Key Points
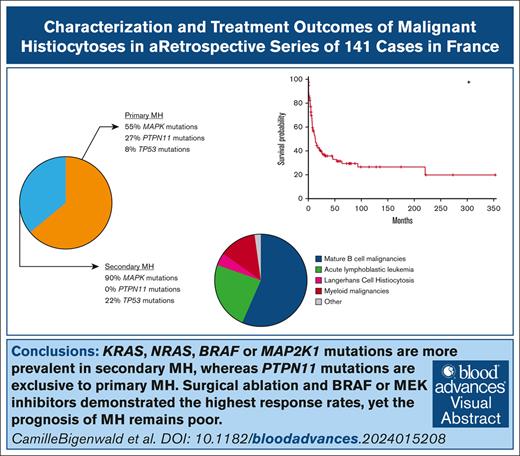
KRAS, NRAS, BRAF, or MAP2K1 mutations are more prevalent in secondary MH, whereas PTPN11 mutations are exclusive to primary MH.
Surgical resection and BRAF or MEK inhibitors demonstrated the highest response rates, yet the prognosis of MH remains poor.
Visual Abstract
Malignant histiocytoses (MH) are rare and poorly understood cancers, with no established therapeutic guidelines. We conducted a national retrospective study of MH diagnosed in France between 2000 and 2023. All cases underwent centralized histological review, and several malignant tumors with a stroma highly enriched in histiocytes were excluded. In total, 141 patients were included, with a median age of 62 years (range, 1-87). The cases comprised either primary MH (64%) or MH associated with other hematologic malignancies (36%). Phenotypes corresponded to histiocytic (43%), interdigitating dendritic cell (37%) or Langerhans cell (12%) sarcomas, or high-grade indeterminate dendritic cell tumors (10%), as per the World Health Organization classification. Tumor cells were almost universally positive for CSF1R and PU.1, and 85% showed phosphorylated extracellular signal–regulated kinase positivity. Next-generation sequencing was performed in 75 cases. Mutations in the MAPK pathway were more frequent in secondary compared with primary MH (90% vs 55%; P = .0012). PTPN11 mutations were exclusively observed in primary MH (P = .0035). Mutations in genes related to DNA methylation mechanisms (TET2, ASXL1, DNMT3A) and TP53 were present in 20% and 14% of cases, respectively. Although therapeutic regimens varied considerably, our results demonstrate that surgical resection in localized cases, and the use of BRAF or MEK inhibitors achieved the highest complete response rates, at 63% and 21%, respectively. The prognosis remains poor, with a 5-year overall survival rate of 31%, which is comparable to that of T/natural killer cell lymphomas. Prospective follow-up and a standardized treatment approach in specialized reference centers are crucial to improving patient survival. This trial was registered at www.clinicaltrials.gov as #NCT04437381.
Introduction
Malignant histiocytosis (MH) is a tumor resulting from the proliferation of histiocytes, which are phagocytic mononuclear cells that include macrophages and dendritic cells.1 The mononuclear phagocyte system represents a subset of leukocytes circulating in the blood as monocytes and less abundantly as dendritic cells, and populating tissues as macrophages or dendritic cells. Macrophages are widely distributed in tissues throughout the body, contributing to both homeostasis and disease. Adult tissue macrophages have dual origins, originating either from embryonic development or from circulating monocytes directly arising from the bone marrow.2-5 Dendritic cells are primarily short-lived hematopoietic cells, continually replaced by blood-derived precursors.5 Monocytes within tissues can differentiate into either inflammatory dendritic cells or macrophages.1
In 2016, the Histiocyte Society proposed to consider as “MH” the malignant neoplasms harboring the morphology and phenotype of macrophages or dendritic cells.6 These neoplasms correspond to cancers classified by the World Health Organization and the International Consensus Classification as histiocytic sarcoma, Langerhans cell sarcoma, interdigitating dendritic cell sarcoma, and indeterminate dendritic cell tumors with high-grade histology.7,8 The Histiocyte Society questioned the clinical relevance of these subtypes, which differ primarily by the expression of S100, CD1a, and/or CD207/Langerin. Instead, the Histiocyte Society considered the association of MH with another hematologic neoplasm (secondary MH) as a distinct and relevant subtype, separate from de novo MH (primary MH). In secondary MH, various associated existing or concurrently diagnosed hematologic malignancy have been documented, including follicular lymphoma, chronic lymphocytic leukemia, and acute lymphoblastic leukemia (ALL).9,10 These cases often exhibit identical clonal antigen receptor gene rearrangements or occasionally identical structural events, such as identical immunoglobulin heavy-chain/B-cell lymphoma 2 (BCL2) rearrangements, as in the associated B-cell lymphoma.11
Given the rarity and complexity of the disease, the histological diagnosis of MH can be challenging. To mitigate the risk of misdiagnosis,12 every case of histiocytosis in France is recommended to undergo a “double check” by the histiocytosis reference center. MH is characterized by pleomorphic histology, featuring large cells with vesicular chromatin, distinct nucleoli, and abundant pale eosinophilic cytoplasm, and abundant cellular atypia. The diagnosis often requires an extensive immunohistochemical panel to confirm the macrophage or dendritic cell lineage and rule out other anaplastic tumors such as carcinoma, melanoma, lymphoma, and sarcoma. Markers commonly used for confirming macrophage or dendritic cell lineage include CD68, CD163, S100, CD1a, and CD207/Langerin.
