Key Points
DBAS is characterized by pure red cell aplasia with 30% of cases undiagnosed likely because of noncoding variants.
Molecular diagnoses of DBAS require careful analysis beyond the coding regions, typically not fully covered or analyzed by clinical testing.
Visual Abstract
Diamond-Blackfan anemia syndrome (DBAS) is a rare congenital disorder with variable penetrance and expressivity and is characterized by pure red cell aplasia that typically manifests as early-onset chronic macrocytic or normocytic anemia and is often associated with other congenital anomalies. DBAS is etiologically heterogeneous with >20 known DBAS-associated genes that encode small and large ribosomal protein subunits and an inheritance pattern that is largely autosomal dominant or sporadic. We report 2 DBAS cases with previous negative genetic testing, which included targeted gene panels, karyotype analysis, chromosome breakage analysis, and whole exome sequencing. Although clinical whole genome sequencing (WGS) was initially negative, in-depth reanalysis identified 2 novel noncoding variants in the RPS gene family, namely a maternally inherited splicing variant at the end of the first noncoding exon in RPS7 (NM_001011.4, c.-19G>C) in family 1 and a deep intronic de novo variant in RPS19 (NM_001022.4, c.172+350C>T) in family 2. In family 1, several maternal relatives were identified who shared the same variant through cascade testing; clinically, they exhibited variable degrees of anemia and elevated erythrocyte adenosine deaminase activity, a marker for DBAS. RNA sequencing analysis demonstrated deleterious functional consequences for both noncoding variants. In case 1, hematopoietic stem cell transplant with an unaffected matched sibling donor who did not carry the variant successfully cured the congenital anemia. This study identified novel noncoding variants and underscores the clinical utility of WGS in accelerating diagnosis and improving care for rare genetic disorders, particularly when timely treatment decisions are critically important.
Introduction
With an overall diagnostic rate of ∼30% to 40%, the benefits of whole genome sequencing (WGS) for individuals with rare diseases are increasingly recognized.1 Unlike whole exome sequencing (WES) and targeted next-generation sequencing (NGS) panels, WGS can identify deep intronic and other noncoding variants, which are often underappreciated causes of undiagnosed disease. This capability is critically important when diagnosing genetic disorders known or suspected to be caused by such variants, as indicated by literature and in silico predictions.2
Diamond-Blackfan anemia syndrome (DBAS) is a very rare (1:500 000 live births) congenital disorder. It often manifests in infancy as pure red cell aplasia and leads to profound macrocytic or normocytic anemia that requires chronic packed red blood cell (pRBC) transfusion support, chronic glucocorticoid treatment, or hematopoietic stem cell transplant (HSCT).3-5 DBAS occurs de novo in two-thirds of the cases and is inherited in the remaining third with variable expressivity and penetrance within families.6 Approximately half of DBAS cases present with additional major or minor congenital anomalies, most commonly craniofacial, skeletal (primarily affecting the upper limbs and hands), genitourinary, and cardiac features.3,7,8 Individuals with DBAS also face an increased risk for cancer, including hematologic malignancies and solid tumors.3,9 Notably, partial expressivity and incomplete intrafamilial penetrance are well documented.3 DBAS is largely caused by heterozygous loss of function variants in various ribosomal genes10 with variants in RPS19, which encodes a 40S ribosomal protein, accounting for ∼25% of DBAS cases.3 In contrast, variants in RPS7, which encodes a different component of the same 40S ribosomal subunit, account for <1% of DBAS cases. However, standard genetic testing of known DBAS genes is negative in ∼20% to 30% of cases although the patients exhibit clinical findings consistent with a DBAS diagnosis.3,4 Importantly, in the 2024 international consensus statement of DBAS,6 this entity is now described as a syndrome with diverse clinical phenotypes not necessarily having anemia manifested, yet with a strong diagnostic emphasis on molecular genetics and morphology. In the same consensus statement, the expert group recommends molecular testing for every patient with suspected DBAS and pursuing familial testing once a pathogenic variant in DBAS genes is found in the proband.6
In this study, we report 2 infants from 2 unrelated families who exhibited clinical and biochemical features of DBAS but remained genetically unresolved despite extensive previous testing, including cytogenetic analysis, gene panel testing, and clinical WGS. Through an in-depth reanalysis of the WGS data, we identified novel noncoding variants in RPS7 and RPS19, which clarified the diagnosis and improved the care plan in both families.
