Key Points
SerpinPC treatment of factor VIII–deficient mice fully restored hemostasis in a tail clip model in a dose- and time-dependent manner.
SerpinPC should not deplete protein C, and the treatment appears to preserve the antithrombotic and signaling functions of APC.
Visual Abstract
The concept of restoring hemostasis by down-tuning anticoagulant pathways holds the promise of treating all forms of hemophilia. Here, we report preclinical efficacy and safety data for SerpinPC, a covalent inhibitor of activated protein C (APC). APC is a serine protease that degrades the enzyme complex that produces thrombin, and its inhibition allows for more thrombin to be produced during the initiation stage of hemostasis. In a hemophilia A (HA) mouse tail clip model, SerpinPC treatment reduced blood loss in a dose- and time-dependent manner to levels comparable with wild-type (WT) mice. SerpinPC was able to treat active bleeds in HA mice and prevented spontaneous internal bleeding when given prophylactically. SerpinPC treatment was not associated with an increased inflammatory response and was well tolerated at high doses in WT animals. SerpinPC is currently being evaluated in individuals with severe hemophilia.
Introduction
Hemostasis is initiated when protein and cellular components of blood come into contact with the subendothelial space, which is rich in collagen and cell surfaces expressing tissue factor (TF).1 TF binds to circulating factor VIIa (fVIIa), forming the extrinsic Xase complex that rapidly converts fX to fXa. fXa interacts with fVa on platelet surfaces to form prothrombinase, the enzyme complex that converts prothrombin to thrombin. This initiation stage of hemostasis is fully functional in persons with severe hemophilia, but it is unable to generate sufficient thrombin to produce a stable clot due to the presence of circulating anticoagulant proteins.2 In the propagation stage, fXa is produced by the intrinsic Xase complex composed of fVIIIa and fIXa, which are deficient in hemophilia A (HA) and HB, respectively. It is, therefore, unsurprising that most treatments for hemophilia function by increasing fXa production. Ultimately, however, the efficacy of any hemophilia treatment is conferred by its ability to increase thrombin generation at sites of vascular damage.3
TF pathway inhibitor4 (TFPI), antithrombin5 (AT; SerpinC1), and protein C6 (PC) are 3 of the principal circulating anticoagulants, and each uses a distinct mechanism and operates at a different stage of hemostasis. All are currently targets for hemophilia treatment.7,8 TFPI acts on the initiation stage of blood coagulation by inhibiting the extrinsic Xase complex and the fXa it produces. AT inhibits fXa and thrombin directly during the propagation stage of blood coagulation. In contrast, PC is not a protease inhibitor but rather the zymogen precursor of activated protein C (APC), a serine protease that exerts its anticoagulant activity by enzymatically inactivating fVa, thereby disabling prothrombinase to shut down thrombin generation. We hypothesized that inhibition of APC would allow prothrombinase generated at the initiation stage more time to produce thrombin and thereby restore hemostasis in the context of hemophilia.9 We previously reported the design and characterization of a variant of the serpin α1-antitrypsin (α1AT; SerpinA1) with specificity for APC over other coagulation proteases (KRK-α1AT).10 KRK-α1AT was found to normalize hemostasis in laser injury and tail clip models in HB mice, albeit only at high doses, likely due to the effect of the lack of glycosylation on pharmacokinetics (PK). Here, we present preclinical efficacy and safety data of SerpinPC, a fully glycosylated version of KRK-α1AT, currently in clinical development.
Methods
SerpinPC production
SerpinPC and the control parent molecule human α1AT were produced in Chinese hamster ovary cells by Lonza (Cambridge, United Kingdom). SerpinPC used in the studies described in the article came from 3 separate batches considered to be identical with respect to purity and endotoxin levels. Details can be found in the supplemental Methods.
Tail clip model
Tails were transected 2 mm from the tip and immediately placed in a 50-mL Falcon tube containing 40 mL of 90% warmed (37°C) sterile saline. The tail was transferred to a fresh pot of warmed saline every 4 minutes, and bleeding was observed for a total of 20 minutes (5 tubes). Total blood loss volume was determined by lysing red blood cells in all tubes and measuring hemoglobin concentration and applying a standard curve. Vehicle-treated wild-type (WT) background strain and HA mice were used as controls every time an experiment was conducted, and the bleeding volumes for these controls were pooled to provide a more accurate representation of the variability and for calculation of statistical significance. Further details are provided in the supplemental Methods.
Spontaneous bleeding model
A total of 48 HA mice aged ∼18 weeks were used in the study. Mice were weighed and allocated to treatment groups based on body weight, to ensure an equal distribution of body weights between the groups. Dosing of vehicle, or 0.01, 0.1, or 1 mg/kg SerpinPC, with dose volume of 10 mL/kg, occurred 3 times per week by subcutaneous injection (Monday, Wednesday, and Friday) for 56 days. Mice that were found dead or were euthanized upon reaching the humane end point (unresponsive) or at the end of the study group (vehicle and 0.01 mg/kg SerpinPC on days 25 and 39, respectively; and at day 56 for 0.1 and 1 mg/kg SerpinPC) were examined for signs of internal bleeding.
