Key Points
CAR-T cell rescue after second line appears to overcome the poor prognosis of HGBL compared with other LBCL all characterized with FISH.
OS from eligibility of HGBL-TH/DH MYC-BCL2 is inferior but identical to other LBCL from the date of infusion.
Visual Abstract
High-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements (double hit [HGBL-DH] or triple hit [HGBL-TH]) or not otherwise specified (HGBL-NOS) are considered to be more aggressive diseases among large B-cell lymphomas (LBCLs). CD19-targeting chimeric antigen receptor (CAR) T cells have changed the prognosis of chemoresistant LBCL. Clinical and pathological data of patients treated for relapsed/refractory LBCL or HGBL in third line or more, all characterized by fluorescence in situ hybridization, were collected from the French DESCAR-T registry. Between January 2018 and November 2022, a total of 228 patients were included across 14 centers, 73 with HGBL (28 HGBL-DH MYC-BCL2, 14 HGBL-TH, 8 HGBL-DH MYC-BCL6, and 23 HGBL-NOS) and 155 with non-HGBL. The median follow-up was 18.5 months (95% confidence interval [CI], 14.3-23.4) from the date of infusion. Progression-free survival and overall survival (OS) were not significantly different between HGBL and non-HGBL, at 3.2 months (95% CI, 2.8-6.0) vs 4.5 months (95% CI, 3.1-8.7; P = .103) and 15.4 months (95% CI, 5.6-32.4) vs 18.3 months (95% CI, 8.5 to not reached), respectively. From the date of eligibility, the median OS was inferior for patients with HGBL-TH/DH MYC-BCL2 at 6.6 months vs 18.5 months for HGBL-NOS vs 13.6 months for HGBL-DH MYC-BCL6 vs 11.8 months for LBCL (P = .037). However, patients who received infusion presented the same outcome. CAR T-cell therapy used in third line or more seems to overcome the poor prognosis of HGBL subtypes, especially in HGBL-TH/DH MYC-BCL2. This observation supports considering the potential benefit of using CAR T cells earlier in disease course.
Introduction
High-grade B-cell lymphomas (HGBLs) represent a particularly aggressive subgroup of large B-cell lymphomas (LBCLs). These patients often present at diagnosis with a deteriorated general condition, elevated lactate dehydrogenase (LDH) level, and extended disease with multiple extranodal localizations.1-3 These high-risk patients are also more likely to have central nervous system involvement.4 This entity was previously considered Burkitt-like lymphoma, but advances in genetic and molecular knowledge have allowed for a better characterization. Representing 5% to 15% of LBCLs and classically of germinal center origin, HGBLs share molecular characteristics with LBCLs and Burkitt lymphoma. In the 2016 World Health Organization (WHO) classification,5 HGBLs are categorized as double hit (DH) and triple hit (TH), defined by the presence of a MYC rearrangement associated with a rearrangement of BCL2 and/or BCL6. Others are HGBL, not otherwise specified (NOS), histologically defined by a Burkitt-like or blastoid morphology, which may present an isolated MYC rearrangement.
Based on recent molecular data,6,7 the new WHO and International Consensus Classification 2022 classifications8,9 distinguish HGBL-DH MYC-BCL6 from HGBL-DH MYC-BCL2 and HGBL-TH. Indeed, HGBL-DH MYC-BCL6 is a more heterogeneous entity than HGBL-DH MYC-BCL2 and is excluded from the group of HGBLs in the WHO classification.8 These new classifications also caution about the subjective nature of assignment, with HGBL-NOS being a diagnosis of exclusion.
Unfortunately, there is a high rate of refractory or relapsed HGBL after standard immuno-chemotherapy based on R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone)/CHOP (cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone)–like regimens. In a large study evaluating the treatment of HGBL-DH and HGBL-TH, 2-year and 4-year progression-free survival (PFS) rates were 40% and 28% with R-CHOP, respectively. It is not clear whether intensive regimen could improve survival of HGBL.10 These chemotherapy-refractory and rapidly progressing diseases often represent a therapeutic challenge. Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 is now the standard of care for LBCL that relapses after 2 lines of treatment or refractory or relapsed early after 1 line of treatment.11-15 However, the efficacy of CAR T cells in HGBL is not well-known, especially in real-life settings.16 Indeed, the complete characterization of LBCL (which requires a fluorescence in situ hybridization [FISH] study at diagnosis) is not always routinely performed; thus, the number of HGBL cases is often underestimated. Additionally, because of their often rapid progression, these diseases are underrepresented in clinical trials. Indeed, even in a recent first-line phase 3 trial conducted on LBCL, FISH testing for MYC rearrangement was performed at diagnosis for only 50.7% of the patients.17 In trials designed for patients with high-risk disease (international prognostic index [IPI] 2-5), the HGBL population is lower than expected, ranging from 3% to 6.8%, depending on the study.18,19
The objective of this retrospective multicenter real-life study was, therefore, to evaluate the efficacy of CAR T cells in a population of HGBL compared with LBCL. Only cases studied by FISH were included.
