Key Points
This post hoc subgroup analysis of older patients enrolled in POLARIX showed consistency with the overall POLARIX population results.
Pola-R-CHP improved PFS vs R-CHOP in patients aged 60 to 70 years and slightly more toxicities in patients aged ≥70 years.
Visual Abstract
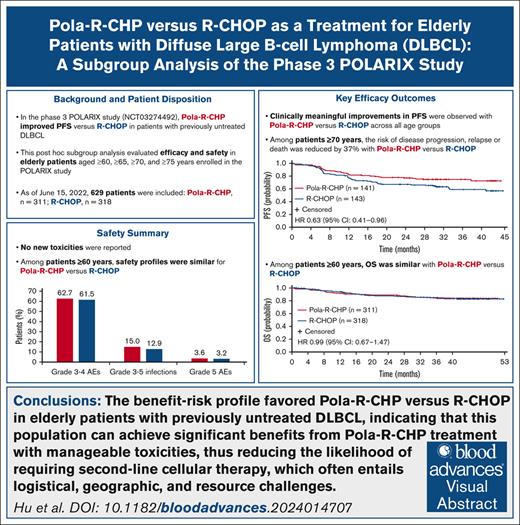
In the phase 3 POLARIX study, Pola-R-CHP (polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisone) improved progression-free survival (PFS) vs R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients with previously untreated diffuse large B-cell lymphoma (DLBCL). This post hoc subgroup analysis of POLARIX evaluated the efficacy and safety of Pola-R-CHP vs R-CHOP in older patients aged ≥60, ≥65, ≥70, and ≥75 years. As of 15 June 2022 (median follow-up, 40 months), 629 patients aged ≥60 years were included (Pola-R-CHP, n = 311; R-CHOP, n = 318). Clinically meaningful improvements in PFS with Pola-R-CHP vs R-CHOP were observed across all age groups, particularly in patients aged ≥70 years whereby the risk of disease progression, relapse, or death was reduced by 37% (unstratified hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.41-0.96). In patients aged ≥60 years, overall survival was similar with Pola-R-CHP vs R-CHOP (unstratified HR, 0. 99; 95% CI, 0.67-1.47). Safety profiles were similar for Pola-R-CHP vs R-CHOP among patients aged ≥60 years, including rates of grade 3 to 4 adverse events (AEs; 62.7% vs 61.5%), grade 3 to 5 infections (15.0% vs 12.9%), and grade 5 AEs (3.6% vs 3.2%); no novel toxicities were reported. Incidence of grade 3 to 4 febrile neutropenia was higher with Pola-R-CHP than R-CHOP (16.3% vs 7.6%), highlighting the importance of granulocyte colony-stimulating factor prophylaxis in older patients receiving Pola-R-CHP. The benefit-risk profile favored Pola-R-CHP vs R-CHOP in older patients with previously untreated DLBCL. This trial was registered at www.ClinicalTrials.gov as #NCT03274492.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is generally a disease of older individuals, with a median age at diagnosis of ∼66 years.1-3 Over the past 20 years, standard first-line treatments for DLBCL were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone)–based regimens. Unfortunately, combination chemotherapy regimens have been associated with higher rates of toxicity in older patients, leading to the use of dose-attenuated regimens, such as R-mini-CHOP,4,5 or regimens with inferior efficacy outcomes vs R-CHOP.6 Thus, when investigating new treatment options for this indication, considering tolerability is particularly critical in older patients. Furthermore, treatment outcomes in the first-line setting are highly important for older patients, given that the likelihood of tolerating intensive second-line therapies (such as chimeric antigen receptor T-cell [CAR-T] therapy or intensive salvage chemotherapy followed by consolidative high-dose chemotherapy with autologous stem cell rescue) is reduced in comparison with younger populations.
In the phase 3 POLARIX study (ClinicalTrials.gov identifier: NCT03274492), Pola-R-CHP (polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone) significantly improved progression-free survival (PFS) compared with R-CHOP in patients with previously untreated DLBCL.7 The types and incidences of grade 3 to 4 adverse events (AEs) reported between treatment arms were generally similar. The safety profile of Pola-R-CHP was consistent with the safety profiles of the individual drugs, and no new safety signals were detected. The results of the POLARIX study led to the approval of Pola-R-CHP in adults with previously untreated DLBCL by many health authorities, including the European Medicines Agency8 and the US Food and Drug Administration.9
The interactions between age and other clinically important considerations of tolerability, including performance status and frailty, are not yet fully understood. Therefore, exploring the safety and tolerability of therapies in older patients is important to allow for proper management of treatment-related toxicities. This post hoc subgroup analysis of POLARIX evaluated the efficacy and safety of Pola-R-CHP vs R-CHOP among older patients aged ≥60, ≥65, ≥70, and ≥75 years.
Methods
Study design and patients
The POLARIX study was a randomized, double-blind study that evaluated Pola-R-CHP vs R-CHOP for patients with previously untreated, CD20+ DLBCL, an Eastern Cooperative Oncology Group performance status of 0 to 2 and an International Prognostic Index (IPI) score of 2 to 5; methods have been described previously.7 This post hoc subgroup analysis evaluated efficacy and safety end points according to age in the subgroup of older patients (aged 60-80 years) at enrollment. The age groups included patients aged ≥60, ≥65, ≥70, and ≥75 years. A summary of methods used are described hereafter.
The POLARIX study was approved by the institutional review board or ethics committee at each participating institution.
Study treatment
Patients were randomized 1:1 to receive 6 21-day cycles of treatment. Each cycle consisted of intravenous rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, and doxorubicin 50 mg/m2 on day 1; and oral prednisone 100 mg once daily on days 1 through 5. In addition, either polatuzumab vedotin 1.8 mg/kg and a placebo of vincristine (Pola-R-CHP treatment arm), or vincristine 1.4 mg/m2 (maximum 2 mg) and a placebo of polatuzumab vedotin (R-CHOP treatment arm) were administered on day 1. Rituximab 375 mg/m2 was administered as monotherapy for 2 additional cycles. A prephase treatment of prednisone at a dose of up to 100 mg every day for up to 7 days before cycle 1, day 1 was permitted at the discretion of the investigator. Additionally, granulocyte colony-stimulating factor (G-CSF) was required as primary neutropenic prophylaxis for all patients during the 6 cycles of treatment.
End points and outcome assessments
Efficacy end points included investigator-assessed PFS, defined as the time from the date of randomization until the first occurrence of disease progression, relapse, or death from any cause; investigator-assessed event-free survival (EFS); fluorodeoxyglucose positron emission tomography–based complete response (CR) rate at end of treatment by blinded independent central review; overall survival (OS); and disease-free survival (DFS) in patients achieving a CR. Additionally, PFS and OS in the older population were evaluated according to IPI score (2 vs 3-5).
Safety and tolerability, and patient-reported outcomes were also assessed. All AEs were reported according to Medical Dictionary for Regulatory Activities thesaurus terms, with AE severity graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.10 Lymphoma symptoms were assessed using FACT-LymS (Functional Assessment of Cancer Therapy Lymphoma Symptom subscale), and EORTC QLQ-C30 (European Organization for Research and Treatment of Cancer Core Quality of Life [QoL] questionnaire) was used to evaluate physical functioning, global health status/QoL, fatigue, constipation, diarrhea, nausea, and vomiting.
Statistical analyses
This analysis was not powered to demonstrate statistical significance in terms of efficacy; thus, no formal hypothesis testing was performed. The analysis methods used in the older subgroups were similar to those previously described for the global population,7 with the exception of hazard ratio (HR) estimates, which were calculated using unstratified Cox regression models. All reported P values are descriptive. To evaluate age-related trends, end points were examined according to the following overlapping age groups: patients aged ≥60 (overall subgroup population), ≥65, ≥70, and ≥75 years.
