted P < .05 and log-fold change >0.58 or less than –0.58 are shown in yellow and blue, respectively. Myeloid/lymphoid program associated genes are labelled in red and green, respectively. (F) Gene set enrichment analysis (GSEA) reaching statistical significance (false discovery rate [FDR] 0.05) from fetal entries in MSigDB C8 (cell type signature gene sets18), CRISPRKMT2A::AFF1 PROM1 high vs low. (G) GSEA of a core stem cell gene set,19CRISPRKMT2A::AFF1 PROM1 high vs low (normalized enrichment score = 1.63, adjusted P ≤ .001). (H) GSEA reaching statistical significance (FDR, 0.05) from entries in MSigDB H1 (hallmark gene sets), CRISPRKMT2A::AFF1 CD133+ vs CD133– blasts. (I) GSEA of a composite core stem cell gene set,19CRISPRKMT2A::AFF1 CD133+ vs CD133– blasts (normalized enrichment score = 2.88, adjusted P ≤ .001). (J) Uniform manifold approximation and projection (UMAP) of single-cell transcriptomic data of CD19+ blasts from 4 samples of patients with KMT2A::AFF1, displaying log1p (normalized PROM1 gene expression) (n = 2, chALL; n = 2, iALL, total of 14 927 cells). (K) Dot plot showing PROM1 expression pattern at a single-cell level in the 4 KMT2Ar patient samples in panel J. (L) Top 25 most enriched gene set entries in MSigDB H1 (hallmark gene sets) by GSEA, PROM1+ vs PROM1– blasts from KMT2A::AFF1 primary patient samples analyzed by single-cell (sc) RNA-sequencing in panel J. AKT, protein kinase B; chALL, childhood ALL; IL, interleukin; mTORC1, mammalian target of rapamycin complex 1; mTOR, mammalian target of rapamycin; ns, not significant; NES, normalized enrichment score; padj, P adjusted; PI3K, phosphoinositide 3-kinase; TPM, transcripts per million; TNFA, tumor necrosis factor alpha.
The CRISPRKMT2A::AFF1 iALL model recapitulates variation in CD133 expression, and CD133 status correlates with differences in functional and molecular signatures. (A) PROM1 TPM from hCD45+CD19+ cells from bone marrow of NSG mice transplanted with Cas9-only (control, n = 4) and CRISPRKMT2A::AFF1 leukemias (transformed, n = 17) fetal liver CD34+ cells (P = .02). PROM1 high (above median) and low (below median) leukemias are indicated by brackets. (B) Correlation of PROM1 gene expression and CD133 protein expression by flow cytometry for CRISPRKMT2A::AFF1 leukemias (n = 17, R2 = 0.92). (C) CD133 positivity in CD19+ blasts in samples of patients with iALL at diagnosis (n = 11) and relapse (n = 5) compared to CRISPRKMT2A::AFF1 leukemias (n = 17). (D) Survival curves of NSG mice with CD133+ (defined as >20% of blasts CD133+) or CD133– primary CRISPRKMT2A::AFF1 leukemias (n = 4 CD133+, n = 12 CD133–, log-rank P = .0004). (E) Volcano plot of differential gene expression between PROM1 high and low CRISPRKMT2A::AFF1 leukemias. Genes with adjusted P < .05 and log-fold change >0.58 or less than –0.58 are shown in yellow and blue, respectively. Myeloid/lymphoid program associated genes are labelled in red and green, respectively. (F) Gene set enrichment analysis (GSEA) reaching statistical significance (false discovery rate [FDR] 0.05) from fetal entries in MSigDB C8 (cell type signature gene sets18), CRISPRKMT2A::AFF1 PROM1 high vs low. (G) GSEA of a core stem cell gene set,19CRISPRKMT2A::AFF1 PROM1 high vs low (normalized enrichment score = 1.63, adjusted P ≤ .001). (H) GSEA reaching statistical significance (FDR, 0.05) from entries in MSigDB H1 (hallmark gene sets), CRISPRKMT2A::AFF1 CD133+ vs CD133– blasts. (I) GSEA of a composite core stem cell gene set,19CRISPRKMT2A::AFF1 CD133+ vs CD133– blasts (normalized enrichment score = 2.88, adjusted P ≤ .001). (J) Uniform manifold approximation and projection (UMAP) of single-cell transcriptomic data of CD19+ blasts from 4 samples of patients with KMT2A::AFF1, displaying log1p (normalized PROM1 gene expression) (n = 2, chALL; n = 2, iALL, total of 14 927 cells). (K) Dot plot showing PROM1 expression pattern at a single-cell level in the 4 KMT2Ar patient samples in panel J. (L) Top 25 most enriched gene set entries in MSigDB H1 (hallmark gene sets) by GSEA, PROM1+ vs PROM1– blasts from KMT2A::AFF1 primary patient samples analyzed by single-cell (sc) RNA-sequencing in panel J. AKT, protein kinase B; chALL, childhood ALL; IL, interleukin; mTORC1, mammalian target of rapamycin complex 1; mTOR, mammalian target of rapamycin; ns, not significant; NES, normalized enrichment score; padj, P adjusted; PI3K, phosphoinositide 3-kinase; TPM, transcripts per million; TNFA, tumor necrosis factor alpha.