Methods
Human subject research statements and ethical considerations
Both patients were referred by the Pediatric Hematology team at the University of Utah/Primary Children’s Hospital to the Penelope Undiagnosed and Rare Disease Program in Utah. The research component of the Penelope Program was approved by the University of Utah institutional review board. Written informed consent for participation in the study and for publication was obtained from the patients’ families and was reconfirmed before article submission. Written consent was obtained from the parents or legal guardians of any participant under the age of 16.
WGS and analysis
The families were enrolled in the Penelope Undiagnosed Disease Program at the University of Utah. We first obtained clinical WGS at a Clinical Laboratory Improvement Amendments-certified laboratory. Genomic DNA was extracted from peripheral blood or buccal samples using the Chemagic Magnetic Separation Module I kit (PerkinElmer, Waltham, MA), and WGS sequencing libraries were prepared and underwent 2 × 150 base pair (bp) paired-end sequencing (Illumina). WGS was performed for the proband, the parents, and an unaffected sibling in case 1 and for the proband and the parents in case 2. The results for both cases were diagnostically negative. We obtained the raw FASTQ files and performed a research reanalysis in parallel at the ARUP Laboratories, using the Emedgene software with its built-in DRAGEN pipeline (Illumina Inc), and at the Utah Center for Genetic Discovery. At the Utah Center for Genetic Discovery, the reads were aligned with the GRCh38 human genome reference using Burrow-Wheeler Aligner, and single nucleotide variant and short insertion/deletion calling was performed using either the Sentieon implementation of Genome Analysis Toolkit algorithms (case 1) or DeepVariant (case 2). Single nucleotide variants and short insertions/deletions were filtered using Slivar11 for nonsynonymous, splicing, and 5′ untranslated region (UTR) start-gain variants with <2% population allele frequency and for cosegregation with the DBAS phenotype among sequenced individuals. Intronic variants with a SpliceAI score of ≥0.1 were included. Structural variants (SVs) were called using Smoove,12 RUFUS, and Manta13. Smoove calls were annotated with population SV allele frequencies using SVAFotate14 and filtered for rare (<1%), cosegregating variants using Slivar. Sanger sequencing was performed clinically for familial segregation studies.
RNA-seq and analysis
RNA sequencing (RNA-seq) was performed at the Huntsman Cancer Institute Genomics Core Laboratory. RNA from whole blood was extracted, quantified, and processed using a TruSeq Stranded Total RNA Library Prep Kit (Illumina). Ribosomal RNA was removed using Ribo-Zero, which targeted ribosomal RNA with biotinylated oligonucleotides. The remaining RNA was fragmented and primed for reverse transcription. DNase treatment was applied to minimize genomic DNA contamination before multiplexing and undergoing 150 bp paired-end sequencing on the NovaSeq Platform (Illumina) with a median of 119 million reads generated per sample. Blood draws were timed to avoid interference from donor-derived RNA from RBC transfusion as much as possible. FASTQ files were aligned to the GRCh37/hg19 reference genome sequence using STAR-2.7.8a. The BAM files that were obtained were analyzed using the Integrative Genomics Viewer v2.11.9.
