Intensified noncross-resistant sequential R-CHOP followed by (R)-ICE may improve outcome in non-GCB DLBCL.
The immunohistochemistry–based Hans algorithm may be used to stratify patients with DLBCL into prognostic subgroups.
Visual Abstract
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is considered the standard-of-care for patients with advanced-stage diffuse large B-cell lymphoma (DLBCL), despite findings that patients with nongerminal center B-cell like (non-GCB) have significantly worse outcome with this regimen. We evaluated the prognostic significance of baseline risk factors, including cell of origin (COO) classified by the Hans algorithm, within an alternative chemoimmunotherapy program. At Memorial Sloan Kettering Cancer Center (MSK), 151 patients with DLBCL received sequential R-CHOP induction and (R)-ICE (rituximab, ifosfamide, carboplatin, and etoposide) consolidation. Outcome analysis based on COO was validated with a propensity score–matched cohort treated with R-CHOP from the Mayo Clinic component of the Molecular Epidemiology Resource (MER). Among the patients with GCB (n = 69) and non-GCB (n = 69) at MSK, event-free survival (EFS) of non-GCB was superior to that of GCB (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.29-0.98). Overall survival (OS) demonstrated an association in the same direction but was not statistically significant (HR, 0.68; 95% CI, 0.33-1.42). Propensity score–matched patients from MSK (n = 108) demonstrated a small attenuation in the HRs for EFS (HR, 0.57; 95% CI, 0.27-1.18) and OS (HR, 0.76; 95% CI, 0.33-1.79) and were no longer statistically significant. In contrast, the matched MER cohort (n = 108) demonstrated an EFS association (HR, 1.17; 95% CI, 0.70-1.95) and OS association (HR, 1.13; 95% CI, 0.64-2.00) in the opposite direction, but were also not statistically significant. R-CHOP induction and (R)-ICE consolidation may overcome the negative prognostic impact of the non-GCB phenotype, per the Hans algorithm, and can be preferentially selected for this population. This trial was registered at www.ClinicalTrials.gov as #NCT00039195 and #NCT00712582.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of B-cell lymphomas. It is the most common type of non-Hodgkin lymphoma, accounting for 30% to 40% of new diagnoses.1,2 Advanced-stage DLBCL is highly variable in its clinical behavior, and treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) is considered the standard for first-line therapy. However, some patients experience disease recurrence or refractory disease soon after treatment with R-CHOP, and numerous chemoimmunotherapy regimens have been attempted to improve event-free survival (EFS) and overall survival (OS) in patients with these aggressive lymphomas.3-6 Strategies to improve outcome include risk-adapted therapies based on baseline prognostic factors that stratify patients into favorable vs less favorable groups.
DLBCL is a diagnostic category that includes morphologically similar tumors based on histology. Gene expression profiling (GEP) through microarray analysis has revealed 3 molecularly distinct subtypes: germinal center B-cell like (GCB), activated B-cell like (ABC), and unclassifiable.7,8 These 3 DLBCL subtypes involve different oncogenic events and varying consequent prognoses, independent of the International Prognostic Index (IPI) score.9,10 However, the expensive technology and limited availability in clinical laboratories make its use impractical for many patients with DLBCL needing therapy. Thus, several surrogates using immunohistochemistry (IHC) algorithms have been developed to approximate GEP. The Hans model, which uses combined immunostaining of CD10, BCL6, and MUM111 to translate the ABC subtype into a similarly behaving group referred to as non-germinal center B-cell (non-GCB) subtype, is the most widely used means of determining cell of origin (COO) in the real world. It has been validated in several studies as predictive of less favorable outcome in patients with non-GCB compared with those with GCB when treated with R-CHOP,12,13 just as with the GEP classification gold standard. The prognostic difference of COO determined by IHC has not been universally reproduceable,14-17 it remains a simple and more accessible method of classifying DLBCL into prognostically significant subgroups, showing a reasonably high concordance with GEP (86%).12
