Key Points
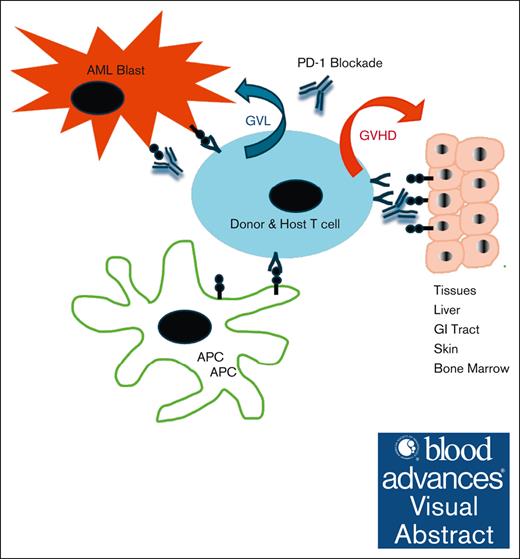
Anti–PD-1 therapy can elicit durable responses in AML/MDS with post-HCT relapse; mixed CD3 chimerism may be a predictive treatment response.
Severe acute GVHD was observed highlighting the need for strategies to mitigate this complication to enable broader use of anti–PD-1 therapy.
Visual Abstract
Relapse of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) remains the primary source of mortality after allogeneic hematopoietic stem cell transplantation (HCT). Targeting programmed death-1 (PD-1) for reversing T-cell exhaustion and restoring the graft-versus-leukemia (GVL) effect may have logistical advantages over donor lymphocyte infusion. In a prospective phase 1B clinical trial, pembrolizumab was administered every 3 weeks to 16 patients with AML (n = 12) and MDS (n = 4) in relapse after HCT to assess graft-versus-host disease (GVHD), clinical response, and survival. The median time to relapse after HCT was 5.5 months and the median pretreatment bone marrow blast percentage was 21.5%. The overall response rate was 31.3% for patients receiving pembrolizumab, consisting of 3 complete remissions (18.8%) and 2 partial remissions (13.5%). The median duration of response was 610 days. A significantly greater proportion of patients with mixed CD3 chimerism had a clinical response than those with full donor chimerism (50% vs 0%; P = .03). Immune toxicities were frequent, with 37.5% of patients developing severe (grade 3-4) GVHD after pembrolizumab, of which most had resistance to corticosteroids and contributed to death in 4 patients (25%). The 1-year overall survival (OS) was 37.5% and event-free survival was 31.3%. For AML, 1-year OS was 50.0%. In this trial, PD-1 inhibition led to durable remission in one-third of the patients experiencing early relapse after HCT, suggesting that this approach may augment the GVL response. Responses were exclusively observed in the setting of mixed CD3 donor chimerism. Immune toxicities (GVHD) were a barrier to successful treatment outcome. This trial was registered at www.ClinicalTrials.gov as #NCT03286114.
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) is critically reliant on unlocking graft-versus-leukemia (GVL) effects to eradicate leukemia.1 Several analyses have found a close link between the incidence of graft-versus-host disease (GVHD) and reduced relapse.2,3 A longstanding strategy for relapse after HCT is to enhance GVL response using therapeutic donor lymphocyte infusion (DLI). Nonetheless, unmanipulated DLI with or without chemotherapy is logistically complex, can precipitate acute GVHD (46%-60%), and is associated with modest response rates (15%-34%) which underpin the dismal survival for acute myeloid leukemia (AML) relapse after HCT.4-6
More recently, immune checkpoint inhibitors have been deployed clinically to elicit GVL. Experimental models suggest that programmed death protein ligand 1 (PD-L1) in tissues can induce exhaustion of donor CD8+ T cells.7 AML blasts also directly contribute to immune escape through upregulation of PD-L1,8-11 and clinical studies suggest the presence of exhausted CD8+ T cells correlated with response to anti–PD-1 treatment.12,13 Anti–PD-1 therapy after HCT occurs in classical Hodgkin Lymphoma, an entity with intrinsic responsiveness in nonallogeneic settings,14 has also been characterized by high remission rates after HCT, albeit at the expense of severe GVHD and immune-related adverse events (irAEs).15-19 In contrast, the effectiveness of PD-1 inhibition after HCT in myeloid malignancies has remained disappointing (0%-21% overall response).18,19
To date, clinical studies of PD-1 inhibitors in AML and myelodysplastic syndrome (MDS) after HCT have included limited patient numbers, heterogeneous populations, and have focused on late HCT relapse for safety considerations.17,18 This poses a challenge in addressing the role of PD-1 in early relapse (<6 months post-HCT), wherein disease progression can be rapidly fatal. We hypothesized that targeting PD-1 with pembrolizumab during early post-HCT relapse would increase the GVL effect, resulting in improved clinical responses in AML and MDS, acknowledging the likely risk of GVHD. To study these effects, we conducted an open-label, prospective, feasibility study of pembrolizumab for early relapse after HCT.