Statistical analysis
A 2-tailed parametric unpaired t test was used for the calculation of statistical significance, with values <0.05 considered significant.
Other methods
Details of other experiments can be found in the supplemental Methods.
Results
Dose-dependent effect of SerpinPC on bleeding
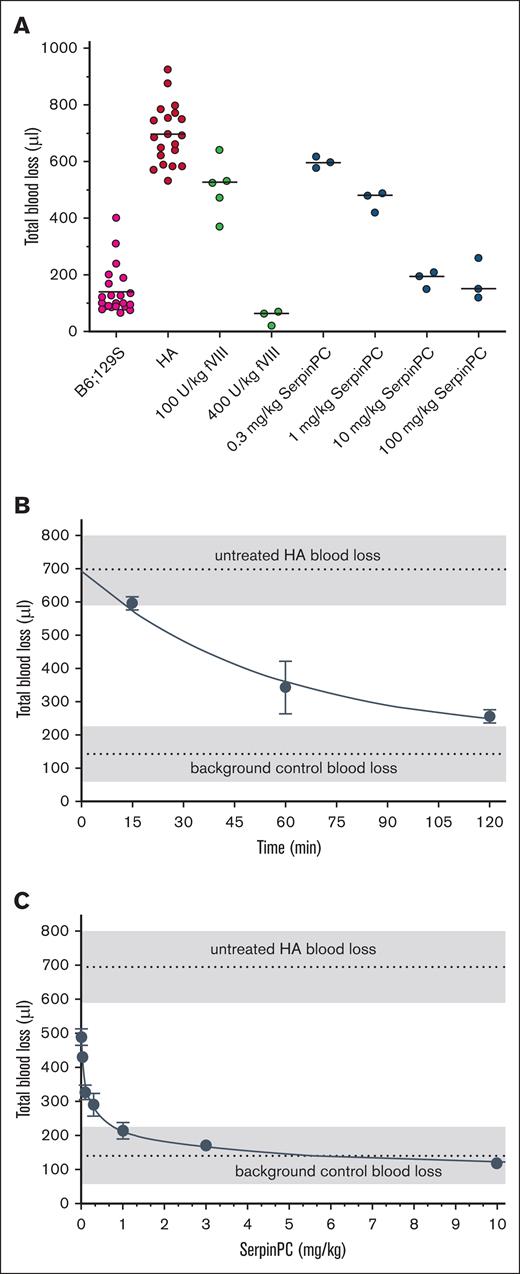
To determine the effect of dose on bleeding in HA mice, SerpinPC was administered via IV bolus at 0.3, 1, 10, and 100 mg/kg, 15 minutes before tail transection, and blood loss over a 20-minute period was measured (Figure 1A). Vehicle-treated WT background strain and HA mice were used as controls every time the tail clip experiment was conducted, and the bleeding volumes for these controls were pooled to provide a more accurate representation of the variability and for the determination of statistical significance. In total, bleeding volumes from 22 WT and 20 HA vehicle-treated mice were obtained, yielding average values of 140 ± 84 μL and 696 ± 105 μL, respectively (P < .0001). Treatment of HA mice with human fVIII decreased blood loss in this model in a dose-dependent manner, with some effect observed at 100 U/kg (508 ± 98 μL; P = .0015) compared with HA controls and an apparent overcorrection at 400 U/kg (51 ± 27 μL) compared with WT controls, although this was not significant (P = .085). SerpinPC reduced blood loss at all doses tested, with the lowest dose of 0.3 mg/kg decreasing blood loss slightly but not significantly to 597 ± 11 μL (P = .16). At 1 mg/kg, SerpinPC reduced blood loss to 463 ± 37 μL (P = .0012), similar to the level seen with 100 U/kg fVIII. The effect of SerpinPC appeared to plateau at 10 mg/kg, with blood volumes similar to WT mice (185 ± 18 μL; P = .43, compared with WT) and no evidence of overcorrection at 100 mg/kg (177 ± 43 μL; P = .53, compared with WT).