Methods
Population
A retrospective nationwide registry-based study was performed on a cohort of 73 patients with HGBL and 155 with LBCL, included in the DESCAR-T registry (ClinicalTrials.gov identifier: NCT04328298), who received CAR T-cell therapy with the commercially available products axicabtagene ciloleucel (axi-cel; Yescarta) or tisagenlecleucel (tisa-cel; Kymriah), having received at least 2 lines of therapy. Only cases with FISH results were analyzed and reviewed.
Patients were included in the DESCAR-T registry if they were eligible for treatment with CAR T cells in an accredited center for a hematologic malignancy covered by the French health care system. CAR T-cell therapy indication had been validated during a multidisciplinary tumor board (MTB). All patients or their representatives were informed of the noninterventional use of personal data before inclusion in DESCAR-T. This study conforms to the Declaration of Helsinki and was approved by the institutional review boards of participating centers.
Characteristics of treated patients
All cases had been studied using FISH for MYC rearrangement (Vysis probe; Abbott, Chicago, IL) and were reviewed by pathologists from the Lymphopath network, a national network aiming at providing an expert pathological review of every newly diagnosed lymphoma in France.20
If a MYC rearrangement was identified, a search for BCL2 and BCL6 rearrangements was conducted. Regarding LBCL, primary mediastinal B-cell lymphomas, primary central nervous system lymphomas, Richter syndrome, T-cell/histiocyte–rich B-cell lymphomas, and posttransplantation B-cell lymphomas were excluded.
Bridging therapy was defined as treatments administered between leukapheresis and lymphodepletion.
Data were collected and analyzed at the time of eligibility (date of MTB) and at the time of CAR T-cell infusion. The response to CAR T-cell therapy, based on 18-fluorodeoxyglucose (FDG) positron emission tomography scan, was evaluated according to the Lugano criteria21 at 1, 3, 6, and 12 months after CAR T-cell infusion. Evaluation considered the best response rate.
Toxicities were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome were assessed according to the consensus criteria of the American Society for Transplantation and Cellular Therapy.22
End points and statistics
This study was designed to analyze the outcomes after treatment with CAR T cells in patients with HGBL compared with those with LBCL (non-HGBL).
Data are presented as medians and range or interquartile range, or as numbers and percentages. For discontinuous variables, a Fisher exact test was used, and for continuous variables, a Wilcoxon test was used.
Concerning outcomes, PFS was calculated from the date of CAR T-cell infusion (day 0) to the next progression/relapse, death without relapse, or last follow-up for surviving patients. Overall survival (OS) was calculated from the dates of decision to treat (MTB date) or CAR T-cell infusion (day 0) until death or last follow-up for surviving patients.
The primary end points of the study were PFS and OS of patients with HGBL compared with those with non-HGBL. Secondary end points were PFS and OS of different subtypes of patients with HGBL compared with patients with non-HGBL. Responses and safety after CAR T-cell infusion were also evaluated.
Estimates of survival were calculated according to the Kaplan-Meier method and compared using the log-rank test. A 2-sided patient P value <.05 was considered significant. In addition, event rates at specific time points were computed, along with 95% confidence intervals (CIs). All analyses were performed using SAS 9.3 (Cary, NC).