Although significant clinical and biological advancements have been made in understanding nonmalignant histiocyte disorders such as Langerhans cell histiocyte and Erdheim-Chester disease, MH remains an enigmatic cancer with no established treatment recommendations.13 Data on these neoplasms are limited to case reports and small series,14-19 and large cohorts of patients are still lacking to determine the most useful classification in clinical practice and to reflect oncogenic mechanisms.
We retrospectively identified cases of MH diagnosed in France since 2000, and describe herein their clinical, histopathological, and molecular characteristics at diagnosis, as well as the therapies received and identified variables associated with outcome.
Materials and methods
Patients
This retrospective study included cases referred as malignant histiocytoses (or histiocytic sarcoma, Langerhans cell sarcoma, and interdigitating dendritic cell sarcoma) in France from 2000 to 2023. The diagnostic criteria followed the World Health Organization8 and Histiocyte Society6 classifications. Cases were identified from 3 main sources: (1) through the pathology samples sent to the Centre de Référence des Histiocytoses (Ambroise Paré hospital, Boulogne, France); (2) Lymphopath (the French Lymphoma Network), and (3) during a national bimonthly multidisciplinary board dedicated to histiocytosis. Clinical details were then gathered by reaching out to all the oncohematology departments implicated in the follow-up of patients with MH. The study was conducted in accordance with the Declaration of Helsinki and with ethical approval from local ethic committee group at Gustave Roussy (institutional review board 2024-235). The molecular study of histiocytosis was approved by the French ethical committee comité éthique et scientifique pour les recherches, les études et les évaluations dans le domaine de la santé (2814848 bis), and declared on www.clinicaltrials.gov as #NCT04437381. The data retrospectively reviewed included age at diagnosis; history of previous neoplasms; clinical, histopathological, and molecular characteristics at diagnosis; type of treatment received; recent updates on patient status; and causes of death. The raw database containing all the clinical, pathological, and molecular information is available as supplemental Table 1.
Histopathological examination
All cases were reviewed by at least 2 pathologists with significant experience in the fields of histiocytosis and/or hematopathology (C.C.-B., M.P., A.T.-G., F.C., and J.-F.E.). Extensive immunostainings were used to exclude other cancer types, such as carcinoma, melanoma, lymphoma, germ cell tumor, and myeloid malignancy or other type of sarcoma. Each case expressed at least 2 markers of macrophages or dendritic cells: CD163, CD68, CD4, CD14, CSF1R, and/or PU.1. PU.1 staining, which was used for the most recent cases, was analyzed in combination with paired box 5 staining. Expression of CD31 or ERG in the absence of expression of CD34 was also considered as a minor criterion. When enough tissue was available, expression of S100, CD1a, and CD207 was also analyzed, for subtyping the MH.
Molecular analyses
The molecular profile was studied by sequential analyses, as previously described.12 First, BRAFV600E analysis was conducted using 1 of the 3 following methods: specific real-time polymerase chain reaction, pyrosequencing, or digital polymerase chain reaction. Subsequently, wild-type BRAF cases were analyzed by deoxyribonucleic acid sequencing next-generation sequencing (NGS) on genomic DNA, if available, as described.20 Two cases were analyzed by DNA sequencing in another laboratory using a small gene panel. In a small number of cases, the presence of specific mutations were analyzed by targeted NGS, including the identification of KRAS (n = 2), MAP2K1 (n = 1), NRAS (n = 1), and PTPN11 (n = 1) mutations.
Statistical analyses
Participant characteristics were reported as numbers and percentages for categorical variables, and as mean, standard deviation, median, and interquartile range (IQR) for continuous variables. Overall survival (OS) was defined as the time from diagnosis to death from any cause, patients still alive were censored at the date of last follow-up. The Kaplan-Meyer method was used to generate survival curves for categorical variables (comparisons with log-rank tests). The Cox proportional hazards model was used to adjust the effects of variables on OS (univariate analysis). Patients in complete remission at last follow-up were indicated as persistent CR. Differences were considered statistically significant when the P value was < .05. SAS 9.4 (SAS Institute Inc, Cary, NC) and R version 4.0.5 (R Foundation for Statistical Computing) were used.