Blood loss volume in a mouse tail clip model is reduced by SerpinPC treatment in a dose- and time-dependent manner. (A) Blood loss volume over a 20-minute observation period is plotted for background strain B6;129S mice (magenta), and fVIII-deficient (HA) mice treated with vehicle (red), human fVIII at doses of 100 and 400 U/kg (green), or SerpinPC at 0.3, 1, 10, and 100 mg/kg (blue). Tails were transected 15 minutes after the injection of vehicle, fVIII, or SerpinPC. Blood loss values for vehicle-treated mice are taken from multiple experiments, and those for HA mice treated with 100 U/kg are taken from 2 separate experiments. For all other conditions, 3 mice were used per group, with median indicated by a line. (B) The effect of time of pre-exposure to SerpinPC on blood loss volume was assessed at a dose of 0.3 mg/kg. SerpinPC was injected 15, 60, and 120 minutes before tail transection, and blood loss was measured over a 20-minute period similar to that in panel A. Vehicle-treated HA and background control blood loss levels are indicated by a dashed line, with a gray area representing 1 standard deviation, calculated from panel A. Three mice were used per group, with average and standard deviation plotted. The line represents a nonlinear fit, forced through the average untreated value at t = 0. (C) To determine the lowest single dose of SerpinPC capable of reducing blood loss volume in the HA mouse, doses of 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mg/kg were given 12 hours before tail transection. The average blood loss volume of untreated HA and control mice are indicated similar to that in panel B. Dose dependency of blood loss is nonlinear, plateauing in the WT blood loss range at a dose of ∼1 mg/kg SerpinPC. Three mice were used per group with average and standard deviation plotted.
Blood loss volume in a mouse tail clip model is reduced by SerpinPC treatment in a dose- and time-dependent manner. (A) Blood loss volume over a 20-minute observation period is plotted for background strain B6;129S mice (magenta), and fVIII-deficient (HA) mice treated with vehicle (red), human fVIII at doses of 100 and 400 U/kg (green), or SerpinPC at 0.3, 1, 10, and 100 mg/kg (blue). Tails were transected 15 minutes after the injection of vehicle, fVIII, or SerpinPC. Blood loss values for vehicle-treated mice are taken from multiple experiments, and those for HA mice treated with 100 U/kg are taken from 2 separate experiments. For all other conditions, 3 mice were used per group, with median indicated by a line. (B) The effect of time of pre-exposure to SerpinPC on blood loss volume was assessed at a dose of 0.3 mg/kg. SerpinPC was injected 15, 60, and 120 minutes before tail transection, and blood loss was measured over a 20-minute period similar to that in panel A. Vehicle-treated HA and background control blood loss levels are indicated by a dashed line, with a gray area representing 1 standard deviation, calculated from panel A. Three mice were used per group, with average and standard deviation plotted. The line represents a nonlinear fit, forced through the average untreated value at t = 0. (C) To determine the lowest single dose of SerpinPC capable of reducing blood loss volume in the HA mouse, doses of 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mg/kg were given 12 hours before tail transection. The average blood loss volume of untreated HA and control mice are indicated similar to that in panel B. Dose dependency of blood loss is nonlinear, plateauing in the WT blood loss range at a dose of ∼1 mg/kg SerpinPC. Three mice were used per group with average and standard deviation plotted.
Time-dependent effect of SerpinPC on bleeding
To assess the effect of pretreatment time, the dose of 0.3 mg/kg was delivered 60 and 120 minutes before the tail clip challenge. The resulting blood loss volumes were significantly lower than those of vehicle-treated HA mice, at 343 ± 79 μL (P = .007) and 255 ± 20 μL (P < .0001), respectively. When total blood loss volume was plotted against the time of pretreatment at a dose of 0.3 mg/kg, there was a clear time dependence that appeared to be nonlinear, with a plateau approaching the WT blood loss range (Figure 1B). Based on the PK profile of SerpinPC in mice (supplemental Table 1; supplemental Figure 1), plasma levels at 15, 60, and 120 minutes are expected to be ∼80%, 50%, and 25% of maximum concentration (Cmax), respectively. Efficacy, as measured by the reduction in blood loss volume, therefore, does not correlate with SerpinPC level at the time of the challenge but rather with the time of exposure to SerpinPC before the tail clip challenge.
Lowest efficacious dose
With this time dependence in mind, we set out to determine the lowest single dose of SerpinPC capable of reducing bleeding in the HA mouse tail clip model. Doses increasing in half-logs from 0.01 to 10 mg/kg were administered via tail vein injection 12 hours before tail transection. This interval was chosen to maximize the pretreatment time for SerpinPC, considering the 10-hour terminal half-life of SerpinPC in mice. We observed a statistically significant reduction in blood loss at the lowest dose tested (0.01 mg/kg; 488 ± 15 μL; P = .0031, compared with HA controls), and the dose of 1 mg/kg achieved blood loss reduction within the WT range (214 ± 24 μL; P = .15, compared with WT controls). When blood loss volume was plotted against dose (Figure 1C), a nonlinear dose dependency was observed, with a plateau of blood loss at WT mouse levels.
To estimate SerpinPC levels at the time of the challenge in this experiment, C57BL/6 mice were treated with the same doses, and blood samples were collected after 12 hours (supplemental Table 2). The plasma concentration of SerpinPC for the 0.01 mg/kg dose was 3.2 ng/mL (64 pM). Assuming pseudo–first-order kinetics and a rate constant of inhibition of mouse APC of 6000 M–1 s–1 (supplemental Table 3), the half-time (t1/2) of inhibition is ∼3 weeks. Yet, the dose of 0.01 mg/kg resulted in a statistically significant 30% decrease in blood loss volume over a time course of 20 minutes after tail transection. Efficacy in this model is, therefore, unlikely to be a function of the plasma concentration of SerpinPC at the time of the challenge.