Results
Patients
Of 879 patients in the overall POLARIX study population (Pola-R-CHP, n = 440; R-CHOP, n = 439), 629 (71.6%) were aged ≥60 years (Pola-R-CHP, n = 311 [49.4%]; R-CHOP, n = 318 [50.6%]) and were included in this analysis. All 629 patients were included in the intention-to-treat population, and 623 were included in the safety-evaluable population (Pola-R-CHP, n = 306; R-CHOP, n = 317). The patient disposition for each treatment arm by age group is provided in Table 1. Baseline patient characteristics were generally similar in both treatment arms and across age groups (Table 2). In the overall subgroup of older patients (aged ≥60 years) included in the analysis, the median age was 69 years (range, 60-80), and 426 patients (67.7%) had an IPI score of 3 to 5.
Patient disposition for each study age group in the ITT and safety-evaluable populations of the POLARIX study
| Study population, n . | Patients aged ≥60 y n = 629 (ITT) n = 623 (safety) . | Patients aged ≥65 y n = 467 (ITT) n = 462 (safety) . | Patients aged ≥70 y n = 284 (ITT) n = 280 (safety) . | Patients aged ≥75 y n = 120 (ITT) n = 117 (safety) . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP . | R-CHOP . | Pola-R-CHP . | R-CHOP . | Pola-R-CHP . | R-CHOP . | Pola-R-CHP . | R-CHOP . | |
| ITT | 311 | 318 | 231 | 236 | 141 | 143 | 60 | 60 |
| Safety evaluable | 306 | 317 | 227 | 235 | 137 | 143 | 57 | 60 |
| PRO evaluable | 291 | 299 | 215 | 221 | 130 | 131 | 54 | 56 |
| FACT-LymS evaluable | 286 | 291 | 210 | 213 | 126 | 128 | 51 | 55 |
| EORTC QLQ-C30 evaluable | 289 | 297 | 214 | 219 | 130 | 129 | 54 | 55 |
| Study population, n . | Patients aged ≥60 y n = 629 (ITT) n = 623 (safety) . | Patients aged ≥65 y n = 467 (ITT) n = 462 (safety) . | Patients aged ≥70 y n = 284 (ITT) n = 280 (safety) . | Patients aged ≥75 y n = 120 (ITT) n = 117 (safety) . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP . | R-CHOP . | Pola-R-CHP . | R-CHOP . | Pola-R-CHP . | R-CHOP . | Pola-R-CHP . | R-CHOP . | |
| ITT | 311 | 318 | 231 | 236 | 141 | 143 | 60 | 60 |
| Safety evaluable | 306 | 317 | 227 | 235 | 137 | 143 | 57 | 60 |
| PRO evaluable | 291 | 299 | 215 | 221 | 130 | 131 | 54 | 56 |
| FACT-LymS evaluable | 286 | 291 | 210 | 213 | 126 | 128 | 51 | 55 |
| EORTC QLQ-C30 evaluable | 289 | 297 | 214 | 219 | 130 | 129 | 54 | 55 |
ITT, intention-to-treat population; PRO, patient-reported outcomes.
Baseline demographics and clinical characteristics of a subgroup of older patients in the POLARIX study
| Characteristic∗ . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 311) . | R-CHOP (n = 318) . | Pola-R-CHP (n = 231) . | R-CHOP (n = 236) . | Pola-R-CHP (n = 141) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 60) . | R-CHOP (n = 60) . | |
| Age, median (range), y | 69 (60-80) | 69 (60-80) | 71 (65-80) | 71 (65-80) | 74 (70-80) | 73 (70-80) | 77 (75-80) | 77 (75-80) |
| Age group, y | ||||||||
| ≥60 to <65 | 80 (25.7) | 82 (25.8) | — | — | — | — | — | — |
| ≥65 to <70 | 90 (28.9) | 93 (29.2) | 90 (39.0) | 93 (39.4) | — | — | — | — |
| ≥70 to <75 | 81 (26.0) | 83 (26.1) | 81 (35.1) | 83 (35.2) | 81 (57.4) | 83 (58.0) | — | — |
| ≥75 | 60 (19.3) | 60 (18.9) | 60 (26.0) | 60 (25.4) | 60 (42.6) | 60 (42.0) | 60 (100) | 60 (100) |
| Sex | ||||||||
| Male | 161 (51.8) | 168 (52.8) | 115 (49.8) | 123 (52.1) | 65 (46.1) | 70 (49.0) | 29 (48.3) | 30 (50.0) |
| Female | 150 (48.2) | 150 (47.2) | 116 (50.2) | 113 (47.9) | 76 (53.9) | 73 (51.0) | 31 (51.7) | 30 (50.0) |
| Ann Arbor stage | ||||||||
| I-II | 42 (13.5) | 47 (14.8) | 27 (11.7) | 34 (14.4) | 17 (12.1) | 22 (15.4) | 10 (16.7) | 12 (20.0) |
| III-IV | 269 (86.5) | 271 (85.2) | 204 (88.3) | 202 (85.6) | 124 (87.9) | 121 (84.6) | 50 (83.3) | 48 (80.0) |
| Extranodal sites | ||||||||
| 0-1 | 180 (57.9) | 178 (56.0) | 125 (54.1) | 133 (56.4) | 77 (54.6) | 77 (53.8) | 35 (58.3) | 31 (51.7) |
| ≥2 | 131 (42.1) | 140 (44.0) | 106 (45.9) | 103 (43.6) | 64 (45.4) | 66 (46.2) | 25 (41.7) | 29 (48.3) |
| Bulky disease (≥7.5 cm) | 128 (41.2) | 126 (39.6) | 98 (42.4) | 94 (39.8) | 59 (41.8) | 60 (42.0) | 22 (36.7) | 24 (40.0) |
| ECOG performance status, n | 311 | 317 | 231 | 235 | 141 | 142 | 60 | 60 |
| 0-1 | 266 (85.5) | 270 (84.9) | 194 (84.0) | 199 (84.7) | 119 (84.4) | 122 (86.0) | 50 (83.3) | 49 (81.7) |
| 2 | 45 (14.5) | 47 (14.8) | 37 (16.0) | 36 (15.3) | 22 (15.6) | 20 (14.0) | 10 (16.7) | 11 (18.3) |
| IPI score, n (%) | ||||||||
| 2 | 99 (31.8) | 103 (32.4) | 66 (28.6) | 72 (30.5) | 42 (29.8) | 44 (30.8) | 17 (28.3) | 20 (33.3) |
| 3-5 | 211 (67.8) | 215 (69.1) | 165 (71.4) | 164 (69.5) | 99 (70.2) | 99 (69.2) | 43 (71.7) | 40 (66.7) |
| Cell of origin, n† | 237 | 252 | 175 | 187 | 108 | 112 | 42 | 45 |
| ABC | 79 (33.3) | 105 (41.7) | 55 (31.4) | 74 (39.6) | 33 (30.6) | 45 (40.2) | 12 (28.6) | 19 (42.2) |
| GCB | 131 (55.3) | 114 (45.2) | 99 (56.6) | 85 (45.5) | 65 (60.2) | 50 (44.7) | 24 (57.1) | 23 (51.1) |