Results
Clinical characteristics at diagnosis
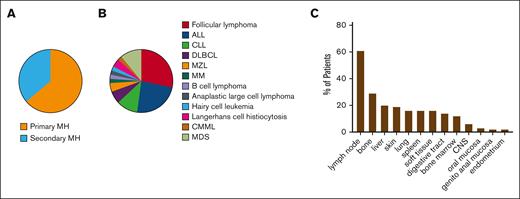
This retrospective study included 141 cases of MH diagnosed between 2000 and 2023. All cases were confirmed by central review. The annual incidence was estimated to be ∼11.25 cases per year in France, extrapolated from the data available for 2020 to 2023, giving an incidence estimate of 0.2 cases per million people. Main clinical and biological characteristics of the whole cohort (n = 141) are summarized in Table 1. The median age at diagnosis was 62 years, ranging from 1 to 87 years (IQR, 25-75%, 40.9-71.8 years). Males constituted 60% of the cohort. Primary MH accounted for 64% of patients (n = 89/138), whereas 36% had secondary MH associated with other hematologic malignancies (n = 49/138; Figure 1A). These hematologic malignancies were diagnosed either previously (n = 31/39, 79%) or concomitantly (n = 8/39, 21%). The hematologic malignancies most commonly associated with this condition, in order of frequency, were follicular lymphoma (n = 13/46, 28%), ALL (n = 11/46, 24%), chronic lymphocytic leukemia (n = 5/46, 11%), myelodysplastic syndrome (n = 5/46, 11%), and diffuse large B-cell lymphoma (n = 3/46, 7%; Figure 1B). Consequently, mature B-cell malignancies accounted for most secondary MH (n = 25/46, 54%). MH secondary to Langerhans cell histiocytosis remained rare (n = 2/46, 4%). At the time of diagnosis, the disease was localized in 33% (n = 44/135) and disseminated in 67% (n = 91/135). The most frequently involved organs were lymph nodes (n = 81/133, 61%), bones (n = 37/127, 29%), liver (n = 25/126, 20%), skin (n = 25/130, 19%), lungs (n = 21/128, 16%), spleen (n = 20/127, 16%), soft tissues (n = 20/129, 16%), and the digestive tract (n = 18/129, 14%; Figure 1C). Central nervous system involvement was observed in 6% of patients (n = 7/127).
Clinical characteristics of MH at diagnosis. (A) Proportion of MH classified into primary or secondary MH. (B) Frequency of hematologic malignancies in secondary MH. (C) Organs affected by MH are listed in order of frequency. CLL, chronic lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MZL, marginal zone lymphoma.
Clinical characteristics of MH at diagnosis. (A) Proportion of MH classified into primary or secondary MH. (B) Frequency of hematologic malignancies in secondary MH. (C) Organs affected by MH are listed in order of frequency. CLL, chronic lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MZL, marginal zone lymphoma.
Histopathological and immunohistochemistry analyses
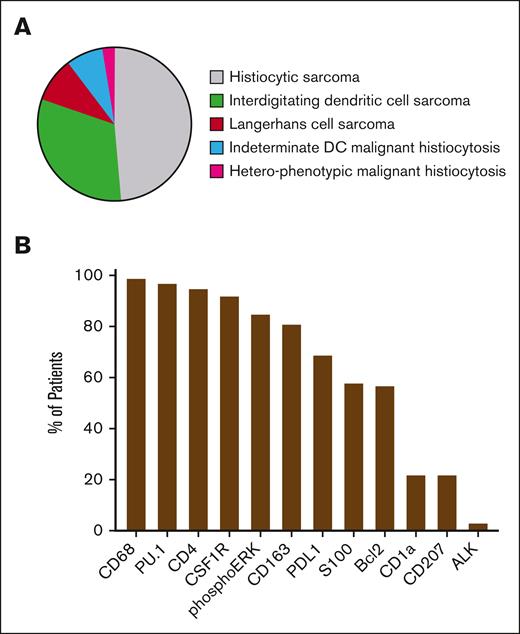
Whenever feasible (n = 100), cases of MH were classified into different histological subtypes based on the phenotypic expression of dendritic cell and macrophage markers (Figure 2A; supplemental Figure 1). The analysis showed that 43% of the cohort had histiocytic sarcoma, 37% interdigitating dendritic cell sarcoma, 12% Langerhans cell sarcoma, and 10% high grade indeterminate dendritic cell tumors. Three cases were heterophenotypic MH (ie, different expression of S100, CD1a, and/or CD207 in different biopsies). Tumoral cells consistently exhibited high expression levels of CD68, CD4, and CSF1R, with rates of 99% (n = 97/98), 95% (n = 79/83), and 92% (n = 23/25), respectively (supplemental Table 2). PU.1 staining (used systematically in the most recent cases) was quasi uniformly positive (n = 33/34, 97%). Phosphorylated extracellular signal–regulated kinase was positive in 85% (n = 41/48) of the samples examined for this marker. Additionally, although conducted in a limited number of cases, programmed death-ligand 1 (PD-L1) staining on tumoral cells was observed in 69% of cases (n = 27/39), and BCL2 staining was noted in 57% of cases (n = 8/14). The proliferation marker Ki67 was estimated to be expressed in a median of 50% (IQR, 25-75, 25-60) of tumoral cells (Figure 2B-C). When separating primary and secondary MH, we observed an underrepresentation of Langerhans cell subtype among primary MH compared with other histological subtypes (n = 4/74, 5%, vs n = 7/41, 17%; P = .05; Fisher exact test).