Methods
Patients
A phase 1B single-center, open-label, prospective clinical trial to assess the safety and feasibility of pembrolizumab was performed in patients with AML and MDS relapse early after allogeneic HCT (ClinicalTrials.gov identifier: NCT03286114). The study procedures involved screening all patients who experienced a relapse of primary hematologic malignancy after HCT for eligibility from December 2017 to October 2020. The study was voluntarily suspended and later closed during the COVID-19 pandemic for logistical reasons.
Eligibility consisted of patients aged ≥18 years with biopsy-confirmed relapse of AML or MDS with measurable disease defined as a marrow blast percentage of >5%. For ≤5% blasts, flow cytometric, cytogenetic, or pre-HCT molecular aberrations were necessary to confirm relapse. There were no eligibility restrictions according to donor type (eg, degree of HLA match), conditioning intensity, or GVHD prophylaxis. Patients were excluded if they received >1 prior line of therapy directed toward post-HCT relapse, did not achieve primary neutrophil engraftment, had active acute or chronic GVHD, had a history of autoimmune condition, noninfectious pneumonitis, or were actively receiving immune suppressive therapy within 7 days of study entry (calcineurin inhibitor or corticosteroids equivalent to ≥10 mg prednisone per day or any other immunosuppressive medications). Participant eligibility required a serum creatinine level ≤1.5× the upper limit of normal (ULN), total bilirubin ≤1.5× ULN, aspartate aminotransferase and alanine aminotransferase ≤5× ULN, and an Eastern Cooperative Oncology Group performance status of ≤1.
The study allowed patients with prior GVHD who were quiescent (grade 0) to enroll. The protocol did not prespecify a duration of GVHD remission for eligibility. An amendment to allow patients to continue GVHD prophylaxis at the start of pembrolizumab treatment (if taken before enrollment) was approved after 4 of the first 6 patients developed stopping events (steroid-refractory [SR] GVHD, n = 3; irAE, n = 1). All patients provided informed consent on an institutional review board–approved protocol. The full details of the protocol are found in the supplemental Appendix.
Study design
Treatment
Pembrolizumab (Keytruda, Merck) was administered at a flat dose of 200 mg IV once every 21 days (±3 days) for up to 4 cycles (induction). Patients with clinical benefit, defined as partial remission (PR) or complete remission (CR), without toxicity were eligible to receive additional dosages for up to 1 year (maintenance). Supportive care was according to the clinical practice guidelines at the University of Michigan. Patients were treated for a minimum of 2 cycles unless toxicity or evidence of a rapidly rising of blast percentage. The development of grade ≥2 acute GVHD resulted in treatment hold and grade ≥3 permanent discontinuation. Evidence of persistence or progressive disease resulted in treatment discontinuation.
Primary and secondary objectives
The primary objectives were to determine the safety, overall response rate (ORR) to pembrolizumab (with or without subsequent chemotherapy), and the rate of GVHD and/or clinically significant immune-mediated toxicity. The secondary objectives were to estimate the 1-year overall survival (OS) and disease-free survival in patients administered pembrolizumab.