Control cohort of patients with non-HGBL
Most centers perform only FISH for MYC rearrangement in some situations such as disease with aggressive presentation, multiple extranodal localizations, or high IPI score. To eliminate any selection bias in choosing the non-HGBL population, the group of “confirmed” patients with non-HGBL (with FISH results available) was compared with the remaining DESCAR-T population reported as non-HGBL by the centers (n = 1337 patients). At eligibility, the control cohort of patients with non-HGBL with a FISH study for MYC rearrangement did not significantly differ from the whole DESCAR-T non-HGBL cohort in terms of median age (62 vs 64 years; P = .313), Eastern Cooperative Oncology Group status ≥2 (14.8% vs 12.4%; P = .442), stage III to IV Ann Arbor (80.6% vs 77%; P = .632), or median number of prior lines of treatment (2 in both groups; P = .632). However, there was a statistically significant difference in the percentage of patients with LDH more than normal range (76.8% vs 64.6%; P = .007) and in the percentage of bridging therapy (91.6% vs 81.1%; P < .001; supplemental Table 1). PFS and OS were not significantly different in the non-HGBL population compared with the population of patients with non-HGBL remaining in the DESCAR-T database, which validates the present non-HGBL cohort (supplemental Figure 2).
This study has received the approval of our institutional review board.
Results
Description of the population
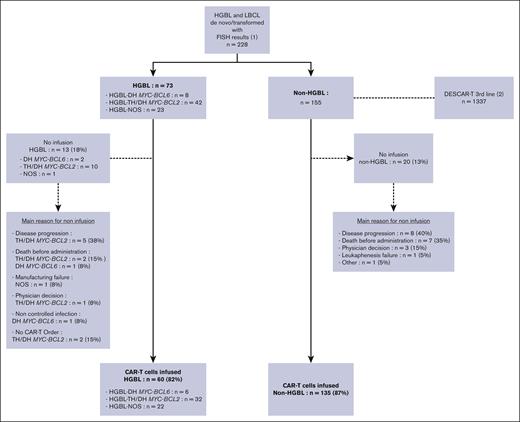
Between January 2018 and November 2022, a total of 228 patients from the DESCAR-T registry, with a FISH MYC rearrangement study, treated in 14 French centers, were analyzed. (Figure 1) Seventy-three patients had HGBL (28 HGBL-DH MYC-BCL2, 14 HGBL-TH, 8 HGBL-DH MYC-BCL6, and 23 HGBL-NOS) and 155 confirmed non-HGBL LBCL, with (n = 19) or without (n = 136) MYC rearrangement alone (MYC-R). Among patients with non-HGBL (n = 155), 122 (79%), 21 (14%), and 12 (7%) were diagnosed with de novo LBCL, transformed Follicular lymphoma (FL), and transformed marginal zone lymphoma (MZL), respectively. These proportions were respectively 66 (90%), 6 (8%), and 1 (1%) for patients with HGBL (n = 73).
The median follow-up was 20.5 months (95% CI, 19.2-25.5) from the date of eligibility and 18.5 months (95% CI, 14.3-23.4) from the date of infusion.
In the whole population (n = 228), most patients received CAR T-cell therapy, that is, 60 of 73 patients with HGBL (82%) vs 135 of 155 patients with non-HGBL (87%; P = .322). The main reason for noninfusion was disease progression for 5 patients with HGBL (39%) vs 8 with non-HGBL (40%), whereas 3 (23%) vs 7 (35%) had died before infusion (P = .354), respectively. Among patients who received infusion, most (55 [92%] in the HGBL group vs 127 [93%] in the non-HGBL group) had received bridging therapy (P = .765). The response to bridging therapy was comparable at 35% vs 37% of complete response (CR) or partial response (P = .378). Among the whole cohort (including patients who did not receive infusion), in both groups, most patients had received a bridge combining an anti-CD20 monoclonal antibody (88% in the HGBL group and 77% in the non-HGBL group) and chemotherapy (91% and 83%, respectively). Two patients with HGBL (3%) and 3 patients with non-HGBL (2%) received a polatuzumab-based regimen. None of the patients received bispecific antibodies for bridging (supplemental Tables 3 and 4).
The characteristics of patients with HGBL or non-HGBL at the date of CAR T-cell eligibility were not significantly different (Table 1). Forty-one patients with HGBL (56%) and 65 with non-HGBL (42%) were of the germinal center phenotype, according to Hans algorithm,23 and respectively, 48 (66%) and 77 (49%) were double expressors (MYC-BCL2). CD19 expression data were missing for 54 patients with HGBL (74%) and 122 with non-HGBL (79%).
Patients had received a median of 2 prior lines of treatment. The median time between the end of the last line of treatment and the date of eligibility was 26 days (range, 0-3420) and 27 days (range, 0-2718) for the HGBL and non HGBL groups, respectively.