Phenotype of MH at diagnosis. (A) MH were classified into World Health Organization subtypes according to expression of macrophages and dendritic cell markers. (B) Immunohistochemistry markers expressed by tumoral cells in order of frequency. DC, dendritic cell.
Phenotype of MH at diagnosis. (A) MH were classified into World Health Organization subtypes according to expression of macrophages and dendritic cell markers. (B) Immunohistochemistry markers expressed by tumoral cells in order of frequency. DC, dendritic cell.
Frequency of wrong diagnosis of MH
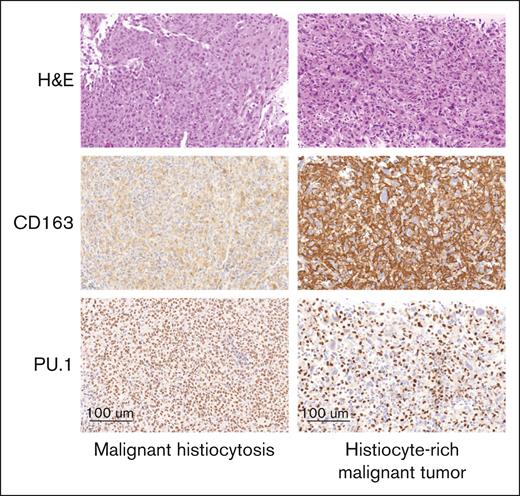
During the central histological review, it was found that a significant number of cases that had been referred with a suspicion or a confirmed diagnosis of MH were not subsequently confirmed. We thus decided to evaluate the frequency of wrong diagnosis in current practice. Between June 2018 and December 2023, biopsies from 121 cases were submitted to the French National Pathology Reference Center for Histiocytoses for potential, probable, or confirmed diagnosis of MH (supplemental Figure 2). In 4 instances, the biopsies were unsuitable for diagnosis evaluation. A diagnosis of MH was confirmed in 61 of 117 cases (52%), whereas in 12 additional cases (10%), the diagnosis of MH was considered possible but could not be established definitively. Nine cases were classified as non-MH. Together, these 21 cases were excluded from further analysis. Most of the other excluded cases (n = 35) corresponded to malignant tumors characterized by a stroma densely populated with reactive histiocytes or dendritic cells (Figure 3). During the same period, 9 cases initially diagnosed as histiocytosis, not otherwise specified (n = 8) or juvenile xanthogranuloma (n = 1), were reclassified as MH upon review. The remaining MH-confirmed cases included in this study were either received between 2000 and May 2018, or retrieved from the French Lymphoma Network (Lymphopath) database, and all were reevaluated for inclusion in this study.
Histological review of referred malignant histiocytoses. Left: liver infiltration by MH, secondary to a large B-cell lymphoma. Tumoral cells only had a weak cytoplasmic staining with CD163 but had a strong nuclear positivity with PU.1. Right: mass involving adrenal glands referred as histiocytic sarcoma. Massive infiltration by reactive DCs with strong CD163 expression, and it was difficult to see that tumor cells were negative. This contrasts with obvious negativity of tumor cells with PU.1. All pictures (original magnification ×300). DC, dendritic cell; H&E, hematoxylin and eosin.
Histological review of referred malignant histiocytoses. Left: liver infiltration by MH, secondary to a large B-cell lymphoma. Tumoral cells only had a weak cytoplasmic staining with CD163 but had a strong nuclear positivity with PU.1. Right: mass involving adrenal glands referred as histiocytic sarcoma. Massive infiltration by reactive DCs with strong CD163 expression, and it was difficult to see that tumor cells were negative. This contrasts with obvious negativity of tumor cells with PU.1. All pictures (original magnification ×300). DC, dendritic cell; H&E, hematoxylin and eosin.