Effect of SerpinPC on spontaneous bleeding
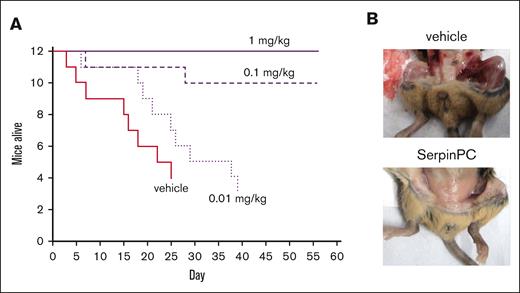
HA mice are known to be susceptible to spontaneous bleeding and subsequently have much shorter life spans than WT mice of the same strain.11 Early death is caused by internal bleeding, although the sites of bleeding vary. The ability of SerpinPC to prevent spontaneous internal bleeding as a prophylactic agent was tested by monitoring the well-being of older HA mice over several weeks. Due to the stochastic nature of spontaneous bleeding, 12 age-matched mice (aged 16-21 weeks) were used per group. Mice were treated with vehicle (phosphate-buffered saline [PBS]), 0.01 mg/kg, 0.1 mg/kg, or 1 mg/kg SerpinPC, administered by subcutaneous injection 3 times per week for a total of 56 days. Groups were not intentionally sex matched and ended up being 6 females and 6 males in the vehicle and 0.1 mg/kg groups, 8 females and 4 males in the 0.01 mg/kg group, and 1 female and 11 males in the 1 mg/kg group. A previous study found that male HA mice had significantly shorter median life expectancy than female mice of the same strain.11 HA mice treated with vehicle died rapidly, with half found dead or moribund by day 18. The group was euthanized on day 25, having reached the humane end point. The 0.01 mg/kg SerpinPC treatment group reached the humane end point on day 39. In contrast, only 2 of 12 mice died in the 0.1 mg/kg treatment group, and all mice receiving 1 mg/kg survived to the end of the 56-day study (Figure 2A). There was no apparent sex difference in survival for any group. Upon termination of the groups, vehicle-treated survivors were found to have widespread internal bleeding, whereas survivors in the 0.1 and 1 mg/kg SerpinPC groups did not (Figure 2B). These data demonstrate the ability of SerpinPC to normalize hemostasis in a spontaneous HA bleeding model as a subcutaneous prophylactic agent.
Effect of SerpinPC on spontaneous bleeding in HA mice. (A) A Kaplan-Meier survival curve is plotted for HA mice treated with either vehicle or 0.01, 0.1, or 1 mg/kg SerpinPC by subcutaneous injection 3 times per week for a maximum of 8 weeks (56 days). The vehicle and 0.01 mg/kg groups were euthanized early after reaching the humane end point. Only 2 mice from the 0.1 mg/kg group died, and none died in the 1 mg/kg group. (B) Surviving mice at the end of the treatment periods were examined to detect overt internal bleeding. Vehicle-treated mice experienced bleeding into many tissues, whereas those treated with 0.1 or 1 mg/kg SerpinPC did not exhibit any signs of internal bleeding.
Effect of SerpinPC on spontaneous bleeding in HA mice. (A) A Kaplan-Meier survival curve is plotted for HA mice treated with either vehicle or 0.01, 0.1, or 1 mg/kg SerpinPC by subcutaneous injection 3 times per week for a maximum of 8 weeks (56 days). The vehicle and 0.01 mg/kg groups were euthanized early after reaching the humane end point. Only 2 mice from the 0.1 mg/kg group died, and none died in the 1 mg/kg group. (B) Surviving mice at the end of the treatment periods were examined to detect overt internal bleeding. Vehicle-treated mice experienced bleeding into many tissues, whereas those treated with 0.1 or 1 mg/kg SerpinPC did not exhibit any signs of internal bleeding.
To estimate SerpinPC exposure in the 56-day prophylaxis experiment, a repeat-dose PK study was conducted in WT C57BL/6 mice, with subcutaneous doses given at the same interval. Trough levels were taken before each dose, and a 24-hour level was taken after the last (fourth) dose. Trough levels were ∼500, 40, and 3 ng/mL for doses of 1, 0.1, and 0.01 mg/kg, respectively. Calculated t1/2 of inhibition of mouse APC by SerpinPC at these exposures is 3 hours, 40 hours, and 3 weeks, respectively. The 24-hour exposure levels were ∼3400, 175, and 10 ng/mL, giving t1/2 values of 28 minutes, 9 hours, and 7 days, respectively. Although peak values are expected to be higher than the 24-hour levels, because time to Cmax (Tmax) is ∼6 hours via the subcutaneous route, it is clear why the 0.01 mg/kg dose failed to show efficacy in this model with a t1/2 of inhibition of 7 days.