Results
Case 1, WGS quad, RPS7/DBAS8
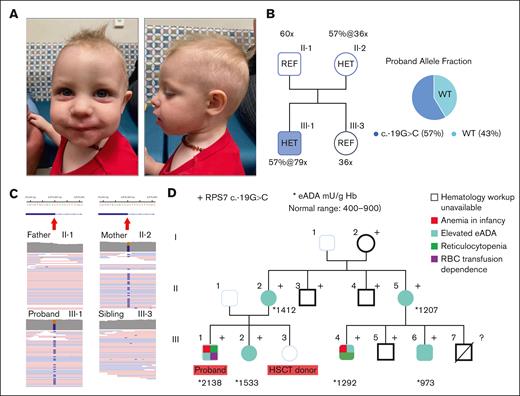
We evaluated a 16-month-old male proband who was clinically diagnosed with DBAS (Figure 1A). Genetic testing, including an inherited bone marrow failure syndrome (iBMFS) panel and WES on peripheral blood samples, was negative. The proband’s clinical findings included being small for gestational age with a weight below the third percentile at 2 weeks of age and profound normocytic anemia at diagnosis (at age 2 months, initial hemoglobin [Hb] = 4.4 g/dL, normal range 10-18 g/dL; mean corpuscular volume, 106.7 fL, normal range for age 85-123 fL; before any transfusion, Figure 1D), accompanied by nearly absent peripheral reticulocyte counts that required regular pRBC transfusions every 3 weeks. Erythrocyte adenosine deaminase (eADA) activity was markedly elevated (2138 mU/g Hb; normal range 400-900). Bone marrow evaluation revealed 30% hypocellularity with markedly decreased and left-shifted erythropoiesis.
The DBAS8 case with proband carrying the RPS7 variant and the maternal segregation studies. (A) Case 1 (DBAS8) proband at the age of 16 months. (B) The WGS depth and variant allele fractions (AF) in heterozygous subjects revealed by Emedgene software. (C) The maternally inherited RPS7 variant viewed by raw reads in IGV software. (D) The maternal 3-generation pedigree with RPS7 genotype/phenotypes and cascade eADA testing values superimposed. WT, wild-type.
The DBAS8 case with proband carrying the RPS7 variant and the maternal segregation studies. (A) Case 1 (DBAS8) proband at the age of 16 months. (B) The WGS depth and variant allele fractions (AF) in heterozygous subjects revealed by Emedgene software. (C) The maternally inherited RPS7 variant viewed by raw reads in IGV software. (D) The maternal 3-generation pedigree with RPS7 genotype/phenotypes and cascade eADA testing values superimposed. WT, wild-type.
The family history was notable for elevated eADA activity in several maternal relatives and a maternal cousin with a history of non–transfusion-dependent anemia and reticulocytopenia that presented at the age of 2 years (Figure 1D). A corticosteroid trial in which the proband participated at 12 months of age was unsuccessful in maintaining transfusion independence and was subsequently discontinued. The proband was otherwise developmentally normal with no obvious congenital anomalies noted by clinical geneticists. Both the renal ultrasound and echocardiogram were negative.
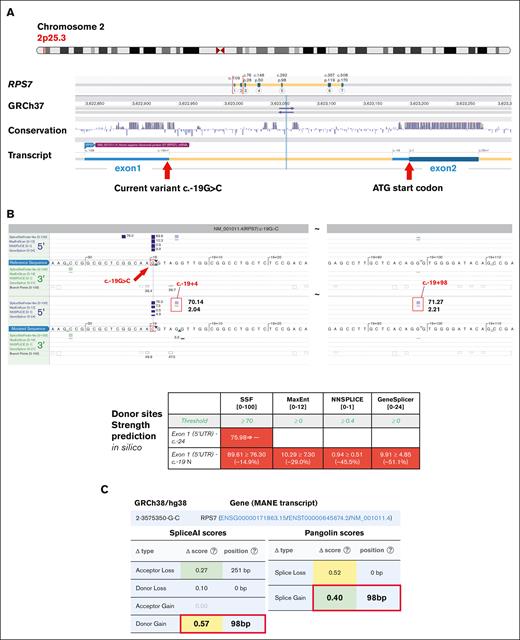
Clinical WGS was initially reported to be negative; however, in a subsequent reanalysis by the Penelope Program team, a novel maternally inherited splicing variant was identified at the last nucleotide of the first noncoding exon in RPS7 (NM_001011.4 c.-19G>C) (Figures 1B-C and 2A). This variant was located at a highly conserved nucleotide (Figure 2A) and was absent from the gnomAD population databases (v2, v3, and v4). Multiple splicing algorithms predicted that it may cause up to a 50% reduction in splicing donor strength (Figure 2B-C). The adjacent c.-19+1 and c.-19+2 canonical splice donor variants have been reported recently in patients with DBAS and are considered pathogenic.15 The c.-19+1 variant has been shown to lead to markedly reduced gene expression with 2 intron retention isoforms detected at positions +4 and +98 downstream of exon 1.16 These 2 intron retention events were precisely predicted in silico for the patient’s c.-19G>C variant (Figure 2B-C), supporting the validity of the predicted donor loss for this variant.