Molecular analyses
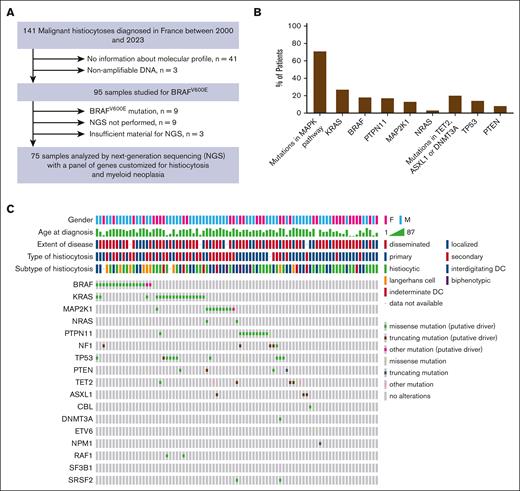
Molecular status was obtained mostly for the recent cases. Before 2017, only BRAF mutation was studied systematically and specific mutations of NRAS, KRAS, and MAP2K1 mutations were selectively examined in isolated cases. Since 2017, targeted NGS was performed whenever material was available (n = 75; Figure 4A). In samples analyzed by targeted NGS, a median of 2 distinct mutations per tumor were observed (range, 0-4; IQR, 25-75, 1-2). These analyses revealed mutations affecting the MAPK pathway in 63% of patients with available information (n = 82; Figure 4B; supplemental Table 3). The frequency order of these mutations included KRAS (27%, n = 18/67), BRAF (18%, n = 17/95), PTPN11 (17%, n = 11/65), MAP2K1 (13%, n = 9/67), and NRAS (3%, n = 2/70; Figure 4C; supplemental Table 4). It is noteworthy that mutations in the MAPK pathway were not always mutually exclusive in MH (conversely to what is observed in other histiocytoses).20 Additionally, 20% of patients were found to have mutations in genes associated with DNA methylation mechanisms (TET2, ASXL1, or DNMT3A; n = 13/64). A TP53 mutation was also present in 14% (n = 9/64) of the cohort. Mutations of KRAS, NRAS, BRAF, or MAP2K1 responsible for extracellular signal–regulated kinase activation were more frequent among secondary vs primary MH (n = 28/31, 90% vs n = 22/40, 55%; P = .0012, χ2 test). Moreover, PTPN11 mutations were detected only in primary MH (P = .0035, Fisher's exact test; Table 2). Additionally, among 11 patients with PTPN11 mutation, 8 had a digestive tract involvement at diagnosis (n = 8/11, 73%), whereas no digestive tract involvement was observed for BRAF, MAP2K1, or KRAS mutated MH.
Molecular characteristics of MH at diagnosis. (A) Flowchart indicating the molecular analysis. (B) Mutations observed in MH cases are listed in order of frequency. (C) The Oncoprint tab of different molecular mutation found in patients with MH analyzed by NGS. Each column represents a tumor sample. The first 5 rows summarized the principal clinicopathological information and each following row represents a gene. DC, dendritic cell.
Molecular characteristics of MH at diagnosis. (A) Flowchart indicating the molecular analysis. (B) Mutations observed in MH cases are listed in order of frequency. (C) The Oncoprint tab of different molecular mutation found in patients with MH analyzed by NGS. Each column represents a tumor sample. The first 5 rows summarized the principal clinicopathological information and each following row represents a gene. DC, dendritic cell.
Type of treatments and clinical responses
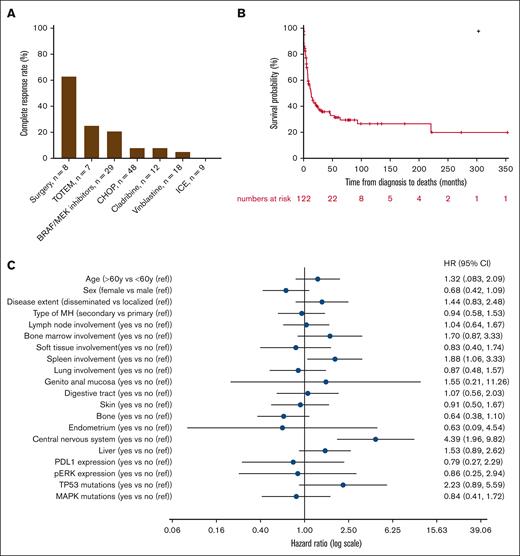
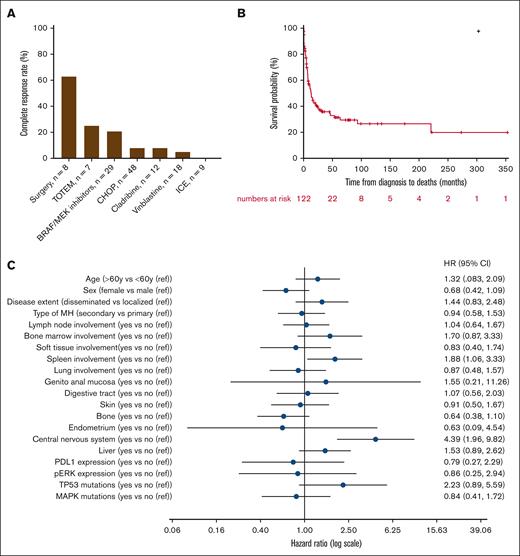
Information on treatments given to patients and clinical responses was available for only 85 patients (60%) and showed wide heterogeneity. The median time from diagnosis to the start of treatment was 0.9 months (IQR, 25-75, 0.3-2.5). Different types of therapies used in first and subsequent lines of treatment are summarized in Table 3. The most common first-line regimen consisted in anthracycline-based chemotherapy (mostly CHOP [cyclophosphamide-doxorubicin-vincristine-prednisolone]; n = 35/85, 41%), vinblastine monotherapy (n = 10/85, 12%), and BRAF or MEK inhibitors (n = 9/85, 11%). Surgical resection of the whole tumor was performed in 8 patients with localized MH, resulting in complete and sustained remission in 5 cases (63%). Clinical characteristics (especially localized/disseminated, primary/secondary disease) did not significantly differ between the largest groups of first-line therapies (data not shown), except for surgery that was only performed in localized MH. Persistent complete response (CR) after first-line therapy was achieved in 12% (n = 10/85) of patients, with a median duration of response of 5.6 years (IQR, 25-75, 2.4-9.6) for these 10 first-line responders. BRAF or MEK inhibitors emerged as the most common second-line regimen, administered to 29% of the cohort (n = 15/52), followed by anthracycline-based regimens (15%, n = 8/52), vinblastine monotherapy (10%, n = 5/52), and cladribine (8%, n = 4/52; Table 3). All patients