Characteristics of patients with HGBL vs non-HGBL at the date of CAR T-cell infusion were not significantly different (Table 2), notably for Eastern Cooperative Oncology Group ≥2 at 18% vs 15% (P = .670) and LDH more than normal range at 62% vs 56% (P = .702). In both groups, approximately two-thirds of patients were treated with axi-cel. All patients who received infusion, except for 1, received a lymphodepletion regimen consisting of a combination of fludarabine and cyclophosphamide for 3 days. Most patients who received infusion were in stable or progressive disease at the time of infusion, that is 62% vs 63% (P = .378).
The characteristics of patients with HGBL-NOS vs non-HGBL (supplemental Table 5) and with HGBL TH/DH MYC-BCL2 vs non-HGBL (supplemental Table 6) at the date of CAR T-cell eligibility were not significantly different, as well as responses to bridging therapy.
Among patients with HGBL MYC-BCL2 (DH and TH) specifically (n = 42), 23 (71.9%) received axi-cel, 9 (28.1%) received tisa-cel, and 10 (23.8%) did not receive reinfusion. The main reasons for nonreinfusion were disease progression (n = 5), death (n = 2), or physician decision (n = 3). Most of the patients (n = 35 [83.3%]) received bridging therapy, which was mostly monoclonal antibody (n = 33 [94.3%]) and chemotherapy (n = 32 [91.4%]; supplemental Table 7). No patient was in CR at the end of bridging therapy. Among patients who received reinfusion (n = 32), the main cause of death was disease progression (n = 21 [65.6%]), whereas only 1 patient presented fatal acute toxicity, and 10 (31.3%) are still alive.
The clinical characteristics of the 19 patients with non-HGBL and a MYC-R alone are summarized in supplemental Figure 8. Among them, 17 (89.5%) received bridging therapy, and 8 (47%) were in CR or partial response after the bridge.
Response and survival outcomes
CR rates between patients with HGBL or non-HGBL were not statistically different, at 60% vs 59% (P = .923), with overall response rates at 68% vs 76% (P = .293). There was no difference between CR rates and overall response rates of the different HGBL subgroups (HGBL-TH/DH MYC-BCL2, HGBL-DH MYC-BCL6, and HGBL-NOS) and non-HGBL (supplemental Figure 9).
The median time between the date of eligibility and the date of CAR T-cell infusion was 51 days (range, 5-207) for patients with HGBL and 54 days (range, 21-195) for patients with non-HGBL, with median times between leukapheresis and infusion of 38 (range, 21-174) and 39 days (range, 29-131), respectively.
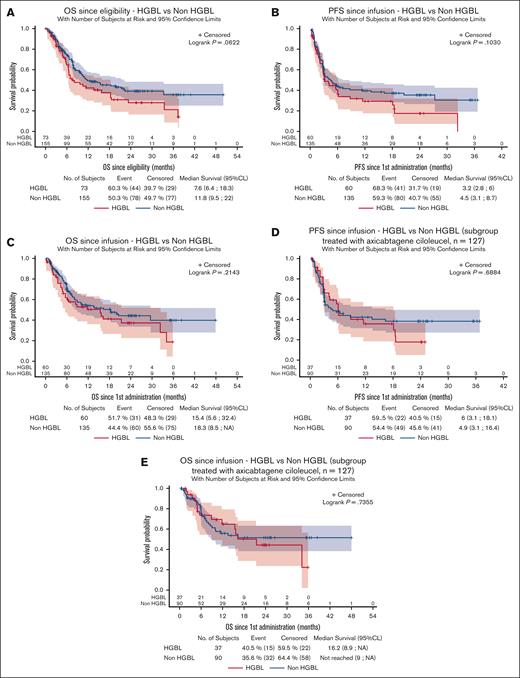
PFS and OS were not significantly different between the 2 groups. Since eligibility, the median OS was respectively 7.6 (95% CI, 6.4-18.3) vs 11.8 months (95% CI, 9.5-22; P = .062) in the HGBL vs non-HGBL groups (Figure 2A). Since infusion, the median PFS was 3.2 months (95% CI, 2.8-6.0) for patients with HGBL vs 4.5 months (95% CI, 3.1-8.7) for patients with non-HGBL (P = .103). The median OS was 15.4 months (95% CI, 5.6-32.4) for patients with HGBL vs 18.3 (95% CI, 8.5 to not reached [NR]; P = .214) for those with non-HGBL (Figure 2B-C).