Assessment of response
Remission was assessed using bone marrow (BM) aspirate and biopsy on day 35 (after cycle 2) and day 77 (after cycle 4) of pembrolizumab. In the analysis of ORR to pembrolizumab monotherapy on day 77, any prior receipt of next-line chemotherapy was considered a treatment failure. In a preplanned (secondary) analysis, we evaluated the day 77 CR/PR rate after allowing the patients to proceed to next-line chemotherapy.
CR was defined as a morphologic leukemia-free state by achieving all of the following criteria: BM blasts <5% by morphologic assessment, absence of circulating blasts with phenotypic or morphologic features of leukemia (eg, Auer rods), and no evidence of extramedullary disease. Patients with ≤5% blasts but possessing flow cytometric, cytogenetic, or leukemia-defining molecular aberrations required resolution after treatment to be classified as CR. PR was defined as a ≥50% reduction in BM blast percentage to 5% to 25% or marrow blasts <5% but with persistent Auer rods, flow cytometric, or cytogenetic disease. Stable disease (SD) was defined as a ≤5% increase in blasts or a decreased blast percentage in the BM that did not meet the criteria for PR. Progression was defined as a >5% increase in BM blast percentage on examination. Not performing a treatment assessment on day 35 or day 77 after HCT was recorded as a lack of response (treatment failure).
Statistics
The estimated sample size was 20 patients to determine whether pembrolizumab would be promising for further study. This sample size was based on probability estimates that with ≥4 patients experiencing clinical benefits, we could be 90% confident that the true clinical benefit rate (CBR) would be between 7% and 40%. The observation of any CRs to pembrolizumab monotherapy without subsequent chemotherapy would be considered promising. The CBR was assessed on day 35 and day 77 based on BM examination and was defined as the ORR plus SD responses. In our primary analysis, SD responses were later excluded from the CBR, given their uncertain clinical benefits. The secondary end points consisted of OS and event-free survival (EFS) at 1 year using the Kaplan-Meier method. OS was recorded from the time of post-HCT relapse until the earliest death or last follow-up. EFS was measured from the time of post-HCT relapse until the earliest occurenece of death, relapse, or last follow-up. GVHD-free, relapse-free survival (GRFS) was estimated from the time of post-HCT relapse until the occurrence of grade 3 to 4 acute GVHD, chronic GVHD, or relapse. Changes in plasma cytokine levels and biomarkers were assessed using paired t tests.
Dose-limiting toxicity events were defined as severe acute GVHD (grade ≥3-4) or grade ≥3 irAEs that did not improve to grade 1 after systemic corticosteroid treatment for 14 days. As toxicity was anticipated, stopping rules for excessive severe immune-related toxicities and acute GVHD unresponsive to steroids were performed after the first 10 and 15 patients.
Chimerism and correlative studies
For chimerism studies, the patients’ post-HCT peripheral blood was analyzed for donor and recipient microsatellite markers using multiplex polymerase chain reaction and differential fluorescence. Full chimerism required the establishment of ≥95% donor cells after HCT. Plasma was collected before pembrolizumab treatment and on day 35 after treatment. Plasma cytokines and GVHD biomarkers were analyzed using Luminex (Invitrogen). Formalin-fixed, paraffin-embedded tissue sections from pretreatment BM examination were cut at 3 to 4 microns and stained with antibodies against CD3, CD4, CD8, and PD-L1. The slides were stained using an automated immunostainer (Ventana Discovery Ultra; Ventana, Tucson, AZ). PD-L1 expression on blast cells was assessed and scored semiquantitively in 5% increments.
Results
Patient characteristics and dosing feasibility
A total of 16 patients were eligible for the study analysis per protocol and treated with ≥1 doses of pembrolizumab for post-HCT relapse for AML (n = 12) or MDS (n = 4). The median age was 55 years (range, 23-69), and the median time to relapse after HCT was 5.5 months. Poor risk cytogenetics were detected in 81%, monosomal karyotype in 56%, and TP53 mutations in 37% of patients. The median BM blast percentage at time of post-HCT relapse was 21.5% (range, 5%-92%) in the total cohort and 26.5% (range, 5%-92%) in AML (Table 1).