R-CHOP has been considered the standard-of-care frontline treatment for patients with advanced-stage DLBCL regardless of COO in the United States, as reflected in the National Comprehensive Cancer Network guidelines,18 despite findings that patients with non-GCB have significantly worse outcome with this regimen than those with GCB.9,12,13,19-22 Recent results of the POLARIX trial suggest that substitution of polatuzumab vedotin with vincristine may improve the outcome of patients with ABC DLBCL.23 Findings from the LNH03-2B trial by the Groupe d’Etudes des Lymphomes de l’Adulte (GELA) demonstrated improved EFS and OS among patients with non-GCB treated with R-ACVBP, an induction or consolidation immunochemotherapy program (supplemental Table 1), compared with R-CHOP, but no impact among patients with GCB.24
We previously conducted 2 highly related phase 2 trials of sequential R-CHOP followed by rituximab plus ifosfamide, carboplatin, and etoposide [(R)-ICE] demonstrating excellent long-term outcome in first-line treatment of DLBCL.25,26 However, this treatment was associated with increased toxicity compared with R-CHOP, so we undertook subsequent analysis to determine if there was a subgroup which had particular benefit to this regimen. R-CHOP followed by (R)-ICE is strikingly similar to the R-ACVBP induction followed by noncross-resistant consolidation regimen in the LNH03-2B trial, leading to the hypothesis that R-CHOP followed by (R)-ICE may have differential outcome based on COO. To examine this question, we undertook a propensity score–matched analysis comparing the patients at Memorial Sloan Kettering Cancer Center (MSK) treated with R-CHOP > (R)-ICE with patients treated with R-CHOP from the Mayo Clinic component of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence Molecular Epidemiology Resource (MER).27
Methods
Key eligibility criteria
Two risk-adapted phase 2 studies treating patients with advanced-stage large cell lymphomas were approved by the MSK Institutional Review Board. Patients were required to have 1 to 3 adverse risk factors according to the age-adjusted IPI (aaIPI).28 All patients were suitable to undergo stem-cell rescue. In the first study, Protocol 01-142,25 eligible patients had a histologic diagnosis of CD20+ DLBCL or primary mediastinal B-cell lymphoma (PMBL). In the second study, MSK Protocol 08-026,26 eligible patients were diagnosed with CD20+ DLBCL, PMBL, or follicular lymphoma grade 3B (FL3B). For both studies, patients were not excluded if the bone marrow demonstrated involvement by small cleaved-cell lymphoma, and all patients had measurable disease as assessed by positron emission tomography with (18F)fluorodeoxyglucose (FDG-PET) scans, normal baseline cardiac function, serum creatinine ≤1.5 mg/dL (or creatinine clearance >60 mL/min), absolute neutrophil count >1 x 103/μL, and platelets >5 x 104/μL. Patients had to be negative for hepatitis B surface antigen and hepatitis C. Exclusion criteria included known pregnancy or breastfeeding, human immunodeficiency virus infection, and central nervous system involvement.
Pathology review and COO assessment
The department of hematopathology at MSK confirmed histologic diagnoses of DLBCL, PMBL, and FL3B, and classified DLBCL COO subtype using the IHC-based algorithm developed by Hans et al.11 Paraffin-embedded tumor cells were stained with antibodies to CD10, BCL6, and MUM1, and cases were considered positive for an antigen if ≥30% of the tumor cells were stained with that antigen. DLBCL cases were classified into the following 2 subtypes: GCB and non-GCB. GCB subtype was defined as any one of the following: CD10+ alone; both CD10+ and BCL6+; CD10−, BCL6+, and MUM1−. Non-GCB subtype was defined as any one of the following: both CD10− and BCL6−; CD10−, BCL6+, and MUM1+; negative for all 3 antigens.