Flowchart. (1) Population included in the study, that is, with a FISH study allowing for the diagnosis of HGBL or non-HGBL and with a pathology report reviewed within the Lymphopath network. (2) Non-HGBL population of the DESCAR-T cohort not included in the study, compared with the included non-HGBL cohort.
Flowchart. (1) Population included in the study, that is, with a FISH study allowing for the diagnosis of HGBL or non-HGBL and with a pathology report reviewed within the Lymphopath network. (2) Non-HGBL population of the DESCAR-T cohort not included in the study, compared with the included non-HGBL cohort.
Survival data in patients with HGBL and non-HGBL. Survival was evaluated from eligibility (A, OS); from CAR T-cell infusion in the whole group (B, PFS; C, OS); and after infusion in the subgroup of patients who received axi-cel (D, PFS; E, OS). NA, non atteint (not reached).
Survival data in patients with HGBL and non-HGBL. Survival was evaluated from eligibility (A, OS); from CAR T-cell infusion in the whole group (B, PFS; C, OS); and after infusion in the subgroup of patients who received axi-cel (D, PFS; E, OS). NA, non atteint (not reached).
PFS and OS were not significantly different among patients treated with axi-cel (HGBL, n = 37 ; non-HGBL, n = 90), with a median PFS of 6 (95% CI, 3.1-18.1) vs 4.9 months (95% CI, 3.1-16.4; P = .688) and a median OS of 16.2 months (95% CI, 8.9 to NR) vs NR (95% CI, 9 to NR; P = .736; Figure 2D-E).
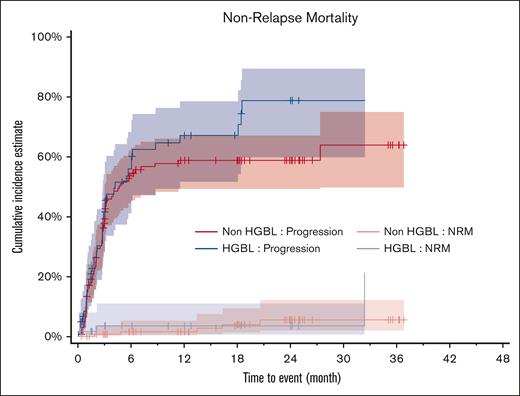
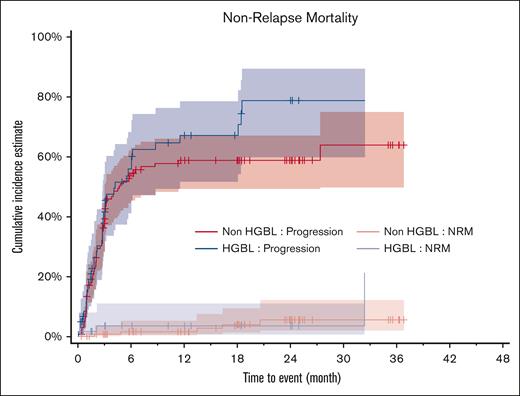
The main cause of death was disease progression in both groups, for 38 (63.3%) and 75 patients (55.6%; P = .206) in the HGBL and non-HGBL groups, respectively. However, nonrelapse mortality was relatively rare at 5% (n = 3) and 3.7% (n = 5), respectively (P = .530; Figure 3). Patients who did not receive reinfusion (13 with HGBL and 20 with non-HGBL) presented a very poor prognosis, and all had died within 6 months (supplemental Figure 10).
Progression and NRM among patients with HGBL and non-HGBL. NRM, nonrelapse mortality.
Progression and NRM among patients with HGBL and non-HGBL. NRM, nonrelapse mortality.