Effect of SerpinPC on established bleeds
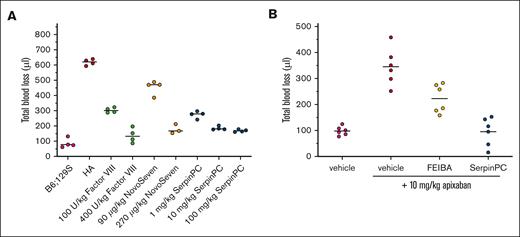
To determine whether SerpinPC might also be effective in treating an established bleeding event, we modified the tail clip method so that SerpinPC or a control hemostatic agent could be given via jugular vein cannula 1 minute after the challenge (Figure 3A). In this model, the measured blood loss volumes from vehicle-treated WT and HA mice were similar to those seen in the pretreatment model (85 ± 31 μL and 618 ± 20 μL, respectively; n = 4). Human fVIII at 100 and 400 U/kg and the bypassing agent NovoSeven at doses of 90 and 270 μg/kg were used as positive controls. Human fVIII reduced blood loss volume to 303 ± 16 μL and 136 ± 48 μL for the low and high doses, respectively (n = 4; P < .0001, for both). Treatment with NovoSeven at 90 and 270 μg/kg reduced blood loss volume to 453 ± 46 μL (n = 4; P = .0025) and 177 ± 31 μL (n = 3; P < .0001), respectively. SerpinPC at 1, 10, and 100 mg/kg reduced blood loss to 273 ± 23 μL, 185 ± 12 μL, and 168 ± 8 μL, respectively (n = 4; P < .0001, for all doses).
Use of SerpinPC to treat an ongoing bleed in HA mice and in WT mice treated with apixaban. (A) Mice were cannulated via the jugular vein to allow for the administration of agent 1 minute after tail transection. Vehicle-treated background control (B6;129S) mice (magenta) and HA mice (red) gave blood loss values similar to those observed in the pretreatment model. Human fVIII at 100 and 400 U/kg (green) and NovoSeven at 90 and 270 μg/kg (orange) reduced blood loss volume in a dose-dependent manner. SerpinPC reduced blood loss volume at doses of 1, 10, and 100 mg/kg (blue), with maximal effect observed for the 2 higher doses. Four mice were used per group, with median indicated. (B) To assess the ability of SerpinPC to reduce excessive bleeding caused by overanticoagulation, WT B6;129S mice were treated with either vehicle (magenta) or 10 mg/kg apixaban via jugular vein cannulas 5 minutes before tail transection. One minute after tail transection, either vehicle (red), 100 IU/kg Factor Eight Inhibitor Bypassing Agent (FEIBA; yellow), or 10 mg/kg SerpinPC (blue) was administered via the jugular vein, and bleeding volume was assessed over 20 minutes (n = 6 per group with averages indicated).
Use of SerpinPC to treat an ongoing bleed in HA mice and in WT mice treated with apixaban. (A) Mice were cannulated via the jugular vein to allow for the administration of agent 1 minute after tail transection. Vehicle-treated background control (B6;129S) mice (magenta) and HA mice (red) gave blood loss values similar to those observed in the pretreatment model. Human fVIII at 100 and 400 U/kg (green) and NovoSeven at 90 and 270 μg/kg (orange) reduced blood loss volume in a dose-dependent manner. SerpinPC reduced blood loss volume at doses of 1, 10, and 100 mg/kg (blue), with maximal effect observed for the 2 higher doses. Four mice were used per group, with median indicated. (B) To assess the ability of SerpinPC to reduce excessive bleeding caused by overanticoagulation, WT B6;129S mice were treated with either vehicle (magenta) or 10 mg/kg apixaban via jugular vein cannulas 5 minutes before tail transection. One minute after tail transection, either vehicle (red), 100 IU/kg Factor Eight Inhibitor Bypassing Agent (FEIBA; yellow), or 10 mg/kg SerpinPC (blue) was administered via the jugular vein, and bleeding volume was assessed over 20 minutes (n = 6 per group with averages indicated).
SerpinPC is thus able to treat an ongoing bleed in the context of this model, with similar efficacy at 1 mg/kg as 100 U/kg human fVIII or 270 μg/kg NovoSeven. The effect size of SerpinPC in this model can be rationalized by the rate of inhibition of APC at each dose. The Cmax at 1 mg/kg SerpinPC by the IV route is 24 μg/mL, which corresponds to a t1/2 of inhibition of 4 minutes, well within the 20-minute duration of the experiment. The Cmax at 10 mg/kg is 267 μg/mL, corresponding to a t1/2 of inhibition of 22 seconds, and the t1/2 of inhibition at 100 mg/kg is expected to be 2.2 seconds, assuming linearity in Cmax.