Treatment
Treatment has been previously described in 01-14225 and 08-02626 (supplemental Figure 1). In 01-142 (NCT00039195), initial therapy consisted of 4 cycles of R-CHOP-14 induction followed by FDG-PET. Patients who were FDG-PET–negative received 3 cycles of ICE consolidative chemotherapy, and those who were FDG-PET–positive received consolidative chemotherapy of 2 cycles of ICE, 1 cycle of (R)-ICE, followed by carmustine, etoposide, cytarabine, and melphalan and autologous stem-cell rescue (HDT-ASCR). In 08-026 (NCT00712582), after induction therapy with 3 cycles of R-CHOP and a fourth cycle of CHOP alone, all patients underwent FDG-PET. Patients who had negative FDG-PET results and a baseline Ki-67 proliferation index of <80% received consolidative chemotherapy with 3 cycles of ICE, whereas those who were FDG-PET–negative and baseline Ki-67 proliferation index of ≥80% received augmented (R)-ICE for 2 cycles. The cutoff point for biomarker Ki-67 was determined using the method of Mazumdar and Glassman.29 If the interim FDG-PET results were positive, patients received consolidative chemotherapy with 2 cycles of augmented (R)-ICE followed by HDT-ASCR. In these 2 studies, patients with positive interim FDG-PET results had a repeat biopsy of the FDG-positive site to verify imaging findings. Only patients with a confirmed positive biopsy went on to receive HDT-ASCR, whereas those with negative biopsy were treated the same as those with FDG-PET–negative imaging. Radiation was not used in any of the patients.
Comparison cohort
An external comparison cohort was assembled from patients enrolled at Mayo Clinic in the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence MER.27 Patients who received frontline R-CHOP in the MER were matched to the MSK cohort based on the following covariates: age, COO by the Hans algorithm (GCB vs non-GCB only), aaIPI, year of diagnosis, sex, and diagnosis to treatment interval (DTI). A 1:1 nearest neighbor matching (NNM) with a 0.2 caliper cutoff30 of MER to patients at MSK was attempted on the 138 patients with non-GCB and GCB subtypes at MSK. Only a subset of 108 patients at MSK were matched within the specified caliper distance.
Statistical analysis
Patient demographics and baseline characteristics were summarized and reported using descriptive statistics. EFS was defined as time from diagnosis until first of disease progression, relapse, initiation of unplanned lymphoma therapy because of lack of efficacy, or death from any cause. Patients alive without an event were censored at their last follow-up. OS was defined as time from disease diagnosis until death from any cause. Patients alive were censored at their last follow-up. EFS and OS rates were estimated using a Kaplan-Meier estimator. The prognostic impact of baseline risk factors on survival were assessed using univariable Cox proportional hazard models. The median follow-up was estimated using the reverse Kaplan-Meier method. Multivariate survival analyses were not performed due to limited power. A 2-sided P value of < .05 was considered statistically significant. Statistical analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC) and R (version 4.1.2) for Windows.
Results
From 26 March 2002 through 3 November 2006, 98 patients were enrolled in MSK protocol 01-142. From 1 July 2008 through 28 May 2013, 99 patients were enrolled in MSK protocol 08-026. For the purposes of this analysis, 44 patients with PMBL and 2 patients with FL3B were excluded. The patients with DLBCL from protocols 01-142 and 08-026 had similar pretreatment characteristics (supplemental Table 2) and similar outcome after a median follow-up of 8.0 years (95% confidence interval [CI], 6.9-8.9; supplemental Figures 2 and 3). This justified combining the 2 cohorts for analysis. A total of 151 patients with DLBCL were evaluable, and their baseline clinical and demographic characteristics are summarized in Table 1. COO subtypes were as follows: 69 GCB, 69 non-GCB, and 13 unclassified. Only 7 patients (4.6%) had a positive interim biopsy and received HDT-ASCR. Of those 7 patients, 3 were GCB, 2 were non-GCB, and 2 were unclassified. As a sensitivity analysis, we examined EFS and OS excluding all patients who underwent HDT-ASCR. This demonstrated very similar EFS and OS compared with the entire group, suggesting that transplantation had minimal impact on the overall outcome (supplemental Figure 4).