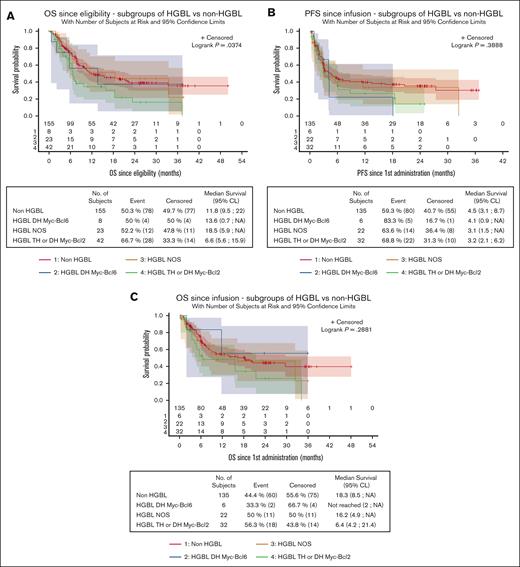
Since eligibility, OS was significantly lower for patients with HGBL-TH/DH MYC-BCL2 (6.6 months; 95 CI, 5.6-15.9) than patients with HGBL-DH MYC-BCL6 (13.6 months; 95% CI, 0.7 to NR), patients with HGBL-NOS (18.5 months; 95% CI, 5.9 to NR), and patients with non-HGBL (11.8 months; 95% CI, 9.5-22; P = .037; Figure 4A). However, OS and PFS since the date of CAR T-cell infusion were not statistically different between the subgroups (OS, P = .288; PFS, P = .389; Figure 4B-C).
Survival data in HGBL subgroups. Survival was evaluated from eligibility (A, OS) or from infusion (B, PFS; C, OS). Patients with HGBL-TH or -DH MYC-BCL2 have a significantly lower OS from eligibility (P = .037). NA, non atteint (not reached).
Survival data in HGBL subgroups. Survival was evaluated from eligibility (A, OS) or from infusion (B, PFS; C, OS). Patients with HGBL-TH or -DH MYC-BCL2 have a significantly lower OS from eligibility (P = .037). NA, non atteint (not reached).
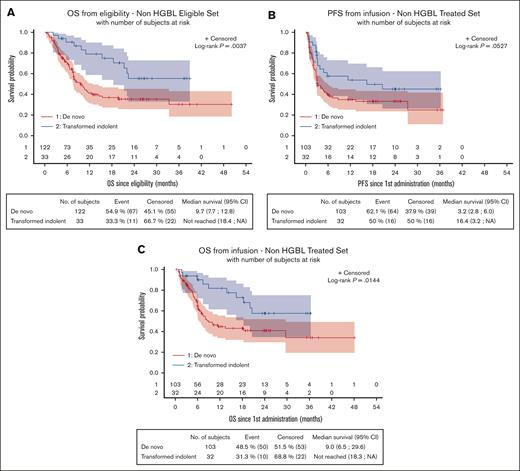
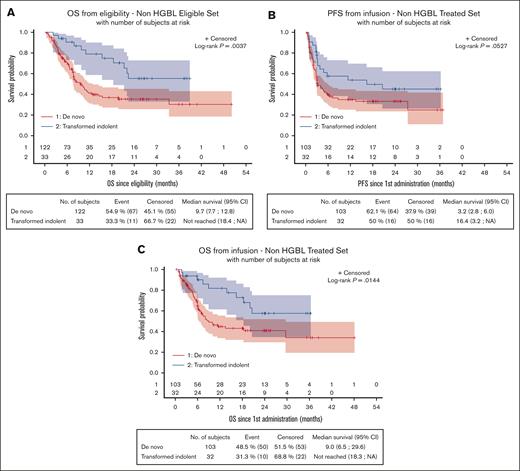
OS from eligibility was significantly lower for patients with de novo non-HGBL (n = 122) than those with non-HGBL transformed from an indolent lymphoma (n = 33), at 9.7 months (95% CI, 7.7-12.8) vs NR (95% CI, 18.4 to NR; P = .0037), respectively, as well as since the date of CAR T-cell infusion, at 9 months (95% CI, 6.5-29.6) vs NR (95% CI, 18.3 to NR; P = .0144; Figure 5A,C). However, since the date of CAR T-cell infusion, PFS was not significantly different, at 3.2 (95% CI, 2.8-6.0) vs 16.4 months (95% CI, 3.2 to NR; P = .0527), between these 2 groups (Figure 5B). Regarding patients with HGBL, survival data of de novo HGBL (n = 66) and HGBL transformed from an indolent lymphoma (n = 7) are presented in supplemental Figure 11, showing the dreadful outcomes for patients with transformed HGBL.
Survival data in patients with de novo non-HGBL and non-HGBL transformed from an indolent lymphoma. Survival was evaluated from eligibility (A, OS) or from infusion (B, PFS; OS, C). Patients with de novo non-HGBL have a significantly lower OS from eligibility (P = .004) and from infusion (P = .014). NA, non atteint (not reached).