Effect of SerpinPC on bleeding associated with overanticoagulation
The ability of SerpinPC to treat an established bleed in HA mice suggests that it might also act as a general hemostatic agent when bleeding is caused by other factors, such as overanticoagulation. Therefore, we tested the ability of SerpinPC to reverse the effect of the fXa inhibitor apixaban (Eliquis) in the treatment tail clip model using WT background strain B6;129S mice. Mice were cannulated, pretreated with vehicle or apixaban 5 minutes before tail transection, and given a rescue agent 1 minute after tail transection (Figure 3B). Apixaban dosed at 10 mg/kg increased the blood loss volume from 98 ± 17 μL to 346 ± 71 μL (n = 6; P = .0002). This effect was only partially reversed with 100 IU/kg of the positive control Factor Eight Inhibitor Bypassing Agent to 223 ± 55 μL (P = .008). In contrast, full reversal of the effect of apixaban on blood loss was achieved with 10 mg/kg SerpinPC (95 ± 54 μL; P < .0001). Neither Factor Eight Inhibitor Bypassing Agent nor SerpinPC administration had a significant effect on blood loss volume in WT mice that were not pretreated with apixaban in this model (data not shown).
SerpinPC in an LPS challenge model
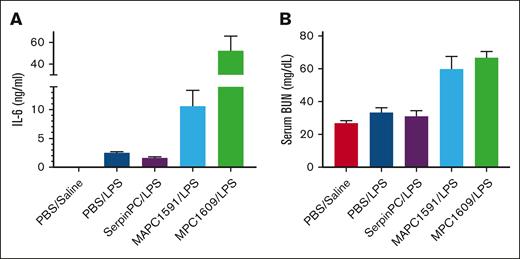
In addition to its anticoagulant activity, the PC system is known to have anti-inflammatory and cytoprotective functions, dependent on the cleavage of protease-activated receptor-1 (PAR-1) on endothelial cells.12 Therefore, it is possible that SerpinPC might exert a proinflammatory effect, especially in the context of infection. To test this, we used a lipopolysaccharide (LPS) challenge model with control antibodies against APC and PC.13 Antibody MPC1609 binds to mouse APC and PC via the N-terminal Gla domain and prevents binding to the endothelial cell PC receptor (EPCR); MAPC1591 only binds to APC and inhibits cleavage of fVa. When these antibodies were given at 10 mg/kg to WT mice in conjunction with a sublethal dose of LPS, only MPC1609 increased interleukin-6 (IL-6) and serum blood urea nitrogen (BUN) levels and conferred lethality.13 We conducted a similar experiment testing the effect of SerpinPC and the 2 control antibodies at 10 mg/kg in a sublethal LPS challenge of WT C57BL/6 mice. Mice dosed with vehicle, SerpinPC, or MAPC1591 became sluggish, with gooey eyes and cold tails after the challenge with LPS but recovered by 24 hours and survived for the full 66-hour study duration. However, all mice treated with MPC1609 were found to be unresponsive, with labored breathing 13 hours after the LPS challenge, and were euthanized after blood sample collection. Three mice from each of the other groups were euthanized at the 13-hour time point for assessment of IL-6 and serum BUN levels (Figure 4). As reported previously,13 IL-6 levels were elevated in the MPC1609-treated mice relative to the LPS control (52.3 vs 2.5 ng/mL; P = .014). LPS-treated mice given SerpinPC had slightly reduced IL-6 levels relative to vehicle (1.6 vs 2.5 ng/mL; P = .032), but levels in MAPC1591-treated mice were elevated relative to vehicle (10.6 ng/mL; P = .043), although still significantly lower than in MPC1609-treated mice (P = .024). Serum BUN levels were doubled when LPS was administered with MPC1609 relative to vehicle (66.8 vs 33.2 mg/dL; P = .0002) and again elevated with MAPC1591 (59.9 mg/dL; P = .031). In contrast, serum BUN levels were unaltered with SerpinPC administration relative to vehicle control (31.0 mg/dL; P = .65). These data suggest that SerpinPC does not interfere with the anti-inflammatory activity of the PC system, likely due to the fact that SerpinPC is mechanistically unable to deplete PC or affect its ability to bind to EPCR.9 The apparent proinflammatory effect of MAPC1591 in this model contrasts with the original study, in which no such effect was observed. This might be due to the fact that antibodies and LPS were coinjected via the IV route in the original method, whereas we injected the antibodies via the IV route 5 minutes before LPS was injected via the intraperitoneal route.
The effect of SerpinPC and anti-PC/APC antibodies in a sublethal LPS model. Serum IL-6 (A) and BUN levels (B) 13 hours after WT mice were given a sublethal dose of LPS and either vehicle (PBS), 10 mg/kg SerpinPC, or 10 mg/kg control antibodies MAPC1591 or MPC1609. MPC1609 conferred lethality in this model and increased serum IL-6 and BUN levels relative to PBS-treated mice. MAPC1591 did not cause lethality but did significantly increase both markers relative to the vehicle control (IL-6, P = .0431; BUN, P = .0309). In contrast, SerpinPC reduced IL-6 relative to vehicle (P = .0309), and had no effect on serum BUN levels relative to PBS controls. Controls from mice treated with PBS and saline, instead of LPS, are included.