who received BRAF or MEK inhibitors had a mutation in the RAF/RAS/MAPK pathway. The median duration of response with BRAF or MEK inhibitors was 7.4 months (IQR, 25-75, 1.2-18.5). We did not observe a distinctly different duration of response to BRAF or MEK inhibitors between primary (9.9 months; IQR, 25-75, 1.1-17.4) and secondary MH (5.1 months; IQR, 25-75, 2.0-18.5; P = .98). Although not statistically significant, a trend was observed for prolonged responses and increased frequency of persistent remissions in patients with BRAF V600E mutations compared with those not harboring this mutation among patients treated with BRAF or MEK inhibitors (supplemental Table 4). Persistent CR after second-line therapy was obtained in 12% (n = 6/52) of patients. When considering first-line and relapse settings, 32% (n = 27/85) of patients exhibited a prolonged remission. BRAF or MEK inhibitors demonstrated the highest rate of persistent CR at 21% (n = 6/29), whereas anthracycline-based regimens and cladribine both led to persistent CR in 8% of cases (n = 4/48 and 1/12, respectively). Vinblastine monotherapy (5%, n = 1/18) and ifosfamide, etoposide, and carboplatin (0%, n = 0/9) almost did not yield any CR (Figure 5A). Interestingly, the combination of topotecan and temozolomide resulted in CR in 2 of 7 pediatric patients (28%) who received this treatment. Such treatment combinations have never been explored in the adult cohort. Five patients underwent allogeneic stem cell transplantation after achieving CR. Two patients experienced a rapid relapse after transplant, whereas the 2 other patients continue to be in CR, >18 months after the procedure. The latter underwent allogeneic transplant too recently for any conclusions to be drawn. Finally, 4 patients received anti–programmed cell death protein 1 therapy (pembrolizumab or nivolumab) alone or in combination with chemotherapy. The treatment failed to control the disease in 3 patients, and the follow-up duration is still too short for the last patient, who is currently in CR.
Treatment characteristics and outcome. (A) The percentage of complete remission is depicted according to the most frequent treatments. (B) OS analysis for the whole cohort of patients with MH. (C) Forest plot representing factors associated with OS. CHOP, cyclophosphamide-doxorubicin-vincristine-prednisolone; ICE, ifosfamide-carboplatin-etoposide; ref, reference; TOTEM, topotecan-temozolomide.
Treatment characteristics and outcome. (A) The percentage of complete remission is depicted according to the most frequent treatments. (B) OS analysis for the whole cohort of patients with MH. (C) Forest plot representing factors associated with OS. CHOP, cyclophosphamide-doxorubicin-vincristine-prednisolone; ICE, ifosfamide-carboplatin-etoposide; ref, reference; TOTEM, topotecan-temozolomide.
OS and associated factors
The median follow-up of our cohort was 58.2 months (95% confidence interval [CI], 33.2-79.0). Median OS was 13.1 months (95% CI, 8.0-21.4) and the 5-year OS was estimated to be 31.3% (95% CI, 22.7-41.4; Figure 5B). Forty-seven patients (33%, n = 47/141) were still alive at last follow-up. Death was related to MH and other causes in 64 (n = 64/66, 97%) and 2 (n = 2/66, 3%) cases, respectively. We searched for clinical and biological factors associated with a poorer prognosis. Age and sex were not identified as factors associated with poorer survival (Figure 5C). However, spleen (median, 4.9 [95% CI, 0.6-16.9] vs 15.6 [95% CI, 9.6-45.4] months; P = .029) and central nervous system involvement (median, 2.5 [95% CI, 0.2-6.8] vs 15.6 [95% CI, 9.2-26.9] months; P < .001) were associated with worse OS. Interestingly, no significant difference in OS was observed between primary and secondary MH. However, the secondary MH group encompassed a wide range of hematologic disorders from ALL to hairy cell leukemia, each associated with markedly distinct clinical outcomes. Additionally, we did not observe any difference in terms of OS between histiocytic sarcoma, Langerhans cell sarcoma, and interdigitating dendritic cell sarcoma (median, 8.5 [95% CI, 5.4-16.9] vs 9.3 [95% CI, 0.5-93.4] vs 9.2 [95% CI, 4.7-24.9] months; P = .751).