The median time from confirmed post-HCT relapse to the initial dosing of pembrolizumab was 18.5 days (range, 7-47), typically reflecting the time for the withdrawal of calcineurin inhibitors. The median number of pembrolizumab treatments administered was 1 (range, 1- 4), with 5 of 16 patients (31%) receiving ≥2 dosages. Progression of malignancy (n = 8), toxicity related to GVHD, immune-related serious AEs (irSAEs; n = 7), and investigator preference (n = 1) were reasons for discontinuation of study treatment.
Response to pembrolizumab with or without chemotherapy
The primary end point of day 35 ORR was 31.3% for patients receiving pembrolizumab monotherapy, consisting of 3 CRs (18.8%) and 2 PRs (13.5%). The day 77 ORR to pembrolizumab monotherapy was 18.8%, which consisted of 3 CRs (Table 2). Therefore, the protocol met its objective for expected response percentage on day 35 but not on day 77. The decline in the response rate from monotherapy from day 35 to day 77 reflected 2 patients becoming unevaluable for assessment (nonresponders) due to the development of GVHD. Conversely, 1 patient showed an improvement in response from PR to CR.
The best ORR on day 77 was 43.8% (CR, 37.5%; PR, 6.3%) when allowing for patients who received next-line reinduction chemotherapy. This analysis included 6 patients who received subsequent induction chemotherapy after pembrolizumab for disease progression. Among these 6 patients, 4 had responses (CR, n = 3; PR, n = 1). At the best ORR, 4 of 7 patients were GVHD-free. GVHD cases in the responding patients included grade 1 (n = 1) and grade 3 (n = 2), 1 of which was SR. In patients with AML (n = 12) who received monotherapy, the day 35 ORR was 34% (CR, n = 2; PR, n = 2) and the day 77 ORR was 25% (CR, n = 3). The day 77 ORR for patients with AML who received pembrolizumab, including next-line chemotherapy, was 58% (supplemental Table 1).
There was no difference in the response rate according to pretreatment median blast percentage; however, no response to monotherapy occurred in patients with >28% blasts (supplemental Figure 1). Two of 9 (22%) patients with a monosomal karyotype and 1 of 6 patients (17%) with a confirmed TP53 mutation responded on day 35. Among the patients who continued immune suppression while initiating pembrolizumab (n = 5), 2 experienced CR on day 35. Patients with a longer median onset of relapse after HCT (≥168 days) had a numerically higher response rate to pembrolizumab monotherapy on day 35 than those with an earlier relapse (50% vs 12.5%; P = .11).
GVHD and irSAEs
A total of 9 patients developed de novo or flare of GVHD after receiving pembrolizumab, 6 (37.5%) patients developed severe (grade 3-4) disease, and 5 were resistant to steroid treatment after 14 days (31.3%; Table 3). Among the patients with grade 3/4 GVHD, 5 had hepatic involvement and 4 had multiple organ involvement (supplemental Table 2). irSAEs were observed in 2 additional patients without GVHD. Therefore, 7 patients (43.8%) experienced either steroid-resistant GVHD or irSAEs, which resulted in discontinuation of treatment and were considered stopping events. The protocol did not meet the predefined stopping criteria for toxicity (see “Methods”). Overall, all patients who developed lower-grade GVHD (n = 3; stage, 1-3 skin) responded after cessation of pembrolizumab; however, only 3 of 8 patients with grade 3/4 and/or irSAEs had an initial response to systemic corticosteroids. In 4 patients, GVHD was determined to be a major contributor to mortality.