The genomic map of the RPS7 noncoding variant and in silico prediction on splicing impact. (A) The genomic location map of the RPS7 c.-19G>C variant in reference to the noncoding exon and the start site (ATG/c.1, Alamut software). (B) In silico algorithms unanimously predicted a donor loss and the precise position of the 2 neo-donors at +4 and +98 loci (Alamut). (C) An independent algorithm (SpliceAI) concurrently confirmed a significant donor loss (at c.-19+1) and a robust new donor gain (at c.-19+98) induced by the c.-19G>C variant (framed red). SSF, SpliceSiteFinder.
The genomic map of the RPS7 noncoding variant and in silico prediction on splicing impact. (A) The genomic location map of the RPS7 c.-19G>C variant in reference to the noncoding exon and the start site (ATG/c.1, Alamut software). (B) In silico algorithms unanimously predicted a donor loss and the precise position of the 2 neo-donors at +4 and +98 loci (Alamut). (C) An independent algorithm (SpliceAI) concurrently confirmed a significant donor loss (at c.-19+1) and a robust new donor gain (at c.-19+98) induced by the c.-19G>C variant (framed red). SSF, SpliceSiteFinder.
Cascade evaluation in the proband’s family identified multiple additional maternal relatives who carried the c.-19G>C variant, some of whom exhibited varying degrees of anemia (see pedigree in Figure 1D; supplemental Table 1), consistent with the phenotype variation described by the recent DBAS international consensus statement.6 Some family members declined a comprehensive hematology workup; however, among those who were evaluated, elevated eADA cosegregated well with the RPS7 variant. Based on these findings, specifically, the rarity of the variant absent from gnomAD, predictions of altered splicing in silico, and cosegregation with a biomarker phenotype, the variant would be considered likely pathogenic according to the American College of Medical Genetics and Genomics/American Association for Molecular Pathology (ACMG/AMP) criteria,17 albeit with reduced penetrance for red cell hypoplasia and other DBAS clinical features.
In investigating why the variant was not reported in the clinical WES and iBMFS panels, we noted that the targeted sequencing lacked capture probe coverage in noncoding exons (1st exon, 5′ UTR of RPS7). This limitation was mentioned in the testing report, and corresponding capture probes are unlikely to be included in other commercial WES or targeted NGS panels because of the current challenges in interpreting variants in such regions and the lack of corresponding literature.
Given the proband’s complicated clinical course that was characterized by transfusion dependency and a failed steroid trial, he underwent HSCT with a variant-negative, unaffected 12/12 ultra-high-resolution HLA-matched sibling donor (Figure 1D, III-3). The transplant was successful; the patient has remained transfusion-independent for >14 months after HSCT, and chimerism analysis showed 100% donor contribution at day +100. The patient is currently asymptomatic with normal hematologic indices.
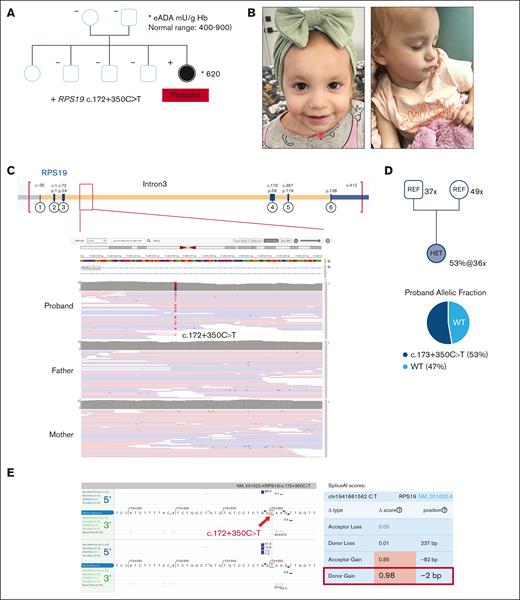
The genomic map of the RPS19 noncoding variant and in silico prediction on splicing impact. (A) The pedigree for the DBAS1/RPS19 family with the eADA level superimposed for the solely affected proband. (B) The DBAS1 proband at the age of 1 year. (C) The genomic position depicting the deep intronic nature of the RPS19 c.172+350C>T variant by IGV. (D) The WGS depth and variant AF in heterozygous subjects. (E) In silico splicing prediction algorithms (Alamut and SpliceAI) independently predicted a profound donor gain (red framed) as a consequence of the deep intronic variant. WT, wild-type.