Follow-up and outcome
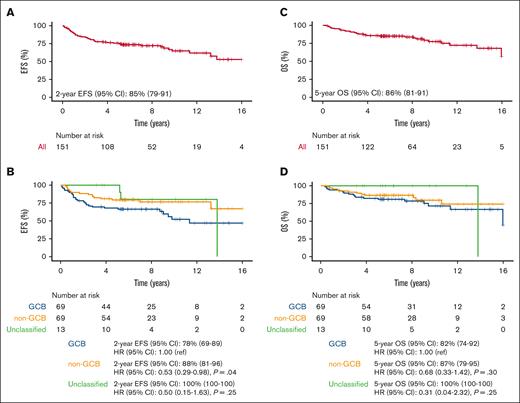
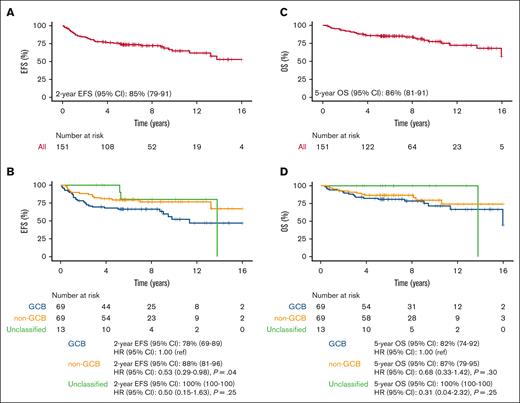
Median EFS and OS have not been reached (Figures 1A,C). The median follow-up for 151 patients is 8.0 years (95% CI, 6.9-8.9). At median follow-up of 8.0 years, 47 and 31 patients in the MSK cohort had an event and died, respectively (4 of the deaths were unrelated to lymphoma and 3 were of unknown causes). The 2-year EFS was 85% (95% CI, 79-91) and 5-year OS was 86% (95% CI, 81-92). Notably, 6 of the 47 events had DLBCL at diagnosis but at progression either had marginal zone lymphoma or follicular lymphoma. Because of the lack of adequate tissue, molecular studies were not performed and clonal relationship to the original tumor remains uncertain. At MSK, patients who remained without evidence of disease at 5 years were referred back to their primary care physician, which truncated the length of follow-up available.
Survival end points for full MSK cohort treated with R-CHOP induction > (R)-ICE consolidation (n = 151). (A) EFS; (B) EFS by COO; (C) OS; (D) OS by COO. MSK, Memorial Sloan Kettering; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; (R)-ICE, rituximab plus ifosfamide, carboplatin and etoposide; EFS, event-free survival; COO, cell of origin; OS, overall survival; GCB, germinal center B-cell like; CI, confidence interval; ref, reference.
Survival end points for full MSK cohort treated with R-CHOP induction > (R)-ICE consolidation (n = 151). (A) EFS; (B) EFS by COO; (C) OS; (D) OS by COO. MSK, Memorial Sloan Kettering; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; (R)-ICE, rituximab plus ifosfamide, carboplatin and etoposide; EFS, event-free survival; COO, cell of origin; OS, overall survival; GCB, germinal center B-cell like; CI, confidence interval; ref, reference.
Impact of baseline prognostic factors on outcome
Associations between baseline clinical characteristics and outcome were calculated by univariate analyses. Among the 151 patients with DLBCL, there was no difference in EFS or OS when stratified by proliferation index Ki-67, aaIPI score, and tumor bulk ≥10 cm (supplemental Figure 5).
Outcome by COO showed superior EFS in the non-GCB subtype (2-year EFS 88%; 95% CI, 81-96) compared with the GCB subtype (2-year EFS, 78%; 95% CI, 69-89), (hazard ratio [HR], 0.53; 95% CI, 0.29-0.98; P = .04; Figure 1B). OS stratified by COO demonstrated an association in the same direction but was not statistically significant: 5-year OS of 87% (95% CI, 79-95) in non-GCB vs 82% (95% CI, 74-92) in GCB (HR, 0.68; 95% CI, 0.33-1.41; P = .30) (Figure 1D).