Patients with clinically active GVHD were prohibited from enrollment; however, 37% of patients had previously resolved GVHD. Patients with resolved GVHD did not have a higher rate of SR GVHD after pembrolizumab than those without GVHD (33.3% vs 30%; P = .89). There was also no significant difference in the proportion of patients who developed SR GVHD based on whether tacrolimus was continued or withdrawn before initiation of pembrolizumab (20% vs 36%; P = .51). A shorter median time to relapse after HCT (<168 days) was not associated with rates of GVHD.
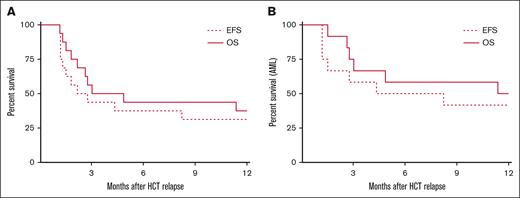
OS, EFS, and time to progression
The OS at 1 year was 37.5% (95% confidence interval (CI), 15.4-59.8) and EFS was 31.3% (95% CI, 11.1-53.7; Figure 1A). At 1 year, 37.5% of the patients were alive and free from GVHD. The 1 year estimated GRFS was 18.75%. For AML, 1 year OS was 50.0% (95% CI, 20.8-73.6) and EFS was 41.7% (95% CI, 15.2-66.5; Figure 1B). Among the patients who achieved CR by day 77 (n = 6), the median duration of response was 610 days (range, 250-875). Of the patients in long-term remission (>6 months) after treatment who subsequently relapsed, all presented initially with extramedullary AML.
Survival. OS and EFS for the entire cohort (n = 16) (A) and patients with AML (n = 12) (B).
Survival. OS and EFS for the entire cohort (n = 16) (A) and patients with AML (n = 12) (B).
Chimerism
Chimerism for myeloid (CD33) and lymphoid (CD3) lineages was obtained from the peripheral blood at the time of relapse before the initiation of pembrolizumab. We analyzed whether the presence of pretreatment mixed chimerism was associated with clinical response and toxicity.
In the CD33 lineage, 1 patient lost donor chimerism (secondary graft failure), 9 patients had mixed chimerism, and 6 had full donor chimerism. As expected, a lower percentage of donor CD33 chimerism was inversely correlated with BM myeloid blasts (r = −0.776; P < .001). The presence or absence of CD33 mixed chimerism was not associated with subsequent SR GVHD or irSAE. The median percentage of donor CD33 chimerism in responders was 100%, but did not significantly differ from that in nonresponders (100% vs 48%; P = .18) (supplemental Figure 2A-B).
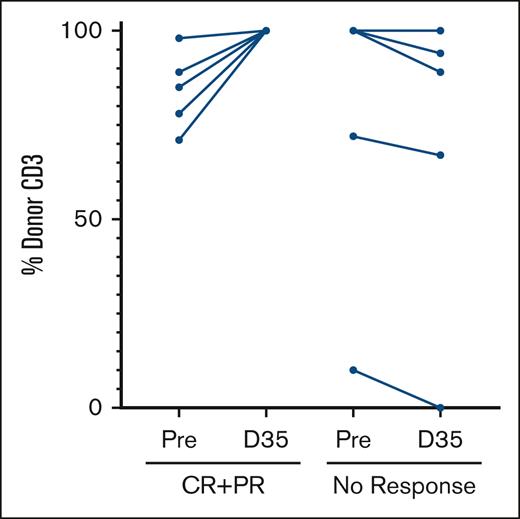
In the CD3 lineage, 10 patients had mixed chimerism, and 6 patients had full donor chimerism. Having any degree of pretreatment CD3 mixed chimerism was associated with clinical response. For example, a significantly greater ORR to pembrolizumab monotherapy on day 35 was observed in patients with any degree of pretreatment mixed CD3 chimerism than in those with pretreatment full donor chimerism (50% vs 0%; P = .03). In addition, 4 patients with mixed CD3 chimerism before pembrolizumab developed SR GVHD (40% vs 60%; P = .3). In comparison to nonresponders, all patients achieving clinical response had subsequent conversion to full CD3 donor chimerism on day 35 after treatment (Figure 2).