The genomic map of the RPS19 noncoding variant and in silico prediction on splicing impact. (A) The pedigree for the DBAS1/RPS19 family with the eADA level superimposed for the solely affected proband. (B) The DBAS1 proband at the age of 1 year. (C) The genomic position depicting the deep intronic nature of the RPS19 c.172+350C>T variant by IGV. (D) The WGS depth and variant AF in heterozygous subjects. (E) In silico splicing prediction algorithms (Alamut and SpliceAI) independently predicted a profound donor gain (red framed) as a consequence of the deep intronic variant. WT, wild-type.
Case 2, WGS trio, RPS19/DBAS1
The proband was a 20-month-old female (Figure 3A-B) who was clinically diagnosed with severe life-threatening anemia at 6 weeks of age (initial Hb = 2.6 g/dL, normal range 10-18 g/dL; mean corpuscular volume 110.3 fL, normal range for age 85-123 fL; before any transfusion). Prenatal history was unremarkable except for being small for gestational age at birth with weight below the third percentile until 7 months of age. Bone marrow aspirate and biopsy revealed pure red cell aplasia with markedly low or absent reticulocytes. In addition, she was found to have congenital heart disease with a fenestrated atrial septal defect, muscular ventricular septal defect, and patent foramen ovale. Clinically, she was transfusion-dependent and required pRBC infusions every 3 weeks to maintain a nadir hemoglobin level >9.0 g/dL.
Clinical genetic testing was negative, including an iBMFS NGS gene panel on peripheral blood, chromosome breakage assay, karyotype analysis of the bone marrow, and a bone marrow oncology microarray. The eADA level was within normal limits (620 mU/g Hb; normal range 400-900; Figure 3A), consistent with eADA’s known sensitivity issue for DBAS.18
Although the clinical WGS was initially reported to be negative, the Penelope Program obtained the FASTQ files for reanalysis. With parental relationships confirmed by the internal Mendelian error matrix in Emedgene and by Peddy,19 WGS reanalysis identified a de novo intronic variant in RPS19 (NM_001022.4 c.172+350C>T) (Figure 3A-C), which was absent from the gnomAD population databases (v2, v3, and v4). The variant is located 350 bp into intron 3 of RPS19 and creates a novel consensus splice donor sequence (GTAAGT; Figure 3C,E, left panel). In silico algorithms predicted that this variant will cause a donor gain (Figure 3E, right panel) with a robust prediction score. Such a deep intronic variant is expected to evade detection by most commercially available NGS panels and WES.
Based on features of this variant (being de novo with a phenotype match (Figure 3A), absent from population databases, and demonstrating strong in silico prediction), we consider it likely pathogenic according to the ACMG/AMP criteria.17 The patient had a 12/12 HLA-matched sibling donor who does not harbor the causative variant and was preparing to undergo HSCT.
Functional affirmation by transcript analyses for case 1 and case 2
RNA-seq can be employed to determine the quantitative and qualitative features of RNA transcripts in a tissue of interest at a given time. In the context of DNA variant classification, RNA-seq can provide strong evidence of the functional impact of genetic variants, thereby enabling many variants of uncertain significance to be classified as pathogenic.17 Both RPS7 and RPS19 are moderately expressed in peripheral blood, providing an in vivo opportunity to confirm the functional impact of these DNA variants at the transcription level.