To interrogate how PROM1/CD133 expression affects the biology of KMT2A::AFF1 iALL, we compared CD133+ and CD133–CRISPRKMT2A::AFF1 ALL. NSG mice developing primary CD133+CRISPRKMT2A::AFF1 leukemia (n = 4) had significantly shorter survival than mice developing CD133– (n = 12) leukemia (Figure 1D; 119 vs 140.7 days median, P = .0004). RNA sequencing of CD19+ blasts showed enrichment of fetal myeloid programs18 and a composite stem cell gene set19 along with reduced expression of lymphoid programs18 in PROM1-high CRISPRKMT2A::AFF1 ALL (Figure 1E-G; supplemental Figure 1A-B; supplemental Table 3). Some of these features were also seen in primary patient samples10 when comparing PROM1-high and PROM1-low KMT2A::AFF1 ALL (supplemental Figure 1C).
As CD19+ blasts from the same leukemia (both in patients and CRISPRKMT2A::AFF1 ALL) have variable expression of CD133, we prospectively isolated CD133+ and CD133– blasts from CRISPRKMT2A::AFF1 leukemias to determine the correlation between CD133 expression and the molecular and functional characteristics of PROM1-high leukemias (supplemental Figure 2A). RNA sequencing showed that of the 162 upregulated and 75 downregulated differentially expressed genes (supplemental Table 4) in CD133+ blasts, most enriched gene sets were associated with cell cycling, cell division, survival, and proliferation, as well as stemness (Figure 1H-I).
We generated single-cell transcriptomic data from CD19+ blasts fluorescence-activated cell sorted (FACS) from 4 samples of patient with KMT2Ar ALL (2 infants: iALL; 2 childhood) (supplemental Figure 2B). Childhood ALL-2 showed no PROM1 expression, whereas the other 3 samples demonstrated a proportion of cells with heterogeneous levels of PROM1 expression (Figure 1J-K), which correlated well with protein expression (supplemental Table 5). PROM1+ blasts enriched for cell proliferation signatures (myelocytomatosis family/early region 2 binding factor targets, mammalian target of rapamycin complex 1 signaling) in comparison to PROM1– blasts (Figure 1L; supplemental Figure 2C), similar to CD133+CRISPRKMT2A::AFF1 blasts. However, PROM1+ patient blasts also enriched for OXPHOS pathway, unlike CD133+CRISPRKMT2A::AFF1 blasts.
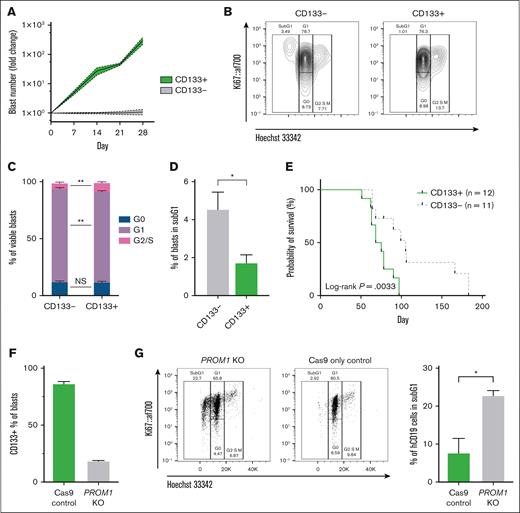
In keeping with these findings, FACS CD133+CRISPRKMT2A::AFF1 blasts underwent ∼100-fold higher expansion in vitro compared to CD133– blasts from the same leukemia (Figure 2A). More CD133+ blasts were in S/G2/M phase after 7 days in vitro (Figure 2B-C; 10.0% vs 5.8%, P = .003), whereas fewer were in subG1 phase (Figure 2D; 1.7% vs 4.5%, P = .01) compared to CD133- blasts, consistent with increased cell cycling and apoptosis resistance. Confirming these findings in vivo, mice transplanted with CD133+CRISPRKMT2A::AFF1 blasts showed more rapid engraftment (supplemental Figure 2D) and reduced survival (Figure 2E; 71 vs 105 days median, P = .0033).