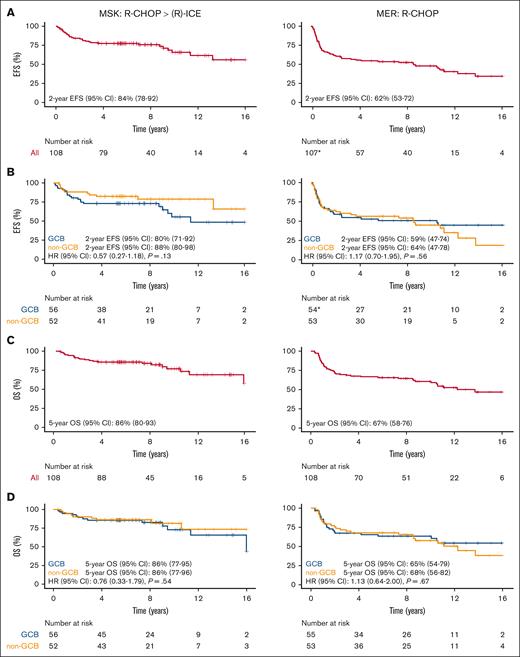
To validate this observation, we used the MER as an external comparison cohort of patients treated with standard-of-care R-CHOP. A 1:1 propensity score matching via the NNM approach with a 0.2 caliper cutoff30 was applied to the 138 patients at MSK with non-GCB and GCB subtypes; ultimately 108 patients were matched within the specified caliper cutoff. Other than aaIPI, there were no clinical, follow-up, or outcome differences between the 108 patients at MSK with available matches and the 30 unmatched patients (supplemental Table 3; supplemental Figure 6). As a consequence of the propensity score matching, there were fewer patients with high and high-intermediate risk disease in the matched MSK cohort. The clinical characteristics of the propensity score–matched cohort from MER and MSK are shown in Table 2. The MER cohort consisted of 108 patients with DLBCL diagnosed between 2002 and 2014 who underwent first-line treatment with R-CHOP. Despite propensity score matching, some minor differences existed between the populations. Compared with the MSK cohort, the MER cohort had a slightly higher proportion of low-intermediate risk aaIPI (27% in MER vs 23% in MSK) and a lower proportion of high-intermediate risk aaIPI (61% in MER vs 64% in MSK). Most prominently, the median DTI was shorter in the MER cohort (15 days in MER vs 21 days in MSK); however, quartile 3 representing the longest delay in treatment initiation was similar (29 days in MER vs 28 days in MSK). At median follow-up of 11 years, 59 and 48 patients in the MER cohort had an event and died, respectively.
Among the 108 matched patients at MSK, the 2-year EFS was 88% (95% CI, 80-98) for non-GCB and 80% (95% CI, 71-92) for GCB (HR, 0.57; 95% CI, 0.27-1.18; P = .13; Figure 2B), and the 5-year OS was 86% (95% CI, 77-96) for non-GCB and 86% (95% CI, 77-95) for GCB (HR, 0.76; 95% CI, 0.33-1.79; P = .54; Figure 2D). Though the non-GCB subtype also showed favorable EFS (and was only slightly attenuated) in the matched subset, the association did not remain statistically significant. Compared with the MSK cohort, the MER cohort demonstrated an EFS association (HR, 1.17; 95% CI, 0.70-1.95; P = .56) and OS association (HR, 1.13; 95% CI, 0.64-2.00; P = .67) in the opposite direction with respect to COO, but these associations were not statistically significant.
Comparing GCB and non-GCB patients from MSK cohort with available matches treated with R-CHOP induction > (R)-ICE consolidation (n = 108) vs matched MER cohort using NNM approach with a 0.2 caliper cutoff treated with R-CHOP (n = 108). (A) EFS; (B) EFS by COO; (C) OS; (D) OS by COO; ∗1 patient at MER had missing EFS data. COO, cell of origin; EFS, event-free survival; GCB, germinal center B-cell like; MER, Molecular Epidemiology Resource; MSK, Memorial Sloan Kettering; NNM, nearest neighbor matching; OS, overall survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone; (R)-ICE, rituximab plus ifosfamide, carboplatin and etoposide.
Comparing GCB and non-GCB patients from MSK cohort with available matches treated with R-CHOP induction > (R)-ICE consolidation (n = 108) vs matched MER cohort using NNM approach with a 0.2 caliper cutoff treated with R-CHOP (n = 108). (A) EFS; (B) EFS by COO; (C) OS; (D) OS by COO; ∗1 patient at MER had missing EFS data. COO, cell of origin; EFS, event-free survival; GCB, germinal center B-cell like; MER, Molecular Epidemiology Resource; MSK, Memorial Sloan Kettering; NNM, nearest neighbor matching; OS, overall survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone; (R)-ICE, rituximab plus ifosfamide, carboplatin and etoposide.