Percentage of donor CD3 chimerism measured in paired peripheral blood samples before treatment and again at day 35 in 5 patients with ORR (CR + PR) and 5 patients without response.
Percentage of donor CD3 chimerism measured in paired peripheral blood samples before treatment and again at day 35 in 5 patients with ORR (CR + PR) and 5 patients without response.
Correlative studies
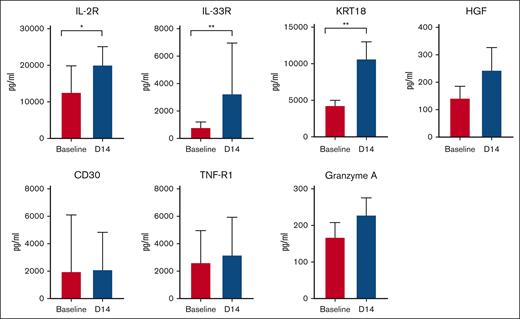
Plasma cytokines, GVHD biomarkers, and immune checkpoint proteins were analyzed in a subset (n = 12) of patients using plasma samples before and 14 days after pembrolizumab. Levels of interleukin-33 receptor (IL-33R; suppression of tumorigenicity 2) and certain GVHD biomarkers (IL-2r [CD25], KRT18) were significantly increased over baseline (Figure 3). No significant changes in plasma inflammatory cytokines were observed (supplemental Figure 3). Most checkpoint proteins (with the exception of CD27 and herpes virus entry mediator) did not increase over baseline levels (supplemental Figure 4).
Plasma levels of selected GVHD biomarkers IL-2R (CD25), IL-33R (suppression of tumorigenicity 2), KRT 18, HGF, CD30, TNR-R1, and granzyme A measured at baseline (pretreatment) and day 14. ∗P < .05; ∗∗P < .01. HGF, hepatocyte growth factor; TNF-R1, tumor necrosis factor receptor 1.
Plasma levels of selected GVHD biomarkers IL-2R (CD25), IL-33R (suppression of tumorigenicity 2), KRT 18, HGF, CD30, TNR-R1, and granzyme A measured at baseline (pretreatment) and day 14. ∗P < .05; ∗∗P < .01. HGF, hepatocyte growth factor; TNF-R1, tumor necrosis factor receptor 1.
Immune staining of BM myeloid blasts for PD-L1 revealed limited expression of PD-L1 at baseline in only 2 of 12 (17%) patients with AML (supplemental Table 3). One patient with 92% BM blasts had <5% PD-L1 expression and did not experience a clinical response after pembrolizumab (91% blasts on day 35). A second patient with 11% BM blasts and 10% PD-L1 expression had CR after pembrolizumab (0% blasts on day 35). Conversely, 3 patients with AML with a response (PR, n = 2; CR, n = 1) had no detectable PD-L1 expression on pretreatment BM blasts.
Discussion
Immune checkpoint inhibition is a modality with unclear safety and efficacy for treatment of AML or MDS relapse after HCT. In this study, we describe the rates of response and major toxicities to pembrolizumab in a homogeneous cohort of patients with myeloid disease who experienced early post-HCT relapse. Several durable responses were observed after PD-1 inhibitor monotherapy and subsequent chemotherapy. These findings suggest that PD-1 blockade may elicit GVL in certain patients with myeloid malignancies; however, several cases of severe GVHD were also observed, suggesting that strategies to reduce toxicity are required for maximum benefit.