| Unclassified | 27 (11.4) | 33 (13.1) | 21 (12.0) | 28 (15.0) | 10 (9.3) | 17 (15.2) | 6 (14.3) | 3 (6.7) |
| Unknown | 74 | 66 | 56 | 49 | 33 | 31 | 18 | 15 |
| Double expressor lymphoma‡ | 104 (40.9) | 119 (45.4) | 80 (42.3) | 89 (45.4) | 48 (42.1) | 55 (46.2) | 18 (40.9) | 24 (49.0) |
| Double-/triple-hit lymphoma§ | 21 (9.0) | 14 (5.7) | 15 (8.7) | 9 (4.9) | 8 (7.6) | 7 (6.1) | 2 (4.8) | 4 (8.7) |
| Characteristic∗ . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 311) . | R-CHOP (n = 318) . | Pola-R-CHP (n = 231) . | R-CHOP (n = 236) . | Pola-R-CHP (n = 141) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 60) . | R-CHOP (n = 60) . | |
| Age, median (range), y | 69 (60-80) | 69 (60-80) | 71 (65-80) | 71 (65-80) | 74 (70-80) | 73 (70-80) | 77 (75-80) | 77 (75-80) |
| Age group, y | ||||||||
| ≥60 to <65 | 80 (25.7) | 82 (25.8) | — | — | — | — | — | — |
| ≥65 to <70 | 90 (28.9) | 93 (29.2) | 90 (39.0) | 93 (39.4) | — | — | — | — |
| ≥70 to <75 | 81 (26.0) | 83 (26.1) | 81 (35.1) | 83 (35.2) | 81 (57.4) | 83 (58.0) | — | — |
| ≥75 | 60 (19.3) | 60 (18.9) | 60 (26.0) | 60 (25.4) | 60 (42.6) | 60 (42.0) | 60 (100) | 60 (100) |
| Sex | ||||||||
| Male | 161 (51.8) | 168 (52.8) | 115 (49.8) | 123 (52.1) | 65 (46.1) | 70 (49.0) | 29 (48.3) | 30 (50.0) |
| Female | 150 (48.2) | 150 (47.2) | 116 (50.2) | 113 (47.9) | 76 (53.9) | 73 (51.0) | 31 (51.7) | 30 (50.0) |
| Ann Arbor stage | ||||||||
| I-II | 42 (13.5) | 47 (14.8) | 27 (11.7) | 34 (14.4) | 17 (12.1) | 22 (15.4) | 10 (16.7) | 12 (20.0) |
| III-IV | 269 (86.5) | 271 (85.2) | 204 (88.3) | 202 (85.6) | 124 (87.9) | 121 (84.6) | 50 (83.3) | 48 (80.0) |
| Extranodal sites | ||||||||
| 0-1 | 180 (57.9) | 178 (56.0) | 125 (54.1) | 133 (56.4) | 77 (54.6) | 77 (53.8) | 35 (58.3) | 31 (51.7) |
| ≥2 | 131 (42.1) | 140 (44.0) | 106 (45.9) | 103 (43.6) | 64 (45.4) | 66 (46.2) | 25 (41.7) | 29 (48.3) |
| Bulky disease (≥7.5 cm) | 128 (41.2) | 126 (39.6) | 98 (42.4) | 94 (39.8) | 59 (41.8) | 60 (42.0) | 22 (36.7) | 24 (40.0) |
| ECOG performance status, n | 311 | 317 | 231 | 235 | 141 | 142 | 60 | 60 |
| 0-1 | 266 (85.5) | 270 (84.9) | 194 (84.0) | 199 (84.7) | 119 (84.4) | 122 (86.0) | 50 (83.3) | 49 (81.7) |
| 2 | 45 (14.5) | 47 (14.8) | 37 (16.0) | 36 (15.3) | 22 (15.6) | 20 (14.0) | 10 (16.7) | 11 (18.3) |
| IPI score, n (%) | ||||||||
| 2 | 99 (31.8) | 103 (32.4) | 66 (28.6) | 72 (30.5) | 42 (29.8) | 44 (30.8) | 17 (28.3) | 20 (33.3) |
| 3-5 | 211 (67.8) | 215 (69.1) | 165 (71.4) | 164 (69.5) | 99 (70.2) | 99 (69.2) | 43 (71.7) | 40 (66.7) |
| Cell of origin, n† | 237 | 252 | 175 | 187 | 108 | 112 | 42 | 45 |
| ABC | 79 (33.3) | 105 (41.7) | 55 (31.4) | 74 (39.6) | 33 (30.6) | 45 (40.2) | 12 (28.6) | 19 (42.2) |
| GCB | 131 (55.3) | 114 (45.2) | 99 (56.6) | 85 (45.5) | 65 (60.2) | 50 (44.7) | 24 (57.1) | 23 (51.1) |
| Unclassified | 27 (11.4) | 33 (13.1) | 21 (12.0) | 28 (15.0) | 10 (9.3) | 17 (15.2) | 6 (14.3) | 3 (6.7) |
| Unknown | 74 | 66 | 56 | 49 | 33 | 31 | 18 | 15 |
| Double expressor lymphoma‡ | 104 (40.9) | 119 (45.4) | 80 (42.3) | 89 (45.4) | 48 (42.1) | 55 (46.2) | 18 (40.9) | 24 (49.0) |
| Double-/triple-hit lymphoma§ | 21 (9.0) | 14 (5.7) | 15 (8.7) | 9 (4.9) | 8 (7.6) | 7 (6.1) | 2 (4.8) | 4 (8.7) |
ABC, activated B cell; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell.
Data presented as n (%) unless otherwise specified. Percentages are based on the total older patient population, unless otherwise specified as an evaluable population.
In the Pola-R-CHP and R-CHOP arms, respective numbers of evaluable patients were 237 and 252 in patients aged ≥60 years, 175 and 187 in patients aged ≥65 years, 108 and 112 in patients aged ≥70 years, and 42 and 45 in patients aged ≥75 years.
In the Pola-R-CHP and R-CHOP arms, respective numbers of evaluable patients were 254 and 262 in patients aged ≥60 years, 189 and 196 in patients aged ≥65 years, 114 and 119 in patients aged ≥70 years, and 44 and 49 in patients aged ≥75 years.
In the Pola-R-CHP and R-CHOP arms, respective numbers of evaluable patients were 233 and 245 in patients aged ≥60 years, 172 and 185 in patients aged ≥65 years, 105 and 114 in patients aged ≥70 years, and 42 and 46 in patients aged ≥75 years.
Efficacy
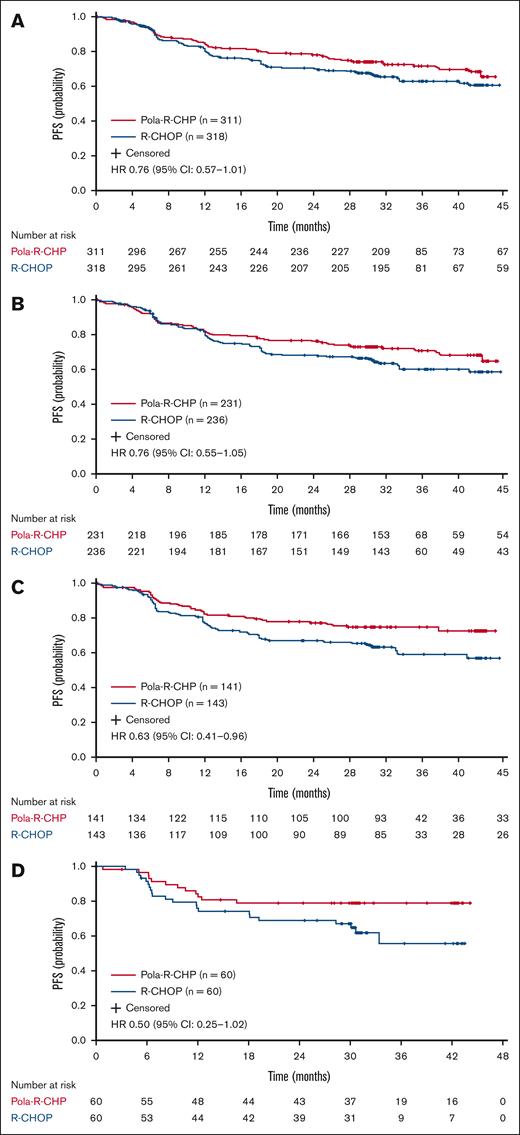
At data cutoff (15 June 2022; median survival follow-up, 40 months), there was a clinically meaningful improvement in PFS in favor of Pola-R-CHP across all older patient age groups (Figure 1). This PFS improvement was particularly noted in the older age group (≥70 years), for whom the risk of disease progression, relapse, or death was reduced by 37% with Pola-R-CHP vs R-CHOP (unstratified HR, 0.63; 95% confidence interval [CI], 0.41-0.96). This also held true for the oldest age group (≥75 years; unstratified HR, 0.50; 95% CI, 0.25-1.02). The proportion of patients surviving without disease progression at 2 years was higher with Pola-R-CHP than R-CHOP across all age groups, with the greatest benefit seen in patients aged ≥70 years (Table 3).