Discussion
In the full MSK cohort treated with R-CHOP > (R)-ICE, the EFS of the non-GCB subtype was superior to that of the GCB subtype, and OS by COO showed an association in the same direction but was not statistically significant. Favorable EFS in the non-GCB subtype was also seen in the 108 patients at MSK with available matches from the MER, though the association was not significant in this subset. Matched patients from the MER cohort treated with R-CHOP alone demonstrated an EFS association in the opposite direction (ie, >1), although HRs were weak and not statistically significant (Figure 2B). Although some minor differences in the baseline characteristics existed between the propensity score–matched patients at MSK and MER, we do not believe they account for the reversed association between EFS and COO observed in the 2 cohorts. We hypothesize that the R-CHOP induction (R)-ICE consolidation chemoimmunotherapy regimens may overcome the negative prognostic impact of the non-GCB phenotype, possibly by selectively targeting oncogenic events activated in non-GCB tumors.
The COO as determined by IHC has historically had a weak association with outcomes in the MER cohort (unpublished data not shown). However, the use of digital gene expression–based algorithms via nanostring and/or RNA sequencing in a subset of 475 patients in the MER has shown significant associations with outcomes (ABC vs GCB EFS HR = 1.41; 95% CI, 1.08-1.83). The lack of significance observed in the MER cohort analyzed in this study may be because of IHC-based approach for COO calling and/or small sample size. Analysis in the full MER cohort should be done to make conclusions regarding COO in the MER cohort which is outside the scope of the present study.
The R-CHOP > (R)-ICE combination therapy in 01-142 and 08-026 have similarities to the induction and noncross-resistant consolidation phase used in the LNH03-2B trial by GELA.31 With only 7 of the 151 patients at MSK (4.6%) receiving HDT-ASCR (2/7 were non-GCB), we do not believe transplantation accounts for the favorable EFS among patients with non-GCB tumors in the MSK cohort compared with the propensity score-matched MER cohort. The LNH03-2B trial was restricted to patients aged between 18 and 59 years with only a single IPI risk factor. The younger age is similar to the age distribution in the MSK cohort: median age of the MSK and LNH03-2B cohorts were 54 and 48 years, respectively. A dose intensity comparison of the 3 regimens is shown in supplemental Table 3. The induction phase in all 3 regimens has 4 cycles with shortened intervals, in which doxorubicin and cyclophosphamide are given at higher doses. The consolidation phase in all 3 regimens consist of sequential treatment with new chemotherapy drugs not included during induction. Therefore, we might suggest that the noncross-resistant consolidation phase–targeted oncogenic pathways specific to non-GCB tumor cells, such as the activation of the antiapoptotic nuclear factor-kappa B (NF-κB) which can inhibit chemotherapy.32-34 Secondary analyses of the GELA LNH03-2B trial showed more favorable outcome in patients with non-GCB treated with an induction regimen of R-ACVBP and consolidated with methotrexate, rituximab, ifosfamide, etoposide, and cytarabine.24 Molina et al suggest that this may be associated with a suppression of NF-κB activity by methotrexate, which sensitized the non-GCB cells to the remaining chemotherapy drugs in the consolidation phase. Because our regimens did not include methotrexate, the current data do not support the conclusion that this drug is the basis for superior outcome in non-GCB tumors.