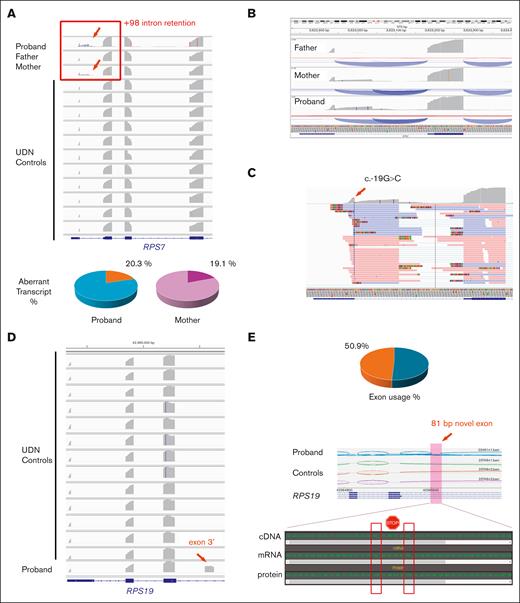
In case 1 (DBAS8/RPS7), family trio RNA-seq from peripheral blood of the proband and both parents showed robust intron retention immediately after the first exon (noncoding) of RPS7, terminating at c.-19+98. Intron retention was present only in the proband and mother, each exhibiting a comparable fraction of ∼20% of all transcripts, and was absent in the father and 11 unaffected controls (Figure 4A-B). In both the proband and mother, there was a 1-to-1 correlation between RNA reads containing the c.-19G>C mutation and those showing intron retention (Figure 4C). Specifically, 26 of 27 unique transcript reads with the c.-19G>C variant extended into the intron, terminating at c.-19+98, whereas only 1 read containing the variant retained normal splicing. This indicates a near-perfect allelic segregation between our variant and the +98 intron retention.
Functional RNA-seq study demonstrated significant transcriptional stability deficiencies for both noncoding variants. (A) For case 2 (DBAS8/RPS7), robust intron retention extending to RPS7 c.-19+98 (red arrow and frame) was revealed in the proband and mother only when compared with father and controls with the aberrant transcript usage rate shown below. (B) Zoomed-in view of the +98 intron retention demonstrating the new transcript usage rate. (C) RNA functional evidence showing that the c.-19G>C variant is confined to those transcripts with retention. (D) For the DBAS1/RPS19 case, a novel exon 3′ (red arrow) immediately upstream of the RPS19 c.172+350C>T variant was identified uniquely in the proband when compared with controls. (E) New exon 3′ usage rate and sequence analysis depicting unique junctional splicing and several stop-gain codons within the 81-bp novel exon 3′. UDN, undiagnosed diseases network.
Functional RNA-seq study demonstrated significant transcriptional stability deficiencies for both noncoding variants. (A) For case 2 (DBAS8/RPS7), robust intron retention extending to RPS7 c.-19+98 (red arrow and frame) was revealed in the proband and mother only when compared with father and controls with the aberrant transcript usage rate shown below. (B) Zoomed-in view of the +98 intron retention demonstrating the new transcript usage rate. (C) RNA functional evidence showing that the c.-19G>C variant is confined to those transcripts with retention. (D) For the DBAS1/RPS19 case, a novel exon 3′ (red arrow) immediately upstream of the RPS19 c.172+350C>T variant was identified uniquely in the proband when compared with controls. (E) New exon 3′ usage rate and sequence analysis depicting unique junctional splicing and several stop-gain codons within the 81-bp novel exon 3′. UDN, undiagnosed diseases network.
These findings suggest that the skewed allelic balance is caused by allele-specific decay of mutant transcripts in blood leukocytes. Together with the cosegregation with eADA levels (Figure 1D), these functional data support the disease association of this novel noncoding variant. Although the variant is not a canonical splice junction variant (±1-2 bp), it is located at the last nucleotide before the splicing junction, thereby impacting the same splice donor site affected by the c.-19+1 and c.-19+2 canonical splicing variants.16
In case 2 (DBAS1/RPS19), proband-only RNA-seq identified an 81-bp novel exon downstream of exon 3 from the MANE (matched annotation from National Center for Biotechnology Information and European Molecular Biology Laboratory-European Bioinformatics Institute) transcript of RPS19 (NM_001022.4). This novel exon was found immediately before the c.172+350C>T deep intronic variant and was absent from all 13 unaffected controls (Figure 4D). This aberrant splicing event was the predicted consequence of the new donor site located 2 bp upstream of the de novo c.172+350C>T variant (Figure 3E). Approximately half of the proband’s transcripts included this novel exon 3′, visually indicated by the extensive junctional reads (Figure 4E) that were present only in the proband and absent in unaffected controls.