The study met the prespecified response target with a day 35 ORR of 31%, which included 3 CRs to pembrolizumab monotherapy. In AML, the ORR was 58% after including the patients who responded to subsequent induction chemotherapy. Among the patients who achieved CR, many were sustained (median, 20 months), with eventual relapse manifesting exclusively as an extramedullary event. This observation suggests that pembrolizumab may be sufficient to initiate GVL, particularly in the BM compartment, and that the absence of GVL is 1 driver of early relapse. The observation of late extramedullary events after pembrolizumab has also been reported in high-risk AML, even in patients experiencing acute and chronic GVHD,20 and may reflect T-cell exhaustion or less effective immunosurveillance in nonhematopoietic host tissues. Although PD-1 inhibition can initiate an antileukemic immune response, further assessments are necessary to ascertain features predictive or response such as depth of response (presence of minimal residual disease), sensitivity of myeloid blasts to GVL (eg, HLA expression), and patient selection.21 It is also important to note that in nontransplant settings, checkpoint inhibition can illicit clinical responses in myeloid malignancies either alone or in combination with other agents such as hypomethylating drugs.22
We observed evidence that mixed CD3 chimerism may be a major factor to predict clinical response to PD-1 inhibition, whereas other patient and disease characteristics did not clearly influence the treatment outcome. For example, any degree of pretreatment host CD3 chimerism was associated with clinical response when compared with patients initiating pembrolizumab with full CD3 donor chimerism (50% vs 0%). This observation is consistent with previous work in mice and humans, suggesting that the DLI response is improved in the context of mixed chimerism, potentially through improved tumor antigen presentation.23 These findings also contrast prior clinical studies where CRs were not observed with either pembrolizumab or nivolumab for AML.18,19 One explanation for this difference may be that early utilization of anti–PD-1 treatment (in the context of mixed chimerism) might facilitate a more potent GVL response and may select patients capable of achieving remission.
Importantly, our ability to determine the extent to which pembrolizumab was able to separate GVL and GVHD was limited in this trial. The overall GRFS was 18.75%; however, 4 of the 7 patients had the best ORR, and 37.5% were free from active GVHD at 1 year. These data suggest that some patients can achieve a response without GVHD, or eventually recover from responses to GVHD after checkpoint inhibition. It is acknowledged that the small sample size may have confounded our interpretation of these and other significant variables. For example, although not statistically significant, a few patients with a high blast percentage, very early relapse (<168 days), monosomal karyotype, or TP53 mutation experienced a response. Therefore, an important aspect of future research will be to better refine parameters that predict the optimal response to anti–PD-1 treatment.
Our study demonstrates that PD-1 inhibition in the post-HCT period is associated with severe acute GVHD, in alignment with previous studies. A total of 37.5% of patients developed grade 3/4 GVHD. This exceeded the de novo rate of grade 3/4 GVHD typically seen at our center (∼10%), but was similar to what has been reported after withdrawing immune suppressive in settings of relapse (∼35%)24; thus, it is possible that pembrolizumab in combination with the rapid withdrawal of tacrolimus may have contributed to the high incidence of GVHD. Most patients had features of high-risk GVHD, including hepatic involvement, which was refractory to corticosteroids. As GVHD was expected, this observation did not trigger stopping criteria; however, it limited our ability to continue pembrolizumab or, in some instances, initiate subsequent lines of treatment. We did not identify any clinical criteria that clearly promoted GVHD, including previously reported factors such as time frame after HCT or prior GVHD. Not surprisingly, a trend toward greater GVHD was also observed in patients with mixed CD3 chimerism before therapy, supporting the known linkage with GVL. To limit the severity of GVHD after pembrolizumab, the protocol was amended to allow continuation of immune suppression for GVHD prophylaxis (vs complete withdrawal before PD-1 treatment). Of the patients who continued immune suppression while initiating pembrolizumab, 20% developed GVHD; however, 2 clinical responses were still observed. Whether continuation of immune suppression in the context of immunotherapies, such as checkpoint inhibitors, can mitigate severe GVHD (while preserving GVL) warrants further study, in addition to other measures to improve safety (dose reductions and combination with chemotherapy).