Some reports have shown enhanced activity of chemotherapy with bortezomib in non-GCB but not in GCB DLBCL,35,36 which also support targeting the NF-κB pathway as an effective treatment approach for the genetically distinct non-GCB subtype. Although the primary analysis of the REMoDL-B trial at a median follow-up of 30 months found no benefit of bortezomib on outcome in the ABC subgroup determined via GEP,37 the updated 5-year survival results demonstrated improved PFS and OS with the addition of bortezomib to R-CHOP in patients with ABC.38 Other large-scale multicenter phase 3 studies have also attempted to improve outcome in untreated non-GCB tumors by adding targeted agents to standard R-CHOP: the PHOENIX trial with ibrutinib39 and the ROBUST trial with lenalidomide.40 Although ABC DLBCL tumors showed promising response to both ibrutinib and lenalidomide in preclinical and phase 1 or 2 studies,41-43 including the ECOG-ACRIN E1412 phase 2 study in which the addition of lenalidomide to R-CHOP demonstrated a potential clinical benefit in newly diagnosed DLBCL regardless of COO (both GCB and ABC),44 results of the PHOENIX and ROBUST phase 3 trials did not demonstrate a definitive benefit in the ABC subgroup. Further investigation of treatment regimens for the various DLBCL molecular groups is needed.
The addition of etoposide to the R-CHOP regimen (R-CHOEP) has been shown to improve outcome in young patients with high-risk DLBCL.45 However, it is uncertain whether baseline biological markers such as COO are prognostic of this effect. Some studies demonstrated no significant difference in outcome between GCB vs non-GCB (as determined by the Hans algorithm) after treatment with R-CHOEP.46,47 Frontzek et al48 also found no OS advantage with R-CHOEP and R-MegaCHOEP (high-dose chemotherapy plus rituximab followed by ASCR) between GCB and ABC, as determined by Lymph2CX.49 Gang et al found that COO (by Hans) predicted that GCB DLBCL had superior outcome after treatment with R-CHOEP.50 In contrast, sequential R-CHOP > (R)-ICE therapy improved the outcome for non-GCB DLBCL. Therefore, we do not think that the impact of sequential therapy is simply attributable to the addition of etoposide.
The recent results of the POLARIX trial suggest that substitution of polatuzumab vedotin for vincristine (pola-R-CHP) may improve PFS in the ABC phenotype as determined by the Nanostring Lymph2Cx assay.23 The magnitude of PFS benefit that Tilly et al observed with pola-R-CHP in the ABC phenotype compared with GCB (HR, 0.4; 95% CI, 0.2-0.6) may be slightly greater than the magnitude of EFS benefit we saw with R-CHOP > (R)-ICE in the non-GCB phenotype compared with GCB (HR, 0.57; 95% CI, 0.27-1.18). However, the high cost of polatuzumab vedotin limits its global availability and the R-CHOP > (R)-ICE regimen may represent a cost-effective alternative for patients with non-GCB DLBCL in certain resource-constrained regions of the world.
Although the Hans model is not a perfect surrogate for COO classification by GEP,12 our results support its use as a means of stratifying patients with DLBCL into prognostic subgroups. R-CHOP is less effective against non-GCB tumors compared with GCB tumors. Similar to the findings in the GELA trial LNH03-2B, we observed improved EFS in patients with non-GCB when treated with R-CHOP induction followed by noncross-resistant (R)-ICE consolidation. This offers a cost-effective treatment approach for this population with a poor outcome. A strength of this analysis is that the improvement in outcome was seen using the Hans algorithm based on IHC rather than the more precise GEP, which is not readily available in clinical laboratories. In conclusion, our results suggest that an intensified noncross-resistant sequential chemoimmunotherapy regimen, such as R-CHOP > (R)-ICE combination therapy, could be preferentially selected for patients with non-GCB tumors as classified by the widely applied Hans algorithm.
Acknowledgments
The research was supported by Memorial Sloan Kettering Cancer Center funding: CORE grant P30 CA008748 and Lymphoma SPORE grant 5 P50 CA192937, and Molecular Epidemiology Resource funding: P50 CA97274 and U01 CA195568.
Authorship
Contribution: K.S.B. and A.D.Z. designed the study; M.J. Matasar, A.J.M., D.J.S., A.N., M.L.P., S.M.H., P.A.H., C.S.P., J.R.C., T.M.H., G.A.S., G.S.N., C.H.M., and A.D.Z. provided study materials and patients; K.S.B., A.N.S., M.J. Maurer, J.R.C., T.M.H., G.S.N., and A.D.Z. coordinated and collected data; K.S.B., A.N.S., M.J. Maurer, J.R.C., G.S.N., and A.D.Z. analyzed and interpreted the data; K.S.B. and A.D.Z. wrote the manuscript; and all authors contributed to the final approval of the manuscript.