CD133 expression marks KMT2A::AFF1 blasts with higher proliferative potential. (A) Proliferation of CD133+ and CD133–CRISPRKMT2A::AFF1 blasts in MS5 coculture. Data shown as fold change in absolute blast count of CD133+ cells compared to CD133– cells at each time point (n = 4 biological replicates, mean values with error bars = standard error of the mean [SEM]). (B) Representative flow plots from cell cycle analysis of CD133– (left) and CD133+ (right) CRISPRKMT2A::AFF1 blasts on day 7 of coculture. (C) Quantification of cell cycle status of CD133– and CD133+CRISPRKMT2A::AFF1 blasts on day 7 of coculture (n = 4 biological replicates, data shown as mean ± SEM). (D) Percentage of CD133– and CD133+CRISPRKMT2A::AFF1 blasts in sub-G1 phase on day 7 of coculture (n = 4 biological replicates, data shown as mean, error bars = SEM). (E) Survival curves of NSG immunodeficient mice transplanted with CD133+ or CD133–CRISPRKMT2A::AFF1 leukemia cells (4 biological replicates, n = 12 CD133+ animals, n = 11 CD133– animals, log-rank P = .0033). (F) Efficiency of PROM1 KO in CRISPRKMT2A::AFF1 blasts assessed by CD133 surface protein expression by flow cytometry, 7 days postelectroporation (error bars = standard deviation). (G) Left: representative flow plots from cell cycle analysis of PROM1 KO and control CRISPRKMT2A::AFF1 blasts 72 hours after electroporation. Right: percentage of PROM1 KO and control CRISPRKMT2A::AFF1 blasts in sub-G1 phase 72 hours after electroporation (n = 4 biological replicates, data shown as mean, error bars = SEM). (H) Proliferation in MS5 coculture of PROM1 KO and control CRISPRKMT2A::AFF1 leukemia cells. Y-axis values expressed as fold change in absolute blast count of PROM1 KO cells compared to control CRISPRKMT2A::AFF1 cells at each time point (n = 4 biological replicates, error bars = SEM). (I) Top row: survival curves of immunodeficient NSG mice transplanted with PROM1 KO and control CRISPRKMT2A::AFF1 blasts for 2 CD133+ leukemias (n = 3 Cas9 controls and n = 3 PROM1 KO animals for each leukemia; P = .06 and P = .02, respectively). Bottom row: CD133 and CD34 status of blast cells recovered from bone marrow of animals at cull.
CD133 expression marks KMT2A::AFF1 blasts with higher proliferative potential. (A) Proliferation of CD133+ and CD133–CRISPRKMT2A::AFF1 blasts in MS5 coculture. Data shown as fold change in absolute blast count of CD133+ cells compared to CD133– cells at each time point (n = 4 biological replicates, mean values with error bars = standard error of the mean [SEM]). (B) Representative flow plots from cell cycle analysis of CD133– (left) and CD133+ (right) CRISPRKMT2A::AFF1 blasts on day 7 of coculture. (C) Quantification of cell cycle status of CD133– and CD133+CRISPRKMT2A::AFF1 blasts on day 7 of coculture (n = 4 biological replicates, data shown as mean ± SEM). (D) Percentage of CD133– and CD133+CRISPRKMT2A::AFF1 blasts in sub-G1 phase on day 7 of coculture (n = 4 biological replicates, data shown as mean, error bars = SEM). (E) Survival curves of NSG immunodeficient mice transplanted with CD133+ or CD133–CRISPRKMT2A::AFF1 leukemia cells (4 biological replicates, n = 12 CD133+ animals, n = 11 CD133– animals, log-rank P = .0033). (F) Efficiency of PROM1 KO in CRISPRKMT2A::AFF1 blasts assessed by CD133 surface protein expression by flow cytometry, 7 days postelectroporation (error bars = standard deviation). (G) Left: representative flow plots from cell cycle analysis of PROM1 KO and control CRISPRKMT2A::AFF1 blasts 72 hours after electroporation. Right: percentage of PROM1 KO and control CRISPRKMT2A::AFF1 blasts in sub-G1 phase 72 hours after electroporation (n = 4 biological replicates, data shown as mean, error bars = SEM). (H) Proliferation in MS5 coculture of PROM1 KO and control CRISPRKMT2A::AFF1 leukemia cells. Y-axis values expressed as fold change in absolute blast count of PROM1 KO cells compared to control CRISPRKMT2A::AFF1 cells at each time point (n = 4 biological replicates, error bars = SEM). (I) Top row: survival curves of immunodeficient NSG mice transplanted with PROM1 KO and control CRISPRKMT2A::AFF1 blasts for 2 CD133+ leukemias (n = 3 Cas9 controls and n = 3 PROM1 KO animals for each leukemia; P = .06 and P = .02, respectively). Bottom row: CD133 and CD34 status of blast cells recovered from bone marrow of animals at cull.