The effect of SerpinPC and anti-PC/APC antibodies in a sublethal LPS model. Serum IL-6 (A) and BUN levels (B) 13 hours after WT mice were given a sublethal dose of LPS and either vehicle (PBS), 10 mg/kg SerpinPC, or 10 mg/kg control antibodies MAPC1591 or MPC1609. MPC1609 conferred lethality in this model and increased serum IL-6 and BUN levels relative to PBS-treated mice. MAPC1591 did not cause lethality but did significantly increase both markers relative to the vehicle control (IL-6, P = .0431; BUN, P = .0309). In contrast, SerpinPC reduced IL-6 relative to vehicle (P = .0309), and had no effect on serum BUN levels relative to PBS controls. Controls from mice treated with PBS and saline, instead of LPS, are included.
Tolerability of SerpinPC in WT animals
The general tolerability of SerpinPC was assessed in WT C57BL/6 mice by delivering daily bolus tail vein injections of 10 and 100 mg/kg for 7 days, with vehicle and 100 mg/kg of the parent serpin (recombinant human α1AT) as controls. Clinical observations, such as body weight and behavior, were made during the 7-day treatment period; additionally, the lungs, livers, and kidneys were assessed at the end of the study for signs of thrombosis. Blood samples were taken for measurement of exposure during the experiment, and terminal samples were taken to determine whether there were any changes in serum markers of inflammation associated with high doses of SerpinPC in WT mice. Three mice were used per group, and 3 naïve mice were used to determine baseline values. All treatments were well tolerated, with no overt difference between groups. Histological examination of the lungs, liver, and kidneys revealed no abnormality or evidence of thrombosis. No increase in a panel of 40 cytokines was observed, with only 12 detectable for any treatment group and none significantly higher than the vehicle (PBS) control (supplemental Figure 2). SerpinPC exposure scaled with dose, with trough plasma levels of ∼19 and 200 μg/mL for the 10 and 100 mg/kg doses, respectively.
The tolerability and PK of SerpinPC were also assessed in nonhuman primates. Single IV doses of 1 and 10 mg/kg were each administered to 2 male cynomolgus monkeys. The doses were well tolerated with no clinical signs. D-dimer levels were taken before and after dosing for 14 days to determine whether a single IV bolus of SerpinPC resulted in excess thrombin generation. Although D-dimer levels varied considerably between animals, no postdose increase was observed for any animal (supplemental Figure 3). In contrast, D-dimer levels in cynomolgus monkeys increased fourfold to fivefold after the administration of the anti-TFPI antibody concizumab.14 The Cmax levels of 29 and 246 μg/mL scaled approximately with the SerpinPC dose, and the terminal half-life was 53 ± 5 hours. A subcutaneous dose of 1 mg/kg was subsequently administered to 2 male cynomolgus monkeys to assess PK. The Tmax of subcutaneously administered SerpinPC was ∼8 hours, with a flat exposure profile maintained for 48 hours and a Cmax value 4.5-fold lower than the corresponding IV dose. Near complete bioavailability (97%) was observed for the subcutaneous route of administration (PK data given in supplemental Table 4).
In vitro assessment of SerpinPC immunogenicity risk
To assess the potential immunogenicity risk of SerpinPC, an EpiScreen time course T-cell assay15 (Abzena) was conducted using the parent serpin, recombinant human α1AT, as a control. Peripheral blood mononuclear cells from a cohort of 50 healthy donors, representing the European and North American populations (based on HLA allotypes), were incubated with the test samples. T-cell responses were measured using proliferation (3H-thymidine uptake) and cytokine secretion (IL-2 enzyme-linked immunospot) assays. Analysis of the frequency and magnitude of the CD4+ T-cell responses was converted into an EpiScreen score and plotted against biologic drugs with known incidence of clinical immunogenicity. Neither α1AT nor SerpinPC were judged to represent an immunogenicity risk (score >10), with scores of 6 and 2, respectively. A score of 2 indicates a single positive response in 50 samples. The apparent low immunogenicity risk of SerpinPC was not unexpected because the parent molecule, human α1AT, is present at a high concentration (∼1.5 g/L) in blood16 and because immune tolerance to a protein is strongly related to its endogenous level.17,18
Discussion
The hemophilia treatment landscape is experiencing a revolution, with extended half-life factors, nonreplacement factors (eg, Hemlibra), gene therapy, and so-called rebalancing agents.19 A potential advantage of the rebalancing approaches is that they should work for both HA and HB, with or without inhibitors, and may also be effective in treating other bleeding disorders. However, unlike replacement therapies, in which there is a large range of effective levels with negligible thrombotic risk, finding the correct reduction in anticoagulant level to normalize hemostasis without incurring a substantial increase in risk of thrombosis is an inherent challenge of rebalancing approaches.20,21 This is illustrated by the anti-TFPI antibody befovacimab, which was discontinued by Bayer due to a combination of poor efficacy and 3 thrombotic episodes that could not be predicted from dose or reduction in TFPI levels.22 Similar issues have been associated with fitusiran, a small interfering RNA molecule that reduces circulating AT levels. Clinical holds have been imposed twice due to thrombotic episodes, including 1 death, and special guidance is required for the treatment of any breakthrough bleed.23 Differing genetic backgrounds and environmental factors, such as age and lifestyle, may contribute to the difficulty in finding the right balance for these 2 approaches, in which a reduction in bleeding requires near-complete depletion of an anticoagulant from the circulation.