Pediatric specificities
Eighteen patients were minors at the time of diagnosis. Among these 18 patients, half had secondary MH after ALL, either from the T-cell or B-cell lineage. Four patients (n = 4/18, 22%) were cured; 3 of whom had primary MH and were treated either with surgery alone (n = 1/18) or with surgery combined with topotecan and temozolomide-based chemotherapy (n = 2/18). This suggests that MH after ALL has a particularly poor prognosis in children.
Discussion
This retrospective study constitutes the largest cohort analysis of MH to date, offering interesting insights into the characteristics and treatment outcomes of this rare disease. However, given the inherent characteristics of this retrospective study, we acknowledge the limitations of our work. Notably, the treatment modalities were highly heterogeneous, and molecular alterations were known for only approximately half of the cohort. However, this work is in line with data previously described by 3 other groups in recent series of 16 to 43 patients (Table 4). Approximately one-third of patients present with localized disease. Most cases exhibit oncogenic mutations that activate the MAPK cell signaling pathway. Prognosis is poor. Treatments are highly variable because of the absence of consensus/recommendations, but surgical resection of localized disease and targeted therapy have the highest CR rates.
Diagnosis of MH can be very difficult. Indeed, almost half of the cases referred recently with possible diagnosis of MH were not confirmed by central review. The main diagnostic challenges arise in the context of anaplastic malignant tumors with a histiocyte-rich stroma. It is possible for reactive macrophages or dendritic cells to be in such close proximity to tumor cells that they appear to be positive for CD68 (or CD163). Our study reinforces the diagnostic value of PU.1.21 This nuclear marker is consistently expressed on tumor cells of MH and allows easy determination of whether atypical nuclei, which are characteristic of tumor cells, are positive or not. PU.1 is not only a marker of macrophages and dendritic cells, but also of B lymphocytes and mast cells, so combination with other markers such as paired box 5 and KIT can sometimes be useful to exclude B-cell lymphoma or mastocytosis.
To the best of our knowledge, no prospective trial has yet been reported in patients with histiocytic sarcoma, Langerhans cell sarcoma, or interdigitating dendritic cell sarcoma. The main reason for this is the rarity of these diseases. In this large series, phenotypic subtypes did not have any impact on OS. Of note, the 2 most recently published series also gathered patients with histiocytic sarcoma, Langerhans cell sarcoma, interdigitating dendritic cell sarcoma, and indeterminate dendritic cell tumors with high-grade histology.14-17 Another large series reported 28 cases of histiocytic sarcoma but did not mention analysis of S100, CD1a, or CD207/Langerin expression.18 So, gathering all these patients in a single entity (ie, MH, as proposed by the Histiocyte Society6) appears to be consensual and appropriate for clinical practice and for future clinical trials.
MH was secondary to another hematologic malignancy in 36% of the patients of the present series, and the most frequent conditions were mature B-cell neoplasms. In the 3 largest recent series, the frequency of secondary MH is similar (Table 4). The respective proportion of the type of associated hematologic neoplasms may depend on the number of children or young adults referred to the expert centers. The distinction between primary and secondary MH is not only clinically relevant but is also strongly supported by molecular characteristics. Indeed, the frequency of KRAS/NRAS/BRAF/MAP2K1 oncogenic alterations is 90% in secondary MH but only 55% in primary MH (P = .0012). This major difference is similar in the largest previous series (Table 4). In contrast, PTPN11 oncogenic mutations have been identified exclusively in primary MH in our work and previously published cases, with an overall frequency of 18% of primary MH cases (18/98; Table 4). Interestingly, 8 of 11 patients of our series with PTPN11 mutation had a digestive tract involvement, and 5 of 8 patients of the published series also had small and large bowel tumors.14,16,18 Finally, simultaneous detection of 2 mutations of KRAS, NRAS, BRAF, and/or MAP2K1, which is extremely rare in nonmalignant histiocytoses, occurred in 2 of 75 cases of our series, as already reported in 9 of 71 in 3 other series14,16,18 (7.5% of all cases).