Survival data in patients with de novo non-HGBL and non-HGBL transformed from an indolent lymphoma. Survival was evaluated from eligibility (A, OS) or from infusion (B, PFS; OS, C). Patients with de novo non-HGBL have a significantly lower OS from eligibility (P = .004) and from infusion (P = .014). NA, non atteint (not reached).
Patients presenting with non-HGBL with MYC-R alone were also analyzed. PFS from infusion in patients with MYC-R (n = 17) was not statistically different from that of patients with HGBL or non-HGBL (P = .088), but OS was surprisingly statistically significantly better (P = .032; supplemental Figure 12). However, these results should be considered with caution, owing to the low number of patients.
Tolerance
As shown in supplemental Figure 13, CAR T-cell treatment was similarly tolerated in both groups. CRS occurred in 56 patients with HGBL (93%) and 115 with non-HGBL (84%; P = .106), with grade ≥3 occurring in 8 (12%) and 6 (5%), respectively (P = .133). Immune effector cell–associated neurotoxicity syndrome were observed in 23 patients with HGBL (38%) and 56 patients with non-HGBL (41%; P = .874), with grade ≥3 occurring in 8 (13%) and 13 (9%; P = .443). Patients with HGBL (n = 22 [38%] vs n = 23 [20%]; P = .009) were statistically significantly more often managed in an intensive care unit for CRS. Yet, there was no difference in the use of vasopressors (n = 6 [10%] vs n = 3 [3%]; P = .137) or in the median length of stay in intensive care (5 days [range, 2-15] vs 5.5 days [range, 2-46]). There was also no difference in the use of corticosteroids or tocilizumab. Macrophage activation syndrome was diagnosed in 2 patients (3%) in the HGBL group.
The incidence of cytopenia was also not statistically different between both groups, with 46 patients with HGBL (77%) vs 107 with non-HGBL (79%) having ≥1 persistent cytopenias 1 month after infusion; with incidence of grade ≥3 of 58% and 54% of the patients, respectively (P = .641). Respectively, 22 (37%) and 31 patients (23%) presented a bacterial infection, whereas hypogammaglobulinemia (<5 g/L) was disclosed in 40 (67%) and 96 (71%) during follow-up.
Discussion
In this real-world study, the outcomes of patients with FISH-defined HGBL and non-HGBL treated by CAR T cells were compared. CAR T cells were prescribed as third line or beyond for HGBL or LGBL. Patient characteristics and their PFS and OS were not statistically different between both the groups from the date of eligibility or from the date of infusion.
However, considering HGBL subgroups, patients with HGBL-TH or HGBL-DH MYC-BCL2 had a statistically significantly poorer prognosis than others, with a nearly halved median OS calculated from the date of eligibility (P = .037). This difference was no longer observed in patients who received infusion. These results suggest that patients with HGBL-TH or HGBL-DH MYC-BCL2 have a worse prognosis, related to resistance to chemotherapy (including bridging therapy), with a highly proliferative disease preventing CAR T-cell infusion. However, for those who were able to receive CAR T cells, this poor prognosis was erased, without excess toxicity, particularly in patients with HGBL-TH/DH MYC-BCL2. These results highlight the importance of a delay as short as possible between leukapheresis and infusion (vein-to-vein delay). It also reinforces the fact that CAR T cells should be used earlier in the course of these diseases, as was shown in the ZUMA-12 trial, evaluating a treatment with CAR T cells in first line in high-risk patients if the response after 2 cycles of R-CHOP was insufficient.24 The recently updated results of this study showed a sustained response over time (4/10 patients with HGBL-DH or -TH and an IPI score ≥3 achieved CR).25
Moreover, patients with non-HGBL with MYC-R alone seem to have a better outcome, but the results are difficult to analyze because of the low number of patients (n = 19). In this study, patients with transformed non-HGBL treated with CAR T-cell therapy had significantly better outcomes than those with de novo HGBL, results similar to those reported by Nydegger et al.26 Unfortunately, the number of patients with transformed confirmed FISH HGBL treated with CAR T cells in the DESCAR-T registry is too small to draw any conclusion.