Importantly, the c.172+350C>T variant created a GT dinucleotide donor site, which was subsequently spliced out. Therefore, no reads containing both the novel exon and the c.172+350C>T variant were available for assessing the allele-specific association. Of note, DNA/RNA sequence analyses revealed 2 stop codons in tandem within this 81-bp new exon 3′ (Figure 4E), indicating a stop-gain mechanism typically associated with nonsense-mediated decay, consistent with the reported molecular etiology of DBAS.3,7
Based on the functional evidence from RNA-seq and confirmed loss-of-function mechanism for DBAS, both RPS7 and RPS19 variants are now reclassified as pathogenic.
Discussion
We report 2 novel, noncoding variants in 2 unrelated infants who had clinical features of DBAS that were identified by in-depth WGS reanalysis following previous negative clinical testing, including WES (case 1 only), targeted iBMFS gene panels, single nucleotide polymorphism microarray (case 2 only), and chromosomal analyses. Clinical WGS in both cases was initially reported to be negative by clinical laboratories.
Both noncoding variants were predicted to cause aberrant splicing and to lead to intron retention in RPS7 and to a novel pseudo-exon in RPS19, respectively. The ability of WGS analysis to identify such variants underscores its clinical utility in detecting cryptic disease-causing variants in rare diseases. The predicted pseudo-exon inclusion, which is supported by existing literature,16 represents an underappreciated pathogenetic mechanism of DBAS.
When we requested the clinical laboratories to include the RPS7 and RPS19 variants in their reports, both variants were classified as variants of uncertain significance, likely because of their noncoding nature. This designation highlights the limited capability of clinical testing laboratories to interpret noncoding variants.
These diagnostically challenging cases underscore the importance of functional data from RNA-seq, which often contributes strong evidence for ACMG/AMP variant classification. For the DBAS8/RPS7 case, we noted that the c.-19+4 intron retention observed by Skorodumova et al through reverse transcriptase-polymerase chain reaction16 on transfected plasmid expression constructs was not present in our RNA-seq data. This discrepancy may be attributed to in vitro artifacts or the limited sensitivity of RNA-seq in our assayed tissue. Our findings also indicate that in silico prediction can help to initially identify suspicious variants that would benefit from functional confirmation, especially when the clinical presentation aligns with the candidate gene of interest.
DBAS is considered a ribosomal disease (ribosomopathy), supported by evidence that many DBAS-associated genes encode ribosomal components or regulators.8,10,16,20 Defective ribosomal protein synthesis triggers apoptosis of erythroid progenitor cells through p53 activation and stabilization,7 a mechanism known as ribosomal stress, thereby leading to erythroid failure and pure RBC aplasia. The increasing reports15,16,20 of causative noncoding variants suggest that these variants may account for some genetically unresolved cases of DBAS by impacting DBAS-associated gene transcript stability.
Our findings have significant implications for clinical laboratories and clinicians. By incorporating noncoding variants into their analytical pipelines, clinical laboratories may enhance their diagnostic sensitivity. Evidence-based updates are particularly crucial for optimizing gene panels, clinical exomes, and WES, especially when the clinical phenotype suggests a disease known to be associated with noncoding variants,6,21 which are typically off-target for the abovementioned molecular platforms.
Clinicians must also be aware of the current limitations of clinical testing and ensure that the requested testing is optimally targeted to the patient’s phenotype. In addition, some Mendelian conditions, such as DBAS, can still exhibit complex clinical genetics that could complicate result interpretation. Approximately 15% to 20% of DBAS cases demonstrate reduced penetrance, variable expressivity (eg, in hemoglobin and eADA activity), and the potential for spontaneous remission because of somatic revertant clones.3,9 These complexities necessitate careful interpretation of diagnostic testing,21 particularly when family cascade testing is based on a more severely affected proband.