In contrast, SerpinPC inhibits the anticoagulant protease APC and is mechanistically unable to affect the circulating levels of its zymogen precursor, PC. This is because the serpin mechanism of inhibition absolutely requires proteolytic activity, which zymogens, such as PC, lack.24 The concentration of PC in the blood is 70 nM, vastly exceeding the concentration of circulating APC of 40 pM (1750 times), and these levels are conserved between humans and mice.25,26 The unusual exposure/time/efficacy profile of SerpinPC in the mouse tail clip model suggests that the observed reduction in bleeding is due to the lowering of levels of preexisting APC and not due to the inhibition of APC formed from PC after the challenge. We propose that preexisting and newly formed APC have distinct activities, with low basal levels of preexisting APC serving an antihemostatic role and APC generated in response to excessive thrombin generation (by the thrombin-thrombomodulin complex on intact endothelial cells) serving signaling and antithrombotic functions. The covalent, irreversible nature of the serpin mechanism of protease inhibition allows for the preexisting APC to be “mopped up” by SerpinPC, even at low doses. The absolute dependence of proteolytic activity for inhibition by serpins also suggests that chronic SerpinPC treatment will not deplete PC from the blood, thus preserving the antithrombotic and signaling functions of the PC system.
The thrombotic potential of SerpinPC was assessed experimentally by administering high doses (10 and 100 mg/kg) to WT mice and nonhuman primates (1 and 10 mg/kg). In these studies, we observed no evidence of thrombosis or elevations in D-dimer. These animals had fully functional hemostatic systems and should therefore be more sensitive to overbalancing toward thrombosis than animals with hemophilia; however, it should be noted that the animals were not challenged. This contrasts with the elevations in D-dimer levels observed preclinically and clinically with the anti-TFPI antibodies, which effect near-complete depletion of TFPI.14,27-30 In addition, cytokine levels were not increased in WT animals after the administration of high doses of SerpinPC. To further assess the proinflammatory potential of SerpinPC, we challenged WT mice and found that a high dose of 10 mg/kg (1000 times the lowest effective dose of 10 μg/kg) did not exacerbate the inflammatory response to LPS, whereas MPC16909, which blocks the APC/PC interaction with EPCR, increased IL-6 and serum BUN levels and conferred lethality.
In conclusion, we have demonstrated that SerpinPC was capable of fully correcting hemostasis at low doses in hemophilia models, and high doses were not associated with overcorrection. Acute high doses of SerpinPC did not cause thrombosis or elevate D-dimer levels in WT animals, nor was the inflammatory response to LPS exacerbated. SerpinPC has a unique mode of action and preclinical profile, which potentially differentiates it from other nonfactor therapies approved or in development. SerpinPC is currently being evaluated clinically in patients with hemophilia.
Acknowledgments
All in vivo experiments were expertly conducted in a blinded manner by the Bioscience team at RxCelerate Ltd, unless otherwise noted. The authors thank Chuck Esmon for supplying antibodies MAPC1591 and MPC1609, and David Grainger and Kevin Johnson for helpful discussions.
Authorship
Contribution: J.A.H. conceived of and interpreted the experiments and wrote the manuscript; J.R. helped in the design and oversaw the execution of the mouse experiments; S.G.I.P. conducted kinetics experiments; P.H. oversaw production and characterization of SerpinPC; and T.P.B. contributed to the design and interpreted the results.
Conflict-of-interest disclosure: J.A.H. and T.P.B. founded ApcinteX Ltd, which was acquired by Centessa Pharmaceuticals in 2021 to develop SerpinPC. S.G.I.P., P.H., J.A.H., and T.P.B. are shareholders in Centessa. J.A.H. is a consultant for Centessa. T.P.B. is an employee of Centessa. J.R. declares no competing financial interests.
Correspondence: James A. Huntington, Department of Haematology, Cambridge Institute for Medical Research, University of Cambridge, The Keith Peters Building, Hills Rd, Cambridge CB2 0XY, United Kingdom; email: jah52@cam.ac.uk.
References
Author notes
Data are available on request from the corresponding author, James A. Huntington (jah52@cam.ac.uk).
The full-text version of this article contains a data supplement.