PFS with Pola-R-CHP and R-CHOP in older patients. (A) Aged ≥60 years, (B) ≥65 years, (C) ≥70 years, and (D) ≥75 years from the POLARIX study (efficacy-evaluable population). Unstratified log-rank analysis.
PFS with Pola-R-CHP and R-CHOP in older patients. (A) Aged ≥60 years, (B) ≥65 years, (C) ≥70 years, and (D) ≥75 years from the POLARIX study (efficacy-evaluable population). Unstratified log-rank analysis.
Efficacy end points (efficacy-evaluable population)
| . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 311) . | R-CHOP (n = 318) . | Pola-R-CHP (n = 231) . | R-CHOP (n = 236) . | Pola-R-CHP (n = 141) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 60) . | R-CHOP (n = 60) . | |
| Clinical response rates,∗n (%) | ||||||||
| ORR | 268 (86.2) | 270 (84.9) | 194 (84.0) | 200 (84.8) | 122 (86.5) | 120 (83.9) | 51 (85.0) | 49 (81.7) |
| CR | 251 (80.7) | 242 (76.1) | 181 (78.4) | 179 (75.9) | 115 (81.6) | 111 (77.6) | 47 (78.3) | 46 (76.7) |
| PR | 17 (5.5) | 28 (8.8) | 13 (5.6) | 21 (8.9) | 7 (5.0) | 9 (6.3) | 4 (6.7) | 3 (5.0) |
| SD | 3 (1.0) | 3 (1.0) | 3 (1.3) | 3 (1.3) | 2 (1.4) | 2 (1.4) | 1 (1.7) | 1 (1.7) |
| PD | 16 (5.1) | 17 (5.4) | 14 (6.1) | 12 (5.1) | 6 (4.3) | 10 (7.0) | 2 (3.3) | 6 (10.0) |
| NE | 6 (1.9) | 9 (2.8) | 4 (1.7) | 6 (2.5) | 2 (1.4) | 3 (2.1) | 1 (1.7) | 1 (1.7) |
| ND/missing | 18 (5.8) | 19 (6.0) | 16 (6.9) | 15 (6.4) | 9 (6.4) | 8 (5.6) | 5 (8.3) | 3 (5.0) |
| 2-y rates, % (95% CI) | ||||||||
| PFS | 77.9 (73.3-82.6) | 70.3 (65.1-75.4) | 76.5 (71.0-82.0) | 68.4 (62.4-74.5) | 77.3 (70.3-84.3) | 67.0 (59.2-74.8) | 78.9 (68.3-89.5) | 68.9 (57.0-80.8) |
| Difference, % | 7.6 | 8.1 | 10.3 | 10.0 | ||||
| OS | 88.6 (85.0-92.1) | 87.4 (83.7-91.1) | 85.9 (81.4-90.4) | 86.5 (82.0-90.9) | 86.3 (80.5-92.0) | 82.8 (76.5-89.1) | 83.1 (73.5-92.6) | 83.1 (73.5-92.6) |
| Difference, % | 1.2 | −0.6 | 3.5 | 0 | ||||
| EFS | 77.3 (72.6-82.0) | 69.7 (64.5-74.9) | 76.1 (70.5-81.6) | 67.7 (61.6-73.8) | 77.3 (70.3-84.3) | 66.3 (58.4-74.2) | 78.9 (68.3-89.5) | 67.2 (55.1-79.3) |
| Difference, % | 7.6 | 8.4 | 11.0 | 11.7 | ||||
| DFS† | 81.8 (77.2-86.4) | 75.4 (70.2-80.7) | 80.4 (74.9-85.9) | 74.4 (68.2-80.5) | 81.3 (74.4-88.2) | 73.0 (65.1-80.9) | 82.7 (72.4-93.0) | 72.8 (60.6-85.0) |
| Difference, % | 6.4 | 6.0 | 8.3 | 9.9 | ||||
| . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 311) . | R-CHOP (n = 318) . | Pola-R-CHP (n = 231) . | R-CHOP (n = 236) . | Pola-R-CHP (n = 141) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 60) . | R-CHOP (n = 60) . | |
| Clinical response rates,∗n (%) | ||||||||
| ORR | 268 (86.2) | 270 (84.9) | 194 (84.0) | 200 (84.8) | 122 (86.5) | 120 (83.9) | 51 (85.0) | 49 (81.7) |
| CR | 251 (80.7) | 242 (76.1) | 181 (78.4) | 179 (75.9) | 115 (81.6) | 111 (77.6) | 47 (78.3) | 46 (76.7) |
| PR | 17 (5.5) | 28 (8.8) | 13 (5.6) | 21 (8.9) | 7 (5.0) | 9 (6.3) | 4 (6.7) | 3 (5.0) |
| SD | 3 (1.0) | 3 (1.0) | 3 (1.3) | 3 (1.3) | 2 (1.4) | 2 (1.4) | 1 (1.7) | 1 (1.7) |
| PD | 16 (5.1) | 17 (5.4) | 14 (6.1) | 12 (5.1) | 6 (4.3) | 10 (7.0) | 2 (3.3) | 6 (10.0) |
| NE | 6 (1.9) | 9 (2.8) | 4 (1.7) | 6 (2.5) | 2 (1.4) | 3 (2.1) | 1 (1.7) | 1 (1.7) |
| ND/missing | 18 (5.8) | 19 (6.0) | 16 (6.9) | 15 (6.4) | 9 (6.4) | 8 (5.6) | 5 (8.3) | 3 (5.0) |
| 2-y rates, % (95% CI) | ||||||||
| PFS | 77.9 (73.3-82.6) | 70.3 (65.1-75.4) | 76.5 (71.0-82.0) | 68.4 (62.4-74.5) | 77.3 (70.3-84.3) | 67.0 (59.2-74.8) | 78.9 (68.3-89.5) | 68.9 (57.0-80.8) |
| Difference, % | 7.6 | 8.1 | 10.3 | 10.0 | ||||
| OS | 88.6 (85.0-92.1) | 87.4 (83.7-91.1) | 85.9 (81.4-90.4) | 86.5 (82.0-90.9) | 86.3 (80.5-92.0) | 82.8 (76.5-89.1) | 83.1 (73.5-92.6) | 83.1 (73.5-92.6) |
| Difference, % | 1.2 | −0.6 | 3.5 | 0 | ||||
| EFS | 77.3 (72.6-82.0) | 69.7 (64.5-74.9) | 76.1 (70.5-81.6) | 67.7 (61.6-73.8) | 77.3 (70.3-84.3) | 66.3 (58.4-74.2) | 78.9 (68.3-89.5) | 67.2 (55.1-79.3) |
| Difference, % | 7.6 | 8.4 | 11.0 | 11.7 | ||||
| DFS† | 81.8 (77.2-86.4) | 75.4 (70.2-80.7) | 80.4 (74.9-85.9) | 74.4 (68.2-80.5) | 81.3 (74.4-88.2) | 73.0 (65.1-80.9) | 82.7 (72.4-93.0) | 72.8 (60.6-85.0) |
| Difference, % | 6.4 | 6.0 | 8.3 | 9.9 | ||||
BICR, blinded independent central review; ND, not done; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
BICR assessed at the end of treatment.
The DFS-evaluable population for Pola-R-CHP and R-CHOP was 276 and 271, respectively, in patients aged ≥60 years; 204 and 201, in patients aged ≥65 years; 125 and 125, in patients aged ≥70 years; and 52 and 53, in patients aged ≥75 years.