To assess drug sensitivity profiles, we performed CellTiter-Glo cell-viability assay on FACS CD133+ and CD133–CRISPRKMT2A::AFF1 blasts after treatment with 4 ALL drugs: prednisolone, L-asparaginase, daunorubicin, and vincristine. This showed no significant difference in drug sensitivities between CD133+ and CD133– blasts (supplemental Figure 3).
Finally, the functional requirement for CD133 was investigated in a relevant primary human fetal hematopoietic stem and progenitor cell–derived model system.17PROM1 KO in CRISPRKMT2A::AFF1 ALL led to a significant loss of CD133 expression by flow cytometry (Figure 2F), and an increased proportion of cells in sub-G1 phase 72 hours after KO (Figure 2G; 22.8% vs 7.7%, P = .02), suggesting increased apoptosis in PROM1 KO cells. In keeping with this, PROM1 KO significantly reduced the proliferative potential of CRISPRKMT2A::AFF1 blasts cultured in vitro (Figure 2H). PROM1 KO or Cas9-only control CRISPRKMT2A::AFF1 blasts were also transplanted into NSG mice 12 to 18 hours after KO (n = 2 CRISPRKMT2A::AFF1 leukemias, 12 mice). Mice transplanted with PROM1 KO blasts had a longer survival duration compared to Cas9-only controls (Figure 2I). Leukemias in the Cas9 control mice had a similar proportion of CD133+ blasts at cull as the original leukemia, but leukemias derived from PROM1 KO blasts also demonstrated some CD133 positivity at the point of culling (Figure 2I). The CD133+ blasts in PROM1 KO xenografts probably represent outgrowth of residual blasts which escaped KO (Figure 2F).
Together, these data show that in CRISPRKMT2A::AFF1 ALL, CD133+ blasts are enriched for a stem cell-like signature and possess almost all the proliferative potential of the leukemia. Enhancement of leukemic proliferation by CD133 can be seen directly in vitro and indirectly in vivo, with animals receiving CD133– blasts having prolonged survival.
Our studies support a role for CD133 in promoting proliferation and resisting apoptosis in KMT2A::AFF1 ALL blasts. Although immunotherapeutic targeting of functionally neutral markers, such as CD19, has been successful in the treatment of iALL,20,21 treatment evasion by downregulation of the target molecule,22 or relapse driven by CD19– upstream progenitors23 remains a serious clinical problem. We conclude that in KMT2Ar leukemias where CD133 is expressed, there is a strong rationale for targeting this molecule as part of a combination approach.24,25
Acknowledgments: The authors are grateful for the technical support received from the WIMM Flow Cytometry Core, WIMM Sequencing Core, WIMM Centre for Computational Biology, and School of Medicine and Biomedical Sciences, John Radcliffe Hospital. The authors are grateful to Kent Fung, Mansour Lab, University College London for providing aliquots of L-asparaginase for their experiments.
This work was funded by grants from the Children's Cancer and Leukaemia Group/Little Princess Trust (CCLGA 2020 23 Roy), and the Azaylia Foundation. N.C. was supported by a Kay Kendall Leukaemia Fund Intermediate Fellowship (KKL1443). T.A.M. and A.S. are both supported by the Medical Research Council (MRC), United Kingdom, Molecular Haematology Unit grant MC_UU_00029/6. A.R. is supported by a Wellcome Trust Clinical Research Career Development Fellowship (216632/Z/19/Z) and MRC, United Kingdom Molecular Haematology Unitgrant MC_UU_00029/7. The human fetal material was provided by the Joint MRC/Wellcome Trust grant 099175/Z/12/Z Human Developmental Biology Resource (http://hdbr.org). Samples from patients with acute lymphoblastic leukemia used in this study were provided by VIVO Biobank, supported by Cancer Research UK & Blood Cancer UK (grant CRCPSC-Dec21\100003).