As a key secondary end point, we measured OS and disease-free survival to estimate the cumulative effects of response and toxicity. The 1-year OS for the entire cohort was 37.5% and 50% for AML, which suggests that PD-1 inhibition might produce similar outcomes when considering historical studies of early relapse after HCT. For example, the largest registry analysis by the Center for International Blood and Marrow Transplant Research (CIBMTR) reported OS in the range of 10% and 30% for AML that relapses <6 months and between 6 months and 2 years after HCT, respectively.25 Among patients receiving DLI in the CIBMTR cohort, OS at 1 year was 13% in early and 35% in intermediate time frames of relapse after HCT. A caveat is that the CIBMTR data were collected from a much larger patient population in the prior treatment era (1990-2010). In addition, we lack a direct comparison of response against DLI, another commonly used modality, but checkpoint inhibition appears to have certain logistical advantages over DLI in terms of timing and ease of administration. Controlled clinical trials in the setting of post-HCT relapse, although difficult to perform, are necessary to provide further clarity in optimizing therapy for relapse.
In correlative studies, we observed increases in certain GVHD biomarkers and plasma proteins early after pembrolizumab. For example, suppression of tumorigenicity 2, a well-validated GVHD biomarker, increased significantly, as did keratin 18, which forms a cytoskeletal element in the hepatocytes. Surprisingly, we did not observe significant increases in inflammatory cytokines (IL-6 and tumor necrosis factor-α) measured early after pembrolizumab administration. It is likely that, in some settings of relapse, donor lymphocytes are primed (but inhibited) from responding to leukemia antigens and/or host alloantigens. As such, early or potentially even planned use of agents that constrain lymphocyte expansion or directly lymphodeplete is needed to curtail GVHD. Another observation was the infrequent and low levels of PD-L1 expression in leukemic blasts. Although PD-L1 expression may herald response (1 patient with expression had CR), its infrequency limits the predictive value of this marker. Thus, pembrolizumab may unleash its effects largely by releasing the suppressive effects of PD-1 expressed in host tissues.26 In addition, other checkpoint inhibitors may provide alternative targets in the postallogeneic setting. For example, CTLA-4 (ipilimumab) had high rates of response in extramedullary relapse and T-cell immunoglobulin and mucin domain-containing protein 3 (sabatolimab) demonstrated activity in AML with minimal residual disease without GVHD or irAEs.27,28
In conclusion, PD-1 inhibition with pembrolizumab in this study led to durable responses in approximately one-third of the patients with early relapse of AML after HCT, suggesting that patients with mixed CD3 chimerism may have the best response. Immune-related toxicity; however, remains a barrier to successful treatment in greater numbers of patients outside of a clinical trial. Given the extremely dismal outcomes of early AML relapse after HCT, ongoing studies are necessary to better understand strategies that apply checkpoint inhibition in the post-HCT setting.
Acknowledgments
The authors thank Lynne Bischoff for research coordination, patients for their courage and commitment to cancer research, and Joel Whitfield for Luminex assays (Rogel Immunology Core).
J.M.M. received support for this project through a National Institutes of Health career development award (K23AI123595) and a Rogel Cancer Center Scholarship. This study was supported in part by a research grant (54053) from the Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC.
The opinions expressed in this article are those of the authors (design, data collection, analysis, and decision to publish this trial) and do not necessarily represent those of Merck Sharp & Dohme LLC.
Authorship
Contribution: J.M.M. and P.R. designed the study; J.M.M., D.G.F., A.M.P., M.R., S.A., M. Geer, J.M., M. Ghosh, and P.R. participated in the recruitment of patients, collection, assembly, analysis, and interpretation of clinical data; J.M.M., A.P., D.G.F., and P.R. designed, performed, and/or analyzed the correlative data; T.B. performed the statistical analysis and designed the trial; and all authors participated in manuscript writing and review, and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John M. Magenau, Transplantation and Cellular Therapy Program, University of Michigan Rogel Cancer Center, 1500 E Center Dr, SPC 5271, Ann Arbor, MI 48109-5271; email: johnmage@med.umich.edu.
References
Author notes
J.M.M. and D.G.F. contributed equally to this study.
Protocol and data sets are available on request from the corresponding author, John M. Magenau (johnmage@med.umich.edu).
The full-text version of this article contains a data supplement.