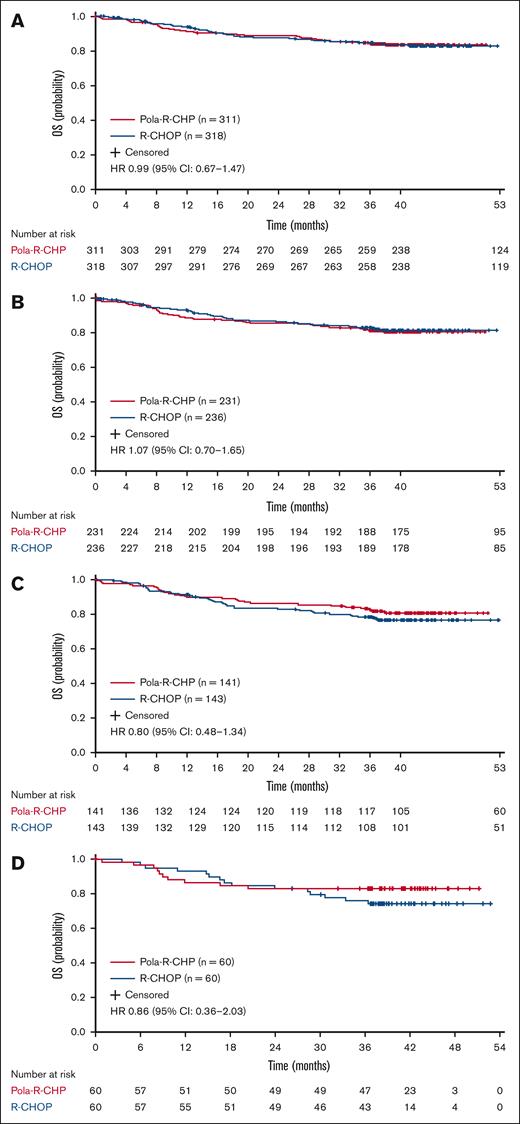
OS did not differ significantly between treatment arms in patients aged ≥60 and ≥65 years. In the Pola-R-CHP and R-CHOP groups, respective numbers of OS events by any cause were 50 (16.1%) and 51 (16.0%), respectively, in patients aged ≥60 years; 43 (18.6%) and 41 (17.4%), respectively, in patients aged ≥65 years; 26 (18.4%) and 32 (22.4%), respectively, in patients aged ≥70 years; and 10 (16.7%) and 15 (25.0%), respectively, in patients aged ≥75 years. OS data showed similar risk of death in patients aged ≥60 years with Pola-R-CHP vs R-CHOP (unstratified HR, 0.99; 95% CI, 0.67-1.47; Figure 2). Median OS was not reached in either arm.
OS with Pola-R-CHP and R-CHOP in older patients. (A) Aged ≥60 years, (B) ≥65 years, (C) ≥70 years, and (D) ≥75 years from the POLARIX study (efficacy-evaluable population). Unstratified log-rank analysis.
OS with Pola-R-CHP and R-CHOP in older patients. (A) Aged ≥60 years, (B) ≥65 years, (C) ≥70 years, and (D) ≥75 years from the POLARIX study (efficacy-evaluable population). Unstratified log-rank analysis.
At data cutoff, EFS and DFS rates favored Pola-R-CHP vs R-CHOP across all age groups, whereas patients who were aged ≥70 and ≥75 years seemed to show the greatest improvement with Pola-R-CHP (supplemental Figures 1 and 2; Table 3). Furthermore, CR rates were numerically higher with Pola-R-CHP vs R-CHOP in patients who were aged ≥60 years (80.7% and 76.1%, respectively), as well as in each older age group (Table 3). Across all age groups, patients who achieved a CR were more likely to maintain a remission with Pola-R-CHP vs R-CHOP, as demonstrated by the improvement in DFS (Table 3).
When analyzing PFS and OS by IPI 2 vs 3 to 5, the results of the older subgroup exploratory analysis were generally consistent with that observed in the overall POLARIX population (aged 18-80 years).7 In patients with an IPI score of 3 to 5, a PFS benefit was observed with Pola-R-CHP vs R-CHOP, with the biggest between-group difference observed in patients aged ≥70 years (2-year PFS difference 13.5%; supplemental Table 1). In patients aged ≥70 years with an IPI score of 2, there was a trend in PFS benefit with Pola-R-CHP vs R-CHOP (supplemental Table 1). There was no observed trend in 2-year OS rates with Pola-R-CHP vs R-CHOP in patients with an IPI score of 2 or 3 to 5 (supplemental Table 1).
Safety and tolerability
Treatment exposure was high in both treatment arms; most patients received all 6 doses of polatuzumab vedotin (90.2%) or vincristine (88.3%). Across the different age groups, polatuzumab vedotin or vincristine exposure varied between 88.3% and 90.2% in the Pola-R-CHP arm, and 88.3% and 92.3% in the R-CHOP arm. The median relative dose intensities (proportions of administered doses relative to planned doses) of rituximab, doxorubicin, and cyclophosphamide were >99% in both treatment arms. Overall, safety profiles were generally similar for Pola-R-CHP and R-CHOP in patients aged ≥60 and ≥65 years, with increased incidence of toxicities observed in patients aged ≥70 and ≥75 years (Table 4). The most common grade 3/4 treatment-emergent AEs across all age groups were neutropenia, febrile neutropenia, and anemia (Table 5). In patients aged ≥70 and ≥75 years, higher rates of serious AEs, and grade 3/4 febrile neutropenia, diarrhea, and infection were observed with Pola-R-CHP compared with R-CHOP (Tables 4 and 5).
Safety summary (safety-evaluable population)
| Adverse event∗ . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 306) . | R-CHOP (n = 317) . | Pola-R-CHP (n = 227) . | R-CHOP (n = 235) . | Pola-R-CHP (n = 137) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 57) . | R-CHOP (n = 60) . | |
| AEs | ||||||||
| Any grade | 301 (98.4) | 312 (98.4) | 223 (98.2) | 232 (98.7) | 136 (99.3) | 142 (99.3) | 57 (100) | 60 (100) |
| Grade 3-4 | 192 (62.7) | 195 (61.5) | 150 (66.1) | 153 (65.1) | 101 (73.7) | 98 (68.5) | 40 (70.2) | 48 (80.0) |
| Grade 5 | 11 (3.6) | 10 (3.2) | 9 (4.0) | 9 (3.8) | 5 (3.6) | 7 (4.9) | 2 (3.5) | 2 (3.3) |
| SAEs | 113 (36.9) | 101 (31.9) | 89 (39.2) | 81 (34.5) | 59 (43.1) | 55 (38.5) | 26 (45.6) | 24 (40.0) |
| Related to any study drug | 86 (28.1) | 67 (21.1) | 66 (29.1) | 53 (22.6) | 48 (35.0) | 35 (24.5) | 20 (35.1) | 18 (30.0) |
| AE leading to | ||||||||
| Study discontinuation | 11 (3.6) | 10 (3.2) | 9 (4.0) | 9 (3.8) | 5 (3.6) | 7 (4.9) | 2 (3.5) | 2 (3.3) |
| Discontinuation of polatuzumab vedotin/vincristine | 17 (5.6) | 19 (6.0) | 15 (6.6) | 15 (6.4) | 11 (8.0) | 6 (4.2) | 4 (7.0) | 3 (5.0) |
| Dose reduction of polatuzumab vedotin/vincristine | 18 (5.9) | 33 (10.4) | 11 (4.8) | 21 (8.9) | 9 (6.6) | 17 (11.9) | 4 (7.0) | 9 (15.0) |
| Interruption of polatuzumab vedotin/vincristine | 57 (18.6) | 56 (17.7) | 42 (18.5) | 43 (18.3) | 27 (19.7) | 31 (21.7) | 13 (22.8) | 12 (20.0) |
| Adverse event∗ . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 306) . | R-CHOP (n = 317) . | Pola-R-CHP (n = 227) . | R-CHOP (n = 235) . | Pola-R-CHP (n = 137) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 57) . | R-CHOP (n = 60) . | |
| AEs | ||||||||
| Any grade | 301 (98.4) | 312 (98.4) | 223 (98.2) | 232 (98.7) | 136 (99.3) | 142 (99.3) | 57 (100) | 60 (100) |
| Grade 3-4 | 192 (62.7) | 195 (61.5) | 150 (66.1) | 153 (65.1) | 101 (73.7) | 98 (68.5) | 40 (70.2) | 48 (80.0) |
| Grade 5 | 11 (3.6) | 10 (3.2) | 9 (4.0) | 9 (3.8) | 5 (3.6) | 7 (4.9) | 2 (3.5) | 2 (3.3) |
| SAEs | 113 (36.9) | 101 (31.9) | 89 (39.2) | 81 (34.5) | 59 (43.1) | 55 (38.5) | 26 (45.6) | 24 (40.0) |
| Related to any study drug | 86 (28.1) | 67 (21.1) | 66 (29.1) | 53 (22.6) | 48 (35.0) | 35 (24.5) | 20 (35.1) | 18 (30.0) |
| AE leading to | ||||||||
| Study discontinuation | 11 (3.6) | 10 (3.2) | 9 (4.0) | 9 (3.8) | 5 (3.6) | 7 (4.9) | 2 (3.5) | 2 (3.3) |
| Discontinuation of polatuzumab vedotin/vincristine | 17 (5.6) | 19 (6.0) | 15 (6.6) | 15 (6.4) | 11 (8.0) | 6 (4.2) | 4 (7.0) | 3 (5.0) |
| Dose reduction of polatuzumab vedotin/vincristine | 18 (5.9) | 33 (10.4) | 11 (4.8) | 21 (8.9) | 9 (6.6) | 17 (11.9) | 4 (7.0) | 9 (15.0) |
| Interruption of polatuzumab vedotin/vincristine | 57 (18.6) | 56 (17.7) | 42 (18.5) | 43 (18.3) | 27 (19.7) | 31 (21.7) | 13 (22.8) | 12 (20.0) |
SAE, serious adverse event.