Contribution: J.W.C. contributed to investigation, formal analysis, and writing the original draft of the manuscript; L.F. performed investigation; A.S. contributed to investigation, formal analysis, and writing, review, and editing of the manuscript; J.H. performed investigation and formal analysis; L.H., E.N., T.J., S.R., N.C., R.E.L., N.E., Q.W., N.E.O., and R.T. performed investigation; S.I. performed formal analysis; J. Bartram, O.S., J. Bond, and I.R. contributed to writing, review, and editing of the manuscript; and T.A.M. and A.R. contributed to conceptualization and writing, review, and editing of the manuscript, and provided supervision.
Conflict-of-interest disclosure: T.A.M. and N.C. are paid consultants for and shareholders in Dark Blue Therapeutics Ltd. The remaining authors declare no competing financial interests.
Correspondence: Anindita Roy, Department of Paediatrics, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, United Kingdom, OX3 9DS; email: anindita.roy@paediatrics.ox.ac.uk.
References
Author notes
T.A.M. and A.R. contributed equally to this study.
The full-text version of this article contains a data supplement.

![The CRISPRKMT2A::AFF1 iALL model recapitulates variation in CD133 expression, and CD133 status correlates with differences in functional and molecular signatures. (A) PROM1 TPM from hCD45+CD19+ cells from bone marrow of NSG mice transplanted with Cas9-only (control, n = 4) and CRISPRKMT2A::AFF1 leukemias (transformed, n = 17) fetal liver CD34+ cells (P = .02). PROM1 high (above median) and low (below median) leukemias are indicated by brackets. (B) Correlation of PROM1 gene expression and CD133 protein expression by flow cytometry for CRISPRKMT2A::AFF1 leukemias (n = 17, R2 = 0.92). (C) CD133 positivity in CD19+ blasts in samples of patients with iALL at diagnosis (n = 11) and relapse (n = 5) compared to CRISPRKMT2A::AFF1 leukemias (n = 17). (D) Survival curves of NSG mice with CD133+ (defined as >20% of blasts CD133+) or CD133– primary CRISPRKMT2A::AFF1 leukemias (n = 4 CD133+, n = 12 CD133–, log-rank P = .0004). (E) Volcano plot of differential gene expression between PROM1 high and low CRISPRKMT2A::AFF1 leukemias. Genes with adjusted P < .05 and log-fold change >0.58 or less than –0.58 are shown in yellow and blue, respectively. Myeloid/lymphoid program associated genes are labelled in red and green, respectively. (F) Gene set enrichment analysis (GSEA) reaching statistical significance (false discovery rate [FDR] 0.05) from fetal entries in MSigDB C8 (cell type signature gene sets18), CRISPRKMT2A::AFF1 PROM1 high vs low. (G) GSEA of a core stem cell gene set,19CRISPRKMT2A::AFF1 PROM1 high vs low (normalized enrichment score = 1.63, adjusted P ≤ .001). (H) GSEA reaching statistical significance (FDR, 0.05) from entries in MSigDB H1 (hallmark gene sets), CRISPRKMT2A::AFF1 CD133+ vs CD133– blasts. (I) GSEA of a composite core stem cell gene set,19CRISPRKMT2A::AFF1 CD133+ vs CD133– blasts (normalized enrichment score = 2.88, adjusted P ≤ .001). (J) Uniform manifold approximation and projection (UMAP) of single-cell transcriptomic data of CD19+ blasts from 4 samples of patients with KMT2A::AFF1, displaying log1p (normalized PROM1 gene expression) (n = 2, chALL; n = 2, iALL, total of 14 927 cells). (K) Dot plot showing PROM1 expression pattern at a single-cell level in the 4 KMT2Ar patient samples in panel J. (L) Top 25 most enriched gene set entries in MSigDB H1 (hallmark gene sets) by GSEA, PROM1+ vs PROM1– blasts from KMT2A::AFF1 primary patient samples analyzed by single-cell (sc) RNA-sequencing in panel J. AKT, protein kinase B; chALL, childhood ALL; IL, interleukin; mTORC1, mammalian target of rapamycin complex 1; mTOR, mammalian target of rapamycin; ns, not significant; NES, normalized enrichment score; padj, P adjusted; PI3K, phosphoinositide 3-kinase; TPM, transcripts per million; TNFA, tumor necrosis factor alpha.