The analysis of treatment modalities is based on data from a subgroup of 85 patients, which may introduce a bias in this retrospective series spanning >23 years. Nevertheless, until now, the largest published series comprised only 43 patients, and thus our results provide significant input for the design of future prospective studies. It was observed that surgical resection, when used for localized disease, resulted in the highest CR rate. In cases in which the disease is localized and surgical resection is feasible, it is recommended that surgical approaches be prioritized, with radiotherapy potentially used subsequently. The use of BRAF or MEK inhibitors yielded encouraging outcomes, with a CR rate of 21%. However, the duration of this response remains uncertain because patients who achieve CR are still undergoing therapy. Other chemotherapeutic agents, such as anthracycline-based chemotherapy or cladribine, have shown disappointing results, with <10% of patients achieving CR. Cladribine, known as the first-line option for hairy cell leukemia, has shown effectiveness in Langerhans cell histiocytosis, despite its primary mechanism of action being well established in lymphocytes.22,23 However, the results observed in the context of MH are limited. Vinblastine monotherapy (or in association with steroids), which mimics the treatment of Langerhans cell histiocytosis should be avoided, except in the context of palliative care. Our series only includes 18 children; however, it also provides some interesting data: (1) prognosis of MH secondary to ALL is unfavorable; and (2) the persistent CR of 2 children after topotecan-temozolomide, which includes temozolomide and topotecan, contrasts with the poor responses to other chemotherapies. Further investigation in this direction is warranted.
Patients receiving MEK or BRAF inhibitors exhibited a response rate of ∼20%, whereas Erdheim-Chester disease and Langerhans cell histiocytosis showed a 100% metabolic response rate.24,25 This suggests that the aggressiveness of MH is not solely dependent on MAPK activation. The antitumor activity of BRAF/MEK inhibitors as monotherapy could pave the way for future exploration of combinations with chemotherapies or other targeted agents to potentially better control the multiple oncogenic pathways of MH and improve long-term disease control.
The identification of recurrent markers, such as CSF1R or BCL2, could lead to the development of new therapies, including CSF1R and inhibitors,26 for further exploration in preclinical models. Pharmacological inhibition of CSF1R has shown promise, with PLX3397 (pexidartinib) and vimseltinib receiving US Food and Drug Administration approval for tenosynovial giant cell tumor treatment.27,28 Axatilimab, another CSF1R inhibitor, has undergone evaluation in a phase 1/2 study for chronic graft-versus-host leukemia.29,30 Notably, axatilimab depletes tissue macrophages, suggesting a potential novel approach for MH. High expression of PD-L1 expression by tumor cells was reported previously,31,32 and we also found PD-L1 in 69% of cases. Check point inhibitors may also be discussed for salvage therapy. Indeed, 1 patient had a CR of >2 years after combination of pembrolizumab and radiotherapy.33
Conducting clinical trials in rare diseases presents challenges, necessitating collaborative efforts and innovative trial designs to improve understanding and develop effective therapeutic interventions. To enhance patient care and treatment standardization, a French multidisciplinary histiocytosis board has been settled since 2017. This multidisciplinary board now meets twice a month to discuss patients with histiocytosis, including malignant cases, fostering collaboration and providing a platform for ongoing prospective patient follow-up.
Acknowledgments
This study was supported, in part, by Programme de Recherche Translationelle PRTK 19-143; in part, by ANR JCJC ANR-22-CE14-0024; and unrestricted grants of Association pour la Recherche et l’Enseignement en Pathologie.
The authors thank Mohamed-Aziz Barkaoui for active contribution to clinical data collection; Catherine Chassagne-Clement, Karima Mokhtari, and Anne Moreau for contribution in the histological review of initial cases; all the pathologists who referred samples; and all clinicians who provided clinical information. The authors also thank patients and their families; and Mariama Bakari, Rim Ben Jannet, Nathalie Terrones, and Thamila Satour for expert technical assistance in molecular analyses.
Authorship
Contribution: C.B., D.R.-W., J. Donadieu, and J.-F.E. designed the study, collected the data, and drafted the manuscript; C.B., C.M., J. Donadieu, and J.-F.E. obtained funding; C.C.-B., M.P., A.T.-G., F.C., and J.-F.E. reviewed histology; Z.H.-R. and J.-F.E. performed molecular analyses; A.P. performed statistical analyses; C.B., D.R.-W., C.C.-B., M.N., P.K., M.P., A.T.-G., I.R.-C., G.B., L.Y., T.M., J.R., F.C., A.N., G.D., J. Dion, E.-M.N.-T., S.T., G.S., H.M., A.I., S.H., J.H., J. Donadieu, and J.-F.E. contributed patients; and all authors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: L.Y. received honoraria as a speaker for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene, Gilead/Kite, Janssen, and Roche. J.-F.E. received honoraria as a speaker for Qiagen. The remaining authors declare no competing financial interests.
Correspondence: Camille Bigenwald, Département d’Hématologie, Gustave Roussy, 114 Rue Edouard Vaillant, 94810 Villejuif, France; email: camille.bigenwald@gustaveroussy.fr; and Jean-François Emile, Département de Pathologie, Hôpital Ambroise Paré, 9 Ave Charles de Gaulle, 92104 Boulogne, France; email: jean-francois.emile@uvsq.fr.
References
Author notes
C.B., D.R.-W., J.D., and J.-F.E. contributed equally to this study.
Data are available on reasonable request from the corresponding authors, Camille Bigenwald (camille.bigenwald@gustaveroussy.fr) and Jean-François Emile (jean-francois.emile@uvsq.fr).
The full-text version of this article contains a data supplement.