Data are presented as n (%).
Grade 3/4 treatment-emergent AEs (safety-evaluable population)
| Adverse event∗ . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 306) . | R-CHOP (n = 317) . | Pola-R-CHP (n = 227) . | R-CHOP (n = 235) . | Pola-R-CHP (n = 137) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 57) . | R-CHOP (n = 60) . | |
| Most common† | ||||||||
| Neutropenia | 90 (29.4) | 104 (32.8) | 69 (30.4) | 82 (34.9) | 42 (30.7) | 50 (35.0) | 16 (28.1) | 26 (43.3) |
| Febrile neutropenia | 50 (16.3) | 24 (7.6) | 40 (17.6) | 18 (7.7) | 29 (21.2) | 12 (8.4) | 12 (21.1) | 5 (8.3) |
| Anemia | 41 (13.4) | 34 (10.7) | 34 (15.0) | 22 (9.4) | 21 (15.3) | 14 (9.8) | 8 (14.0) | 8 (13.3) |
| Leukopenia | 19 (6.2) | 27 (8.5) | 17 (7.5) | 18 (7.7) | 10 (7.3) | 9 (6.3) | 5 (8.8) | 3 (5.0) |
| Diarrhea | 14 (4.6) | 7 (2.2) | 12 (5.3) | 5 (2.1) | 10 (7.3) | 4 (2.8) | 3 (5.3) | 3 (5.0) |
| Thrombocytopenia | 12 (3.9) | 15 (4.7) | 10 (4.4) | 11 (4.7) | 7 (5.1) | 6 (4.2) | 2 (3.5) | 6 (10.0) |
| Pneumonia | 11 (3.6) | 12 (3.8) | 9 (4.0) | 9 (3.8) | 5 (3.6) | 6 (4.2) | 4 (7.0) | 3 (5.0) |
| Lymphopenia | 7 (2.3) | 8 (2.5) | 7 (3.1) | 5 (2.1) | 5 (3.6) | 3 (2.1) | 2 (3.5) | 2 (3.3) |
| Syncope | 7 (2.3) | 8 (2.5) | 7 (3.1) | 8 (3.4) | 6 (4.4) | 6 (4.2) | 4 (7.0) | 4 (6.7) |
| Hyponatremia | 5 (1.6) | 7 (2.2) | 2 (0.9) | 6 (2.6) | 1 (0.7) | 3 (2.1) | 0 | 2 (3.3) |
| Fatigue | 4 (1.3) | 10 (3.2) | 4 (1.8) | 7 (3.0) | 4 (2.9) | 5 (3.5) | 3 (5.3) | 2 (3.3) |
| Hypertension | 3 (1.0) | 10 (3.2) | 3 (1.3) | 8 (3.4) | 2 (1.5) | 5 (3.5) | 1 (1.8) | 4 (6.7) |
| AEPI | ||||||||
| Infection | 43 (14.1) | 35 (11.0) | 35 (15.4) | 30 (12.8) | 22 (16.1) | 18 (12.6) | 13 (22.8) | 10 (16.7) |
| Urinary tract infection | 6 (2.0) | 5 (1.6) | 5 (2.2) | 4 (1.7) | 4 (2.9) | 3 (2.1) | 3 (5.3) | 2 (3.3) |
| Asthenia | 4 (1.3) | 1 (0.3) | 3 (1.3) | 1 (0.4) | 3 (2.2) | 1 (0.7) | 0 | 1 (1.7) |
| Peripheral neuropathy | 2 (0.7) | 3 (0.9) | 1 (0.4) | 3 (1.3) | 1 (0.7) | 1 (0.7) | 1 (1.8) | 1 (1.7) |
| Pyrexia | 2 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sepsis | 1 (0.3) | 7 (2.2) | 1 (0.4) | 7 (3.0) | 1 (0.7) | 4 (2.8) | 1 (1.8) | 3 (5.0) |
| Septic shock | 0 | 1 (0.3) | 0 | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 |
| Adverse event∗ . | Patients aged ≥60 y . | Patients aged ≥65 y . | Patients aged ≥70 y . | Patients aged ≥75 y . | ||||
|---|---|---|---|---|---|---|---|---|
| Pola-R-CHP (n = 306) . | R-CHOP (n = 317) . | Pola-R-CHP (n = 227) . | R-CHOP (n = 235) . | Pola-R-CHP (n = 137) . | R-CHOP (n = 143) . | Pola-R-CHP (n = 57) . | R-CHOP (n = 60) . | |
| Most common† | ||||||||
| Neutropenia | 90 (29.4) | 104 (32.8) | 69 (30.4) | 82 (34.9) | 42 (30.7) | 50 (35.0) | 16 (28.1) | 26 (43.3) |
| Febrile neutropenia | 50 (16.3) | 24 (7.6) | 40 (17.6) | 18 (7.7) | 29 (21.2) | 12 (8.4) | 12 (21.1) | 5 (8.3) |
| Anemia | 41 (13.4) | 34 (10.7) | 34 (15.0) | 22 (9.4) | 21 (15.3) | 14 (9.8) | 8 (14.0) | 8 (13.3) |
| Leukopenia | 19 (6.2) | 27 (8.5) | 17 (7.5) | 18 (7.7) | 10 (7.3) | 9 (6.3) | 5 (8.8) | 3 (5.0) |
| Diarrhea | 14 (4.6) | 7 (2.2) | 12 (5.3) | 5 (2.1) | 10 (7.3) | 4 (2.8) | 3 (5.3) | 3 (5.0) |
| Thrombocytopenia | 12 (3.9) | 15 (4.7) | 10 (4.4) | 11 (4.7) | 7 (5.1) | 6 (4.2) | 2 (3.5) | 6 (10.0) |
| Pneumonia | 11 (3.6) | 12 (3.8) | 9 (4.0) | 9 (3.8) | 5 (3.6) | 6 (4.2) | 4 (7.0) | 3 (5.0) |
| Lymphopenia | 7 (2.3) | 8 (2.5) | 7 (3.1) | 5 (2.1) | 5 (3.6) | 3 (2.1) | 2 (3.5) | 2 (3.3) |
| Syncope | 7 (2.3) | 8 (2.5) | 7 (3.1) | 8 (3.4) | 6 (4.4) | 6 (4.2) | 4 (7.0) | 4 (6.7) |
| Hyponatremia | 5 (1.6) | 7 (2.2) | 2 (0.9) | 6 (2.6) | 1 (0.7) | 3 (2.1) | 0 | 2 (3.3) |
| Fatigue | 4 (1.3) | 10 (3.2) | 4 (1.8) | 7 (3.0) | 4 (2.9) | 5 (3.5) | 3 (5.3) | 2 (3.3) |
| Hypertension | 3 (1.0) | 10 (3.2) | 3 (1.3) | 8 (3.4) | 2 (1.5) | 5 (3.5) | 1 (1.8) | 4 (6.7) |
| AEPI | ||||||||
| Infection | 43 (14.1) | 35 (11.0) | 35 (15.4) | 30 (12.8) | 22 (16.1) | 18 (12.6) | 13 (22.8) | 10 (16.7) |
| Urinary tract infection | 6 (2.0) | 5 (1.6) | 5 (2.2) | 4 (1.7) | 4 (2.9) | 3 (2.1) | 3 (5.3) | 2 (3.3) |
| Asthenia | 4 (1.3) | 1 (0.3) | 3 (1.3) | 1 (0.4) | 3 (2.2) | 1 (0.7) | 0 | 1 (1.7) |
| Peripheral neuropathy | 2 (0.7) | 3 (0.9) | 1 (0.4) | 3 (1.3) | 1 (0.7) | 1 (0.7) | 1 (1.8) | 1 (1.7) |
| Pyrexia | 2 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sepsis | 1 (0.3) | 7 (2.2) | 1 (0.4) | 7 (3.0) | 1 (0.7) | 4 (2.8) | 1 (1.8) | 3 (5.0) |
| Septic shock | 0 | 1 (0.3) | 0 | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 |
AEPI, adverse events of particular interest.