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/18/10.1182_bloodadvances.2024015185/2/m_blooda_adv-2024-015185-gr1b.jpeg?Expires=1769106323&Signature=ICO31eXQdjwxHQCKVpu4hW5uQ9kU2yOwYXsBg4M2mpsY7MKc1oBd2zx61zQTg-gIU4aeSuYhzaHxKR6UkGd0GJXJWOQ5NHZp0x6sRtbHdgb7avAMESekQVw8HYyi68YlGDci2sluvSXekCQtcv3ioOSEUpQmPff3jQkBURDH-QRHHUFhFiVv4Jt1AQGIE8jqwSzMtp-yzoaQKTQQWczdMruz7VLKq94oB-CYweEsa-8htaj~vte-9~YYLn9ZY~RHk9W9MfJ68jmPmjLJvWUFxry3oI96AZpXLLy-HDKFyuy5Xc7MFRMPL4Jqp5J5gtQdzsU7viRISfwvR6ir-tfEhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD133 expression marks KMT2A::AFF1 blasts with higher proliferative potential. (A) Proliferation of CD133+ and CD133–CRISPRKMT2A::AFF1 blasts in MS5 coculture. Data shown as fold change in absolute blast count of CD133+ cells compared to CD133– cells at each time point (n = 4 biological replicates, mean values with error bars = standard error of the mean [SEM]). (B) Representative flow plots from cell cycle analysis of CD133– (left) and CD133+ (right) CRISPRKMT2A::AFF1 blasts on day 7 of coculture. (C) Quantification of cell cycle status of CD133– and CD133+CRISPRKMT2A::AFF1 blasts on day 7 of coculture (n = 4 biological replicates, data shown as mean ± SEM). (D) Percentage of CD133– and CD133+CRISPRKMT2A::AFF1 blasts in sub-G1 phase on day 7 of coculture (n = 4 biological replicates, data shown as mean, error bars = SEM). (E) Survival curves of NSG immunodeficient mice transplanted with CD133+ or CD133–CRISPRKMT2A::AFF1 leukemia cells (4 biological replicates, n = 12 CD133+ animals, n = 11 CD133– animals, log-rank P = .0033). (F) Efficiency of PROM1 KO in CRISPRKMT2A::AFF1 blasts assessed by CD133 surface protein expression by flow cytometry, 7 days postelectroporation (error bars = standard deviation). (G) Left: representative flow plots from cell cycle analysis of PROM1 KO and control CRISPRKMT2A::AFF1 blasts 72 hours after electroporation. Right: percentage of PROM1 KO and control CRISPRKMT2A::AFF1 blasts in sub-G1 phase 72 hours after electroporation (n = 4 biological replicates, data shown as mean, error bars = SEM). (H) Proliferation in MS5 coculture of PROM1 KO and control CRISPRKMT2A::AFF1 leukemia cells. Y-axis values expressed as fold change in absolute blast count of PROM1 KO cells compared to control CRISPRKMT2A::AFF1 cells at each time point (n = 4 biological replicates, error bars = SEM). (I) Top row: survival curves of immunodeficient NSG mice transplanted with PROM1 KO and control CRISPRKMT2A::AFF1 blasts for 2 CD133+ leukemias (n = 3 Cas9 controls and n = 3 PROM1 KO animals for each leukemia; P = .06 and P = .02, respectively). Bottom row: CD133 and CD34 status of blast cells recovered from bone marrow of animals at cull.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/18/10.1182_bloodadvances.2024015185/2/m_blooda_adv-2024-015185-gr2b.jpeg?Expires=1769106323&Signature=jSqRxzVKu8eAw4k7Nj5i5b7Ks2WV09YXuxDjal29NmJAp3ta57GrxdOCyP05xW8LT9W3yRK5NSo5pdJz9wKOm4a3z5PL9A74kS55aJquQJGSCckvhMFgt13lPPrWzNRl9tuKTHWooV4GkK9PzocVnhy1nvsZkyHgllZP38VCGXt9BauueOwpXlGEv0769cWlx0fQgCaMNXgWODTSTc~JGKUxgrdsUsX0oa3PhYfRp5A6yuXP3uJ2cRWu6arsg2MwAB-LN9DJbCh~Jc~VumKhreTZJ78i7xNWFFEG8TSdJQ4iPDabiJOgkl3qmlV6ZbUoolEdc2gL4Qy8Uc~wdcc1hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)