The difficulty in managing patients with HGBL partly lies in the rarity and heterogeneity of this disease, which includes several subgroups, as recognized in recent classifications.8,9 To our knowledge, the work presented here is the first real-world clinical study comparing the outcomes of well-characterized patients with HGBL and non-HGBL, with a subgroup analysis of HGBL. Recently published large series provide little information on HGBL subtypes, because the cohorts were relatively small, with ∼30 patients in 2 of them27,28 and 64 in the third.29 Zurko et al presented a cohort of 80 patients with HGBL-DH or -TH treated in the second (n = 16) or third line or more with CAR T cells in several US centers.30 Three different CAR T cells (axi-cel, tisa-cel, or lisocabtagene maraleucel) and 25 patients (31.3%) were enrolled in clinical trials. Responses in patients with HGBL mirror those of patients with other LBCL, with a median PFS of 6.2 months in HGBL vs 7.5 months in LBCL and a median OS of 21 months vs NR, respectively. Patients with HGBL-NOS were not included, and there was no analysis of HGBL subgroups. Indeed, the definition of the HGBL group varies between cohorts, often including DH/TH, excluding NOS, and omitting the specification of the proportion of HGBL-DH MYC-BCL6.27,28,31 In the study reported here, all patients defined as HGBL according to the WHO 2016 classification were considered, including HGBL-NOS and HGBL-DH MYC-BCL6, allowing for the identification of the worse prognosis associated with HGBL-DH MYC-BCL2 and HGBL-TH, which was rescued by CAR T-cell infusion.
The weakness of this study relies on the relatively small number of patients studied by FISH compared with the number of patients treated with CAR T cells in France. However the DESCAR-T registry allowed for the collection of a large enough cohort of patients with HGBL and compare them with a greater number of patients with non-HGBL. Additionally, the patients in this cohort received 2 different CAR T-cell therapies, either axi-cel or tisa-cel. It has been shown that the efficacy of these treatments, in third line, differs in patients with LBCL, with axi-cel providing better results than tisa-cel.32 Here, the outcome is identical in HGBL vs non HGBL after axi-cel, but the sample size is too small to compare the different subgroups of HGBL in this context. It would be interesting to study a more homogeneous cohort treated exclusively with axi-cel.
Factors intrinsic to the tumor and the microenvironment are major determinants of CAR T-cell efficacy and persistence.33 Some studies suggest that the deregulation of the host innate immune system, contributing to the poor prognosis of HGBL, may actually lead to better CAR T-cell treatment effectiveness, due to a better expansion and longer CAR survival.33-36
Finally, this real-world study focused on CAR T-cell treatment in third line and beyond to have a sufficient median follow-up and a substantial number of patients. However, it would remain interesting to analyze data from less heavily pretreated patients.
In conclusion, HGBL, especially HGBL-DH MYC-BCL2 and HGBL-TH, are confirmed here to be diseases of poor prognosis. Infusion of CAR T cells in the third line seems to overcome this poor outcome, and it might be worth considering moving to CAR T-cell therapy as soon as possible in such refractory diseases. Reducing the vein-to-vein delay should be a priority in these aggressive diseases.
Acknowledgments
The authors thank the patients whose data were collected in the DESCAR-T registry and their families. The authors thank each member of the LYSARC DESCAR-T study group who actively participated in the study and notably E. Gat from the biostatistics department.
The DESCAR-T registry was partly funded by Gilead and Novartis; however, they did not participate in the study design, data collection, statistical analysis, or interpretation and did not provide assistance for manuscript writing or editorial support. Medical writing for this manuscript was assisted by My Pen is Your Pal (MPIYP, MC Béné), Paris, France.
Authorship
Contribution: X.P.-Z. and L.R. conceived and designed the study, wrote the article, and performed data analysis and interpretation; E.G. performed statistical analyses; and all authors provided study material and patients, collected and assembled data, provided final approval for the article, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: E.B. received honoraria from Kite, a Gilead Company, and Novartis. F.-X.G. received consulting fees and travel and accommodation expenses from Kite/Gilead and Novartis. R.D.B. received honoraria from Kite, a Gilead Company, and Novartis. O.C. received honoraria from Kite, a Gilead Company. F.J. received honoraria from Gilead. M.M. received honoraria from Kite, a Gilead company, and Novartis. L.R. received travel grants from Kite/Gilead. The remaining authors declare no competing financial interests.
Correspondence: Louise Roulin, Department of lymphoid hematology, University hospital Henri Mondor 1 av Gustave Eiffel, 94000 Créteil, France; email: louise.roulin@aphp.fr.
References
Author notes
Data from this study are available on reasonable request from the corresponding author, Louise Roulin (louise.roulin@aphp.fr).
The full-text version of this article contains a data supplement.