Data are presented as n (%).
Occurring in ≥2% of patients in any treatment arm.
Although all patients were required to receive G-CSF during the first 6 cycles of treatment as primary prophylaxis against neutropenia, it is important to note that among the patients who experienced grade 3/4 febrile neutropenia (Pola-R-CHP, n = 50; R-CHOP, n = 24), 16 patients (32.0%) in the Pola-R-CHP arm and 8 patients (33.3%) in the R-CHOP arm did not receive prophylactic G-CSF. In patients aged ≥70 years, who experienced grade 3/4 febrile neutropenia (Pola-R-CHP, n = 29; R-CHOP, n = 12), 9 patients (31.0%) in the Pola-R-CHP arm and 3 patients (25.0%) in the R-CHOP arm did not receive G-CSF prophylaxis before a febrile neutropenia event. In patients aged ≥60 years, the incidence of grade 3/4 febrile neutropenia was higher with Pola-R-CHP than with R-CHOP (16.3% vs 7.6%), but the incidence of grade 3 to 5 infection was comparable (15.0% vs 12.9%, respectively). This trend was particularly noticeable in patients aged ≥70 years, with corresponding rates of grade 3/4 febrile neutropenia in the Pola-R-CHP vs R-CHOP arm of 21.2% vs 8.4%, and rates of grade 3 to 5 infection of 17.5% vs 16.1%.
Rates of grade 5 AEs in patients aged ≥60 years were similar between Pola-R-CHP (3.6%) and R-CHOP (3.2%); this similarity was consistently observed in the other age groups (≥65 years: 4.0% and 3.8%, respectively; ≥70 years: 3.6% and 4.9%; ≥75 years: 3.5% and 3.3%; Table 4). The grade 5 AEs in patients aged ≥60 years included death (no known cause; 1.3%), pneumonia (0.7%), acute kidney injury (0.3%), cardiac death (0.3%), gastrointestinal perforation (0.3%), respiratory failure (0.3%), and sepsis (0.3%) in the Pola-R-CHP group, and pneumonia (0.9%), septic shock (0.6%), complete atrioventricular block (0.3%), death (no known cause; 0.3%), injury (car accident; 0.3%), multiple organ dysfunction syndrome (0.3%), and sepsis (0.3%) in the R-CHOP group.
QoL
After 24 months of follow-up, improvements in lymphoma symptom scores by FACT-LymS and global health status/QoL, fatigue, and physical functioning by EORTC QLQ-C30 were similar between treatment arms in the older patient population (supplemental Figures 3-6). In patients aged ≥60 and ≥65 years, FACT-LymS scores were generally similar between the Pola-R-CHP and R-CHOP treatment arms. In patients aged ≥70 years, FACT-LymS scores improved from baseline; however, patients in the Pola-R-CHP arm generally showed less improvement in FACT-LymS scores vs the R-CHOP arm (supplemental Figure 3). After 24 months of follow-up, mean improvements from baseline in QoL were similar between the Pola-R-CHP and R-CHOP treatment arms and were more pronounced in patients aged ≥60 vs ≥70 years (supplemental Figure 4). Patients in the Pola-R-CHP arm showed more improvement in fatigue vs patients in the R-CHOP arm at 24 months follow-up in patients aged ≥60 vs ≥70 years (supplemental Figure 5). Mean improvements in physical functioning from baseline were generally similar between the Pola-R-CHP and R-CHOP treatment arms; however, patients ≥70 years in the Pola-R-CHP treatment arm showed slightly less improvement in physical functioning vs the R-CHOP arm (supplemental Figure 6).
Discussion
In this analysis of older patients (aged ≥60 years) with previously untreated DLBCL in the phase 3 POLARIX study, the benefit-risk profile favored Pola-R-CHP over R-CHOP. Efficacy end points favored Pola-R-CHP vs R-CHOP across all age groups studied, including superior PFS in the older age groups (≥70 and ≥75 years). In the primary analysis of the POLARIX study, 2-year PFS was significantly higher with Pola-R-CHP vs R-CHOP (76.7% [95% CI, 72.7-80.8] vs 70.2% [95% CI, 65.8-74.6]).7 In this analysis of patients aged ≥60 years, 2-year PFS rates were also higher with Pola-R-CHP vs R-CHOP (77.9% [95% CI, 73.3-82.6] vs 70·3% [95% CI, 65.1-75.4]). When analyzing PFS and OS by IPI 2 vs 3 to 5, the results were generally consistent with that observed in the overall population. In patients with an IPI score of 3 to 5, a PFS benefit across all age groups was observed with Pola-R-CHP vs R-CHOP. Importantly, these analyses on a subgroup of older patients were not statistically powered to assess efficacy based on IPI score; thus, these results should be considered exploratory.
In the overall POLARIX population, the safety profile was generally similar between treatment arms, and the most common grade 3/4 AE was neutropenia. Although there was a higher rate of grade 3/4 AEs observed in this analysis with Pola-R-CHP than with R-CHOP, the safety profile in older patients was similar across all age groups and generally consistent with that reported in the overall population of POLARIX (aged 18-80 years).7 In patients aged ≥60 years in this analysis, grade 3/4 febrile neutropenia occurred in 50 (16.3%) patients who received Pola-R-CHP vs 24 (7.6%) patients who received R-CHOP, and approximately one-third (n = 16 and n = 8, respectively) did not receive G-CSF primary prophylaxis. Importantly, the risk of grade 3/4 febrile neutropenia was much higher in older patients aged ≥60 years compared with younger patients aged <60 years treated with Pola-R-CHP (16.3% vs 7.8%). Therefore, older patients have an increased risk of febrile neutropenia after Pola-R-CHP treatment, and they should receive G-CSF per the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) guidelines.11,12 Overall, Pola-R-CHP was well tolerated, and treatment-related toxicities should be manageable with supportive medical care.
Several prior studies have demonstrated poor efficacy and safety outcomes in older patients with DLBCL when treated with R-CHOP alone or in combination with an additional treatment.13-16 In the LNH03-6B study (ClinicalTrials.gov identifier: NCT00144755), PFS did not differ between patients treated with R-CHOP given every 14 days (R-CHOP14) vs those treated with R-CHOP given every 21 days (R-CHOP21) in patients aged 60 to 80 years.13 Grade 3-4 neutropenia occurred in 64% of patients in the R-CHOP21 arm, which was higher than the rate observed in patients treated with Pola-R-CHP in the POLARIX study.13 However, in the LNH03-6B study, G-CSF was only used in 60% of patients from cycle 1 of R-CHOP2113; hence, caution must be taken when making comparisons between trials because of differences in study design.