Conflict-of-interest disclosure: M.J. Maurer reports research funding from Roche/Genentech, Bristol Myers Squibb, and GenMab, and serves on the advisory boards of GenMab and Adaptive Biotechnologies. J.T.-F. is a full-time employee of Histowiz. M.J. Matasar reports compensation from Epizyme, Genentech, Roche, Kite, Bayer, Seagen, Celgene, ADC Therapeutics, IMV Therapeutics, and AstraZeneca. A.M. receives research support from Seattle Genetics, Merck, Bristol Myers Squibb, and Incyte, and receives honorarium from Kyowa Hakko Kirin Pharma, Miragen Therapeutics, Takeda Pharmaceuticals, ADC Therapeutics, Seattle Genetics, Cell Medica, Bristol Myers Squibb, and Erytech Pharma. D.J.S. receives compensation from InPractice Elsevier and Seattle Genetics, and is on speaker’s bureau for Medical Crossfire. A.N. received honoraria from Janssen, Pharmacyclics, and Prime Oncology; consults for Medscape; serves on an advisory board for Janssen; serves on the speaker's bureau for Prime Oncology; and receives research funding for Rafael Pharma and Pharmacyclics. S.M.H. received research funding from ADCT Therapeutics, Aileron, Forty-Seven, Verastem, Kyowa Hakko Kirin, Millennium Pharmaceuticals Inc, Celgene, Trillium, and Daiichii Sankyo, and consults for Astex, Affimed, Merck Sharp & Dohme, Kyowa Hakko Kirin Pharma, Corvus Pharmaceuticals Inc, Celgene, Portola Pharmaceuticals, Takeda Millennium, Innate Pharma, Verastem, Miragen Therapeutics Inc, Seattle Genetics, and ADCT Therapeutics. P.A.H. receives research support from Portola, Novartis/GlaxoSmithKline, Molecular Templates, and Janssen Pharmaceuticals, and served as a consultant for Karyopharm, Juno, Portola, Celgene, and AstraZeneca. T.M.H. reports research support from Genentech and Sorrento; serves on the data monitoring committee of Seagen, Tess Therapeutics, and Eli Lilly & Co; and serves on the scientific advisory boards of Eli Lilly & Co, MorphoSys, Incyte, BeiGene, and Loxo Oncology. G.A.S. reports advisory boards/consulting fees from AbbVie, ATB Therapeutics, Bayer, BeiGene, Bristol Myers Squibb/Celgene, Debiopharm, Epizyme, F. Hoffmann-La Roche Ltd/Genentech, Inc, GenMab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo/Lilly, Molecular Partners, MorphoSys, Nordic Nanovector, Novartis, Regeneron, and Takeda, and is a shareholder of Owkin. C.H.M. has held an employment/leadership position/advisory role for Celgene, Genentech, Merck & Co, and Seattle Genetics Inc; participated in an advisory board for Molecular Templates; and has received research funding from Pharmacyclics, Genentech, Merck & Co, Inc, and Seattle Genetics. A.D.Z. received research grants from AbbVie, Adaptive Biotechnologies, Bristol Myers Squibb, BeiGene, Genentech/Roche, and MEI Pharma; consulting fees from Amgen, AstraZeneca, BeiGene, Genentech/Roche, Janssen, JUNO/Celgene/Bristol Myers Squibb, Kite/Gilead, MEI Pharma, Pfizer, Pharmacyclics, and Sandoz/Novartis; and serves on the scientific advisory board of Adaptive Biotechnologies, Lymphoma Research Foundation. The remaining authors declare no competing financial interests.
Correspondence: Andrew D. Zelenetz, Department of Medicine, Division of Hematologic Malignancies, Lymphoma Service, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: zeleneta@mskcc.org.
References
Author notes
Data will be shared upon reasonable request from the corresponding author, Andrew D. Zelenetz (zeleneta@mskcc.org).
The full-text version of this article contains a data supplement.