It should also be noted that the 2024 DBAS consensus statement6 emphasized the following 3 concepts that are highly pertinent to our reported cases: (1) because of the variable syndromic manifestations, DBAS diagnoses do not mandate the presence of anemia (family 1, maternal I, II, III); (2) sibling donors, even if proven clinically asymptomatic, require genetic testing to rule out the mutation identified in the proband (family 1, HSCT donor/III-3); (3) the presence of a pathogenic variant in DBAS genes is now sufficient for DBAS diagnosis, regardless of the presence of clinical symptoms such as anemia, and life-long malignancy monitoring is warranted even for these asymptomatic patients with DBAS (family 1, cascade study). Collectively, these new updates underscore the importance of the genomic testing approach in DBAS clinical management.
Although our study is limited by clinical data availability and cohort size, it illustrates the need to search beyond the coding regions for DBAS/ribosomopathy. The challenge of timeliness is further highlighted by the limited documentation of noncoding variants and deep intronic variants in disease summaries, such as GeneReviews (accessed in March 2023).3 Notably, noncoding variants that affect splicing account for 75% (12/16) of the reported pathogenic RPS7 variants and 14% (28/194) of the reported pathogenic RPS19 variants (HGMD version 2023.422).
In conclusion, the diagnostic journeys of these 2 children emphasize the value of clinical assessment and persistence, even in the face of repeated negative genetic results. Collaborative support from an interdisciplinary diagnostic team, combined with in-depth WGS reanalysis on a research basis, resolved 2 difficult-to-diagnose cases and informed critical care decisions, including donor selection for HSCT and familial care management. Scrutinizing such cases also generated new insights into disease mechanisms that could benefit additional patients and families, thereby laying the groundwork for new treatments that target splicing disruption caused by noncoding variants.
Acknowledgments
The authors thank the families for their participation and willingness to share their stories to help other families with rare and undiagnosed disorders and their providers in the future. The authors express their gratitude to the Penelope Undiagnosed and Rare Disease Program for their invaluable support and collaboration throughout this project. The authors also extend thanks to the Center for Genomic Medicine, Intermountain Healthcare Center for Personalized Medicine, Utah Center for Genetic Discovery, and ARUP Laboratories for supporting the research efforts. Special thanks to the Miller Genomic Fund for their generous gift, which has enabled the authors to push the boundaries of genomic medicine in the pursuit of better understanding and treating undiagnosed and rare diseases. Genome sequence alignment and variant calling were performed by the Utah Center for Genetic Discovery Core, which is part of the Health Sciences Center Cores at the University of Utah.
This work used resources and support from the Center for High Performance Computing at the University of Utah, partially funded by the Office of The Director, National Institutes of Health Biomedical Research Support Shared Instrumentation grant 1S10OD021644-01A1.
Authorship
Contribution: T.W. drafted the initial manuscript; T.W., S.E.B., R.M., P.B.-T., J.A.M., and L.D.B. conceptualized and designed the study; T.W., R.G.L., S.E.B., T.J.N., A.C., R.M., and P.B.-T. analyzed and interpreted the genomic and RNA sequencing data; E.E.B., A.A., and J.V. collected samples and provided genetic counseling; C.M.H., E.M.F., J.A.M., and L.D.B. recruited patients and provided clinical care; and all authors reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for T.W. is Pathology and Laboratory Medicine, Henry Ford Hospital, Detroit, MI.
Correspondence: Ting Wen, Department of Pathology, Henry Ford Hospital, 2799 W Grand Blvd, Detroit, MI 48202; email: twen1@hfhs.org; and Jessica A. Meznarich, Department of Pediatrics, University of Utah, 295 Chipeta Way, Salt Lake City, UT 84108; email: jessica.meznarich@hsc.utah.edu.
References
Author notes
Other than the limited genetic information revealed in this study, the patients’ whole genome data sets are subjected to the protection of Protected Health Information and patients’ privacy, and hence are not releasable under our study consenting agreements. Therefore, genomic data sharing is not applicable in this case, which will not hinder the comprehension of the topic discussed in the article.
The full-text version of this article contains a data supplement.