In the SENIOR study (ClinicalTrials.gov identifier: NCT02128061), the addition of lenalidomide to R-mini-CHOP vs R-mini-CHOP did not improve OS in patients aged ≥80 years and resulted in higher grade 3/4 AEs.15 In the PHOENIX study (ClinicalTrials.gov identifier: NCT01855750), in patients aged ≥60 years with non–germinal center B-cell DLBCL treated with ibrutinib plus R-CHOP, there was a trend toward poorer EFS, PFS, and OS vs those treated with R-CHOP plus placebo.16 Ibrutinib plus R-CHOP was also associated with increased toxicity vs R-CHOP plus placebo; serious AEs occurred in 63.4% vs 38.2% of treated patients aged ≥60 years, respectively. There was also a decrease in treatment exposure of R-CHOP with the addition of ibrutinib in these patients. These studies underscore the need for innovative treatment combinations for this clinical population. Attenuated doses of chemotherapy within the Pola-R-CHP regimen have also been shown to be tolerable in older and frail populations, as demonstrated in the POLAR BEAR study (ClinicalTrials.gov identifier: NCT04332822) with similar rates of peripheral neuropathy; however, patients in the R-Pola-mini-CHP arm had twice as many gastrointestinal toxicities than those in the R-mini-CHOP arm.17
Older patient populations are more challenging to treat in the relapsed setting given increased incidence of comorbidities and reduced eligibility for more intensive therapies with curative intent, such as stem cell transplantation or CAR-T therapy.4,18,19 Although older patients are potentially eligible for CAR-T therapy, barriers to treatment do exist including toxicity risks and patient disease kinetics. Additionally, there may be logistic, geographic, or resource constraints, further limiting access to cellular therapies, particularly for patients in urgent need of treatment. These potential barriers highlight the important role of achieving a long-term remission in the first-line setting.4 Given this paradigm, improving clinical outcomes with first-line therapy, while limiting toxicity, would represent important progress for older populations with DLBCL, underscoring the importance of the current analysis.
The strengths of this analysis include the large population size of older patients, the balanced baseline demographics, and the clinically meaningful efficacy and safety outcomes. Potential limitations of the study include the arbitrary age classifications used in the analysis and the lack of quantified clinical factors such as frailty and comorbidities.
Conclusions
Overall, the benefit-risk profile favored Pola-R-CHP vs R-CHOP in older patients with previously untreated DLBCL. Pola-R-CHP demonstrated improved PFS vs R-CHOP with a similar safety profile in patients across all the age groups studied; this PFS benefit was also observed in patients with an IPI score of 3 to 5. The results were consistent with those of the overall POLARIX population and suggest that older populations can benefit from treatment with Pola-R-CHP with manageable toxicities.
Acknowledgments
The authors acknowledge Piotr Smolewski (1960-2023) for his contributions to this research, and his dedication to his patients in the field of internal medicine and hematology.
The POLARIX study (NCT03274492) was sponsored by F. Hoffmann-La Roche Ltd and Genentech, Inc. Third-party editorial assistance, under the direction of the authors, was provided by Jonike Dreyer and Anna Nagy of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd.
This study was designed by F. Hoffmann-La Roche Ltd and academic authors. Data were collected and analyzed by the academic authors and their research teams, which were interpreted by the authors and the sponsor.
Authorship
Contribution: L.H.S., J.P.S., C.H., J.H., C.L., and H.T. designed the study; B.H., S.C., R.K., J.H., M.S., C.L., and H.T. analyzed the data; S.C. and M.S. directly accessed and verified the underlying data; and all authors executed the study, wrote the manuscript, and provided final approval.
Conflict-of-interest disclosure: B.H. has served in a consultancy or advisory role for ADC Therapeutics, BeiGene, and Janssen; has acted in a leadership role for F. Hoffmann-La Roche Ltd/Genentech Inc; and has received research funding from Juno/Bristol Myers Squibb (BMS), and F. Hoffmann-La Roche Ltd/Genentech, Inc. P.M.R. has served in a consultancy or advisory role for Kite Pharma (a Gilead company) and Caribou Biosciences; has received honoraria from Kite Pharma; and has received research funding from Seagen, Genentech Inc, and F. Hoffmann-La Roche Ltd. L.H.S. has received grants or contracts from Cargo Therapeutics; has received honoraria from F. Hoffmann-La Roche Ltd/Genentech Inc, AbbVie, Janssen, Incyte, BeiGene, AstraZeneca, BMS/Celgene, Genmab, Merck, Seagen, and Kite/Gilead; and has received research funding from F. Hoffmann-La Roche Ltd/Genentech, Inc. J.P.S. has served in a consulting or advisory role for AbbVie, AstraZeneca, BeiGene, BMS, Lilly, Merck, and Genentech Inc. M.H. has served in a consulting or advisory role for F. Hoffmann-La Roche Ltd, Takeda, Otsuka, Gilead Sciences, Menarini, and BeiGene; and has received travel, accommodations, or expenses from Takeda. D.M. has received honoraria from Merck Sharp and Dohme (MSD), Zenyaku, BMS Japan, AstraZeneca, Nippon Shinyaku, Takeda, Janssen, Eisai, Celgene, Kyowa Kirin, Genma, Novartis, Ono Pharmaceutical, Sanofi, Mundipharma, AbbVie, and Chugai Pharma; and has received research funding from Astellas Pharma, AstraZeneca, MSD, Otsuka, Novartis, Kyowa Kirin, AbbVie, Sanofi, Symbio, BMS, Chugai Pharma, Ono Pharmaceutical, Janssen, Pfizer, Genmab, Zenkayu, Eisai, Takeda, and Taiho. F.S. has received payment for presentation at F. Hoffmann-La Roche Ltd/CH; has received travel, accommodation, or expenses from F. Hoffmann-La Roche Ltd (International Conference on Malignant Lymphoma [ICML] conference 2022); and has received payment for participation on an advisory board by F. Hoffmann-La Roche Ltd/CH. C.L.B. has served as an employee of Genentech, Inc/F. Hoffmann-La Roche Ltd; reports stock and other ownership interests from F. Hoffmann-La Roche Ltd; has received research funding from Epizyme, Autolus, Roche, and Vincerx; has received consulting fees from BMS, Seagen, Kite, Karyopharma, TG Therapeutics, ADC Therapeutics, AbbVie, Genentech, Treeline Biosciences; and has received honoraria from Dava Oncology, Touch Independent Medical Education (TouchIME), and Medscape. J.H. reports stock and other ownership interests from F. Hoffmann-La Roche Ltd. D.S. has served as an employee of F. Hoffmann-La Roche Ltd; and reports stock and other ownership from F. Hoffmann-La Roche Ltd. C.L. has served as an employee of Genentech, Inc/F. Hoffmann-La Roche Ltd, and reports stock and other ownership from F. Hoffmann-La Roche Ltd. M.S. reports stock and other ownership interests from Genentech, Inc/F. Hoffmann-La Roche Ltd. H.T. has received support for the present manuscript from F. Hoffmann-La Roche Ltd; has received honoraria from F. Hoffmann-La Roche Ltd and Incyte; has received travel, accommodation, or expenses from F. Hoffmann-La Roche Ltd and Janssen; has received honoraria from BMS and F. Hoffmann-La Roche Ltd; and has received payment for participation on an advisory board by BMS and Karyopharm. The remaining authors declare no competing financial interests.
The current affiliation for J.H. is BeOne Medicine, San Carlos, CA.
The current affiliation for C.L. is Gilead Sciences, Foster City, CA.
Correspondence: Bei Hu, Malignant Hematology, Atrium Health Levine Cancer Institute, Building 2, 1021 Morehead Medical Dr, Charlotte, NC 28204; email: bei.hu@atriumhealth.org.
References
Author notes
The data in this article represent updated data previously presented, in part, at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, 2 to 6 June 2023; and, as an encore, at the 17th International Conference on Malignant Lymphoma, Lugano, Switzerland, 13 to 17 June 2023.
For eligible studies, qualified researchers may request access to individual patient level clinical data through a data request platform. At the time of writing this request, the platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's global policy on the sharing of clinical information and how to request access to related clinical study documents (available at https://go.roche.com/data_sharing). Anonymized records for individual patients across >1 data source external to Roche cannot, and should not, be linked because of a potential increase in risk of patient reidentification.
The full-text version of this